Abstract
When patients with chronic kidney disease are infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) they can face two specific problems: virus‐specific immune responses may be impaired and remdesivir, an antiviral drug described to shorten recovery, is contraindicated. Antiviral treatment with convalescent plasma (CP) could be an alternative treatment option. In this case report, we present two kidney transplant recipients and two hemodialysis patients who were infected with SARS‐CoV‐2 and received CP. Antibodies against the receptor‐binding domain in the S1 subunit of the SARS‐CoV‐2 spike protein were determined sequentially by immunoglobulin G (IgG) enzyme‐linked immunosorbent assay (ELISA) and neutralization assay and specific cellular responses by interferon‐gamma ELISpot. Before treatment, in both kidney transplant recipients and one hemodialysis patient antibodies were undetectable by ELISA (ratio < 1.1), corresponding to low neutralizing antibody titers (≤1:40). ELISpot responses in the four patients were either weak or absent. After CP treatment, we observed an increase of SARS‐CoV‐2‐specific antibodies (IgG ratio and neutralization titer) and of specific cellular responses. After intermittent clinical improvement, one kidney transplant recipient again developed typical symptoms on Day 12 after treatment and received a second cycle of CP treatment. Altogether, three patients clinically improved and could be discharged from the hospital. However, one 83‐year‐old multimorbid patient deceased. Our data suggest that the success of CP therapy may only be temporary in patients with chronic kidney disease; which requires close monitoring of viral load and antiviral immunity and possibly an adaptation of the treatment regimen.
Keywords: cellular immunity, convalescent plasma, COVID‐19, ELISpot, hemodialysis, kidney transplantation
Highlights
After treatment with convalescent plasma we observed an increase of specific humoral and cellular immunity in two kidney transplant recipients and two haemodialysis patients with SARS‐CoV‐2 infection.
However, the success of convalescent plasma therapy was only be temporary in one transplant recipient.
Short‐term monitoring of viral load and antiviral immunity appears as mandatory for this patient group.
1. INTRODUCTION
In patients with chronic kidney disease and infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) treatment can be complicated because their immune function is suppressed due to medication to prevent allograft rejection and/or the underlying kidney disease. Thereby, the formation of specific antibodies and of T‐cell immunity is impaired; which can result in a prolonged persistence of SARS‐CoV‐2 (for up to 2 months 1 ). Furthermore, remdesivir, an antiviral nucleoside analog that shortened the time to recovery in adults hospitalized with coronavirus 2019 (COVID‐19) disease, 2 is contraindicated in this special cohort. Antiviral treatment with convalescent plasma (CP) could be an alternative treatment option. Data on patients with chronic kidney disease infected with SARS‐CoV‐2 and receiving CP treatment are still limited. We are aware of only 14 described kidney transplant recipients who received CP. 3 , 4 , 5 , 6 , 7 Whereas clinical improvement after CP has been shown for all six kidney transplant recipients included in three studies, 3 , 4 , 5 in the fourth study 6 a mortality rate for solid organ recipients (including six with kidney allograft) in the range of recipients without CP treatment 8 , 9 , 10 was reported (23% 6 vs. 24%–32%, 8 , 9 , 10 respectively). In the fifth study describing HIV‐infected kidney transplant recipients 7 one of the two patients died after having received CP treatment. However, the previous reports did not present data on the course of SARS‐CoV‐2‐specific antibodies or cellular responses in the patients.
It was the aim of the current study to follow‐up up virus‐specific humoral and cellular immunity in patients with chronic kidney disease who were infected with SARS‐CoV‐2 and received CP therapy. We functionally analyzed the antibodies (by neutralization assay) and measured specific cellular responses by the highly sensitive ELISpot method, using various protein antigens of SARS‐CoV‐2 as specific stimuli. Finally, in one transplant recipient who again developed typical COVID‐19 symptoms after initial clinical improvement, we had the chance to modify the treatment regimen and to apply the second cycle of CP therapy.
2. MATERIALS AND METHODS
2.1. Patients and blood donors
The current case report includes two renal transplant recipients and two hemodialysis patients (Table 1) and their respective CP donors. Within the study period (July 27 to September 9, 2020), all SARS‐CoV‐2 infected renal transplant and hemodialysis patients with an estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2 were included. The four patients included in the current study had chronic kidney disease according to the eGFR of 7–29 ml/min/1.73 m2. Both transplant recipients received tacrolimus, mycophenolate mofetil, and prednisone, both hemodialysis patients dexamethasone. The kidney transplant recipients were treated with prednisone to prevent organ rejection (which was not changed due to COVID‐19 infection), whereas the dialysis‐requiring patients were specifically treated with dexamethasone for 5 days to prevent an exaggerated immune response during COVID‐19 infection. Treatment with CP started when patients with chronic kidney disease without detectable immunoglobulin G (IgG) antibodies against SARS‐CoV‐2 showed increasing oxygen demand/clinical deterioration (RTX01, RTX02, and HD01) or when oxygen supply via nasal cannula was no longer sufficient in a patient with chronic kidney disease with detectable antibodies (HD02). One patient suffered from moderate (RTX02) and three from severe COVID‐19 disease. 11 More detailed information on the patients and the therapy used can be found in Table 1. One cycle of CP consisted of three units, separated with the Amicus™ (Fresenius Kabi), each containing 200–280 ml, which was applied at Days 1, 3, and 5. The study was approved by the local ethics committee (20‐9256‐BO for the patients and 20‐9225‐BO for the donors) and the study participants provided written informed consent. The procedures were in accordance with the institutional and national ethical standards as well as with the Helsinki Declaration of 1975, as revised in 2013. Four donors were selected based on their SARS‐CoV‐2 IgG ratio after polymerase chain reaction (PCR)‐confirmed SARS‐CoV‐2 infection and additional parameters like blood group and weight (Table 2). Details on the donor selection criteria have been described recently. 12
Table 1.
Clinical characteristics of patients with chronic kidney disease
| ID | Sex/age/blood group | CP intervala (days) | CP units/cycles | (Pre‐existing) comorbidity/cause of death | COVID‐19 therapy | Severity of COVID‐19 disease/outcome (discharge from hospitalb) |
|---|---|---|---|---|---|---|
| RTX01 | F/63/O | 3 | 6/2 | RTX 1997 and 2001, chronic antibody‐mediated rejection, hypertension, asthma bronchial, reactive arthritis | Oxygen administration via nasal cannula | Severec/A (d28) |
| RTX02 | F/62/A | 13 | 3/1 | RTX 14.08.2020 (13 days before SARS‐CoV‐2 infection), steroid‐induced diabetes, hypertension | No oxygen necessary (minimum oxygen saturation 92%) | Moderate (CT: pneumonia, but clinically asymptomatic)/A (d16) |
| HD01 | F/83/A | 4 | 2d/1 | HD since 02/2012, type II diabetes, coronary heart disease, atrial fibrillation, apoplexy, acute event of fall (no evidence of stroke)/COVID‐19 pneumonia | Oxygen administration first via nasal cannula, then 50–60 L/min high‐flow ventilation (FiO2 60%), dexamethasone | Severec/D |
| HD02 | F/78/O | 7 | 3/1 | HD since 01/2020, type II diabetes, hypertension, chronic obstructive pulmonary disease, adipositas | Oxygen administration via nasal cannula, dexamethasone | Severec/A (d8) |
Abbreviations: A, alive; COVID, coronavirus; CP, convalescent plasma; CT, computed tomography; D, deceased; HD, hemodialysis; RTX, renal transplantation; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Interval between the onset of symptoms or positivity to SARS‐CoV‐2 polymerase chain reaction and treatment with CP.
Discharge from hospital given as days after initiation of CP treatment.
Oxygen supplementation but no mechanical ventilation.
The patient deceased due to COVID‐19 pneumonia after having received two CP units.
Table 2.
Characteristics of convalescent plasma donors
| ID | Sex | Age | Blood group | Antibody ratio | Neutralizing antibody titer | HLA antibodies |
|---|---|---|---|---|---|---|
| D‐RTX01 | F | 55 | O | 5.83 | 1:1280 | neg |
| D‐RTX02 | M | 53 | A | 7.33 | 1:320 | neg |
| D‐HD01 | M | 40 | A | 10.44 | 1:160 | neg |
| D‐HD02 | F | 48 | O | 3.39 | 1:320 | neg |
Abbreviations: D, donor; neg, negative.
2.2. Antibody enzyme‐linked immunosorbent assay
To assess SARS‐CoV‐2‐specific humoral immunity, IgG antibodies in donor and patient sera were determined by a CE marked anti‐SARS‐CoV‐2 IgG semi‐quantitative enzyme‐linked immunosorbent assay (ELISA; Euroimmun), according to the manufacturer's instructions. The ELISA plates were coated with recombinant SARS‐CoV‐2 spike (S) 1 protein (receptor binding domain). Serum samples were analyzed automatically at a 1:100 dilution, using the Immunomat™ (Virion\Serion). Results are given as a ratio (patient sample/control sample). An antibody ratio of ≥1.1 was considered positive, of ≥0.8 to <1.1 borderlines and of <0.8 negative.
2.3. Virus neutralization assay
The function of specific antibodies was measured by a cell‐culture based neutralization assay, using Vero E6 cells (ATCC® CRL‐1586™) and a clinical isolate of SARS‐CoV‐2 in a biosafety level 3 laboratory. 12 , 13 Neutralization capacity was determined by endpoint dilution assay, expressed as 50% tissue culture infective dose (TCID50)/ml. Serial dilutions (1:20 to 1:1280) of the respective sera were preincubated with 100 TCID50 of SARS‐CoV‐2 for 1 h at 37°C and added afterward to confluent Vero E6 cells cultured in 96‐well microtiter plates. On Day 3 after infection, the cells were stained with crystal violet (Roth) solved in 20% methanol (Merck) and the appearance of cytopathic effects (CPE) was analyzed by light microscopy. The neutralizing titer was defined as the reciprocal of the highest serum dilution at which no CPE breakthrough in any of the triplicate cultures was observed.
2.4. ELISpot assay
To assess SARS‐CoV‐2‐specific cellular immunity, we performed ELISpot assays, using peptide pools of the S1/S2 protein, the S1 protein, and the membrane (M) protein (PepTivator®, Miltenyi Biotec) and an S1 protein antigen of SARS‐CoV‐2 (Sino Biological). The peptide pools consist mainly of 15‐mer sequences with 11 amino acids overlap. We tested 250,000 peripheral blood mononuclear cells per cell culture and measured interferon‐gamma (IFN‐γ) production after 19 h, as published recently in detail. 12 Spot numbers were analyzed by an ELISpot reader (AID Fluorospot; Autoimmun Diagnostika GmbH). Mean values of duplicate cell cultures were considered. SARS‐CoV‐2‐specific spots were determined as stimulated minus nonstimulated (background) values (spots increment). We defined threefold higher SARS‐CoV‐2‐specific spots versus background together with at least three spots above background as a positive response. This cut‐off was set based on negative control values as specified previously. 12
3. RESULTS
In both kidney recipients and one hemodialysis patient with undetectable SARS‐CoV‐2‐specific IgG (ratio < 1.1) and low neutralizing antibody titers ( ≤ 1:40; RTX01, RTX02, and HD01; Table 1) we observed an increase of antibody titers (Figure 1A‐C). A 63‐year‐old female who was transplanted twice (RTX01) initially showed a clinical response to CP therapy, but at Day 12 again developed typical symptoms of COVID‐19 disease (fever and shortage of air; Figure 2A,B). Therefore, she received another cycle of CP therapy (from the same donor). SARS‐CoV‐2 antibodies increased after both CP cycles and SARS‐CoV‐2 viral load decreased (C t value to the PCR increased from 17.8 to 25.8 after the first and to 34.9 after the second CP cycle; Figure 2C). The patient could be discharged from the hospital on Day 28 after initiation of CP treatment. Since Day 13 after initiation of CP therapy oxygen supplementation via nasal cannula could be completely stopped and at Day 29 viral load became undetectable. The second kidney transplant recipient (RTX02), a 62‐year‐old female who received her graft 13 days before the detection of SARS‐CoV‐2 infection, also showed a decrease of the viral load (C t value to the PCR increased from 21.6 to 30.3 after the CP cycle and to 35.4 on Day 16 after initiation of CP treatment, when the patient was discharged from the hospital). On Day 39 after CP therapy, SARS‐CoV‐2 viral load became undetectable in the nasopharyngeal swab. The third patient, an 83‐year‐old multimorbid female (HD01), showed no clinical improvement despite increasing neutralizing antibody titers and decreasing C‐reactive protein and deceased due to COVID‐19 pneumonia on Day 4 after initiation of CP therapy. She had been on hemodialysis for 8 years, suffered from diabetes mellitus, coronary heart disease, had apoplexy in 2010, and an acute event of fall.
Figure 1.
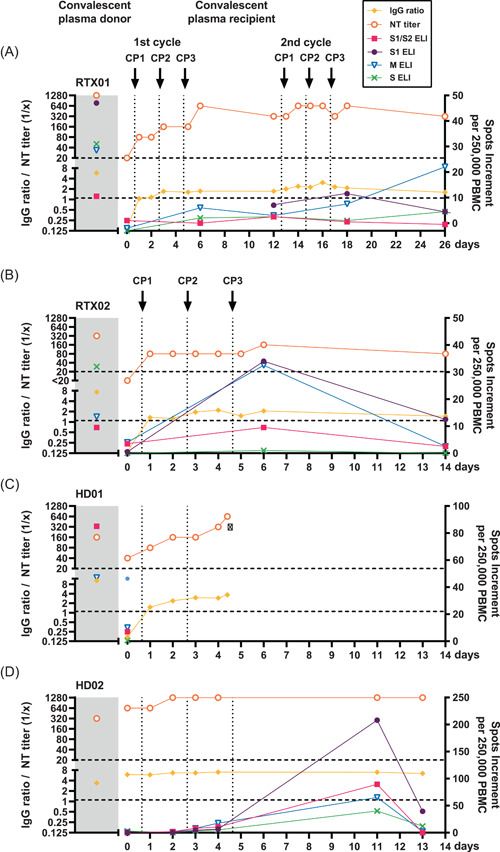
Course of specific humoral and cellular immunity in four patients with chronic kidney disease infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and receiving convalescent plasma treatment. Antibodies were determined by an S1 specific immunoglobulin G (IgG) enzyme‐linked immunosorbent assay (Euroimmun) and by cell‐culture based neutralization assay (NT titer). Cellular responses were analyzed by an interferon‐gamma (IFN‐γ) ELISpot assay, using peptide pools of the S1/S2, S1, and M protein and an S1 protein antigen as specific stimuli (depicted as S1/S2, S1, M, and S ELI). We here present data on two kidney transplant recipients (RTX01, RTX02) and two patients on hemodialysis (HD01, HD02) and compared their immune responses with those of the corresponding donors of convalescent plasma (CP; shaded area). SARS‐CoV‐2‐specific antibody data (IgG ratio and NT titer) are given on the left Y‐axis and ELISpot data on the right one. Horizontal dashed lines represent the cut‐off values for positive reactions (IgG ratio of 1.1 and NT titer of 1:20). Vertical dotted lines indicate the time points of convalescent plasma applications (CP1, CP2, and CP3). Related data points are connected. PBMC, peripheral blood mononuclear cells
Figure 2.
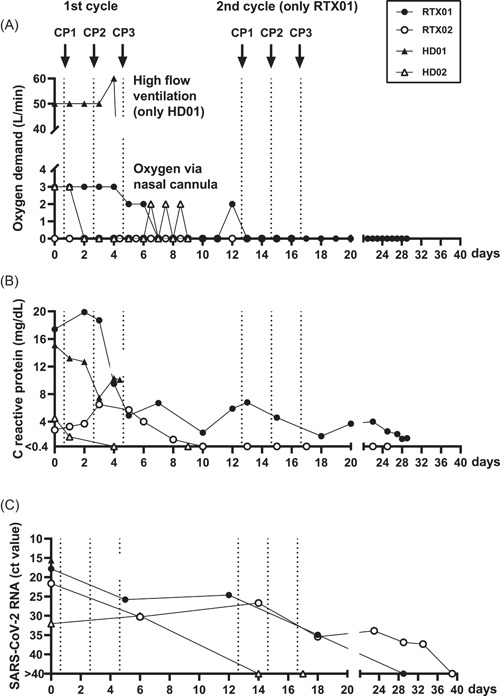
Course of oxygen demand, C‐reactive protein, and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) viral load in four patients with chronic kidney disease infected with SARS‐CoV‐2 and receiving convalescent plasma treatment. We here present data on two kidney transplant recipients (RTX01, RTX02) and two patients on hemodialysis (HD01, HD02) which were tested up to Day 39 after receiving convalescent plasma (CP). Vertical dotted lines indicate the time points of convalescent plasma applications (CP1, CP2, and CP3). Of note, only RTX01 received two cycles of CP while the remaining three patients received one cycle. A ct value of SARS‐CoV‐2 RNA > 40 was considered negative
The antibody ratios in these first three patients before the CP therapy were 0.15, 0.14, and 0.17, and the respective neutralizing antibody titers 1:20, <1:20, and 1:40. After CP therapy, antibodies in the patients reached a maximum ratio of 3.07, 2.19, and 3.70, corresponding to a neutralizing titer of up to 1:640, 1:160, and 1:640, respectively. In the donors, the antibody ratios were 5.83, 7.33, and 10.44, and the neutralizing titers 1:1280, 1:320, and 1:160, respectively.
The fourth patient, a 78‐year‐old female with pre‐existing antibodies (HD02), showed rapid clinical improvement and could be discharged from the hospital on Day 8 after initiation of CP treatment. Before CP treatment, SARS‐CoV‐2 was detectable by PCR at a low level (C t value of 31.1). On Day 14 after CP therapy, viral load was undetectable. The patient also showed an increase of specific immunity (ratio 5.96 → 7.01; neutralizing titer 1:640 → 1:1280; Figure 1D). However, SARS‐CoV‐2‐specific antibodies in the CP donor of the fourth patient were lower than in the patient (ratio: 3.39, neutralizing titer: 1:320).
Cellular immunity could be followed‐up by IFN‐γ ELISpot, using four different SARS‐CoV‐2‐specific antigens (peptide pools of the S1/S2, S1, and M protein and an S1 protein antigen). Before CP treatment, one patient was negative to the ELISpot (HD02) and three showed weak responses (RTX01, RTX02, and HD01). Three patients could be followed‐up after CP treatment. In these three patients, IFN‐γ production to the ELISpot intermittently increased, reaching a maximum at Days 6–14 after CP therapy.
4. DISCUSSION
Our data show an increase of specific humoral and cellular immunity in two kidney transplant recipients and two hemodialysis patients with SARS‐CoV‐2 infection after treatment with CP. This may represent the natural course of infection. However, the increase of immune responses occurred very close in time to the administration of CP; which suggests that there may be a causal relationship between treatment with CP and the increase in humoral and cellular immune responses. CP contains neutralizing antibodies as well as anti‐inflammatory cytokines and other immunomodulatory proteins. This combination could improve virus control in immunocompromised patients. 3 CP therapy thus could bridge the phase of acute COVID‐19 disease. However, presumably due to drug‐induced immunosuppression or impaired kidney function, the immune responses were not as long‐acting as expected. In one patient with two prior kidney transplantations (RTX01) two cycles of therapy were necessary for successful treatment. It can be supposed that the patient herself was unable to mount an adequate antibody response and that the passively transferred antibodies partly bound the virus that resides in the affected organs and in the respective lymphoid tissue. 14 Theoretically, it is possible that CP therapy mitigates the native humoral immune response and leaves an individual vulnerable to subsequent reinfection with SARS‐CoV‐2. 3 , 15 This phenomenon appears more likely in immunosuppressed versus otherwise healthy individuals. Concerning ELISpot data, we observed a maximum of IFN‐γ responses shortly after completion of the CP cycle. Of note, cellular immunity is regarded as important for recovery from SARS‐CoV‐2 infection 16 and appears as short‐lived in the current cohort. As CP therapy is a form of passive immunization, an increase in cellular responses is not expected at first glance. After an initial increase, IFN‐γ production decreased again, which could reflect the fact that proinflammatory immune responses shifted to anti‐inflammatory responses. 17 It has already been shown that there was a reduction in proinflammatory cytokines like IL‐6 and an increase in anti‐inflammatory cytokines after CP was administered. 18 , 19 , 20 Moreover, chronic kidney disease suppressed T‐cell function, which could impede long‐term protection against reinfection. 3 , 21
Three out of four patients with chronic kidney disease showed clinical improvement; which is in the range of previous reports. 3 , 4 , 5 , 6 However, due to the low patient number, it was beyond the aim of our study to answer the question of CP therapy was effective. This answer can only be given by large randomized clinical studies such as the Randomized Evaluation of COVID‐19 Therapy (RECOVERY) trial 22 ; which is currently underway.
In conclusion, our data suggest that despite an increase of SARS‐CoV‐2‐specific immunity the success of CP therapy may only be temporary in patients with chronic kidney disease. Thus, short‐term treatment control (monitoring of viral load and antiviral immunity) appears mandatory for this patient group. If necessary, the treatment regimen has to be adapted.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
The study was approved by the local ethics committee (20‐9256‐BO for the patients and 20‐9225‐BO for the donors) and the study participants provided written informed consent.
AUTHOR CONTRIBUTIONS
Monika Lindemann, Adalbert Krawczyk, Veronika Lenz, Ulf Dittmer, Peter A. Horn, and Oliver Witzke conceived and designed the study. Laura Thümmler, Sina Schwarzkopf, Leonie Schipper, Maren Bormann, and Lukas van de Sand performed the experiments and analyzed the data. Sebastian Dolff, Margarethe Konik, Hana Rohn, Maximillian Platte, Marianne Breyer, Hannes Klump, Dietmar Knop, Veronika Lenz, Christian Temme, Peter A. Horn, and Oliver Witzke took care of the patients or convalescent plasma donors and participated in the collection and interpretation of data. Monika Lindemann had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. Monika Lindemann, Adalbert Krawczyk, Sebastian Dolff, Peter A. Horn, and Oliver Witzke wrote the manuscript. All authors gave final approval of the manuscript.
ACKNOWLEDGEMENTS
This study was supported by the Stiftung Universitätsmedizin Essen (Adalbert Krawczyk) and the Rudolf Ackermann Foundation (Oliver Witzke). The authors would like to thank Babette Große‐Rhode and Martina Filipovic for their excellent technical assistance. The authors, furthermore, thank all volunteers for their participation and the donation of blood samples. Open Access funding enabled and organized by Projekt DEAL.
Lindemann M, Krawczyk A, Dolff S, et al. SARS‐CoV‐2‐specific humoral and cellular immunity in two renal transplant and two hemodialysis patients treated with convalescent plasma. J Med Virol. 2021;93:3047–3054. 10.1002/jmv.26840
REFERENCES
- 1. Gajurel K. Persistently positive severe acute respiratory syndrome coronavirus 2 (SARS‐COV2) nasopharyngeal PCR in a kidney transplant recipient. Transpl Infect Dis. 2020;22:e13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—Preliminary report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PubMed] [Google Scholar]
- 3. Naeem S, Gohh R, Bayliss G, et al. Successful recovery from COVID‐19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl Infect Dis. 2020:e13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiang J, Miao Y, Zhao Y, et al. Convalescent plasma therapy: helpful treatment of COVID‐19 in a kidney transplant recipient presenting with serve clinical manifestation and complex complications. Clin Transplant. 2020;34(9):e14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fung M, Nambiar A, Pandey S, et al. Treatment of immunocompromised COVID‐19 patients with convalescent plasma [published online ahead of print September 29, 2020]. Transpl Infect Dis. e13477 10.1111/tid.13477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rahman F, Liu STH, Taimur S, et al. Treatment with convalescent plasma in solid organ transplant recipients with COVID‐19: experience at large transplant center in New York City. Clin Transplant. 2020;34:e14089. [DOI] [PubMed] [Google Scholar]
- 7. Mehta SA, Rana MM, Motter JD, et al. Incidence and outcomes of COVID‐19 in kidney and liver transplant recipients with HIV: report from the National HOPE in Action Consortium. Transplantation. 2021;105(1):216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cravedi P, Mothi SS, Azzi Y, et al. COVID‐19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140‐3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO . Clinical management of severe acute respiratory infection when COVID‐19 is suspected. 2020; https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed November 26, 2020.
- 12. Schwarzkopf S, Krawczyk A, Knop D, et al. Cellular Immunity in COVID‐19 convalescents with PCR‐confirmed infection but with undetectable SARS‐CoV‐2‐specific IgG. Emerg Infect Dis. 2021;27(1):122‐129. [DOI] [PubMed] [Google Scholar]
- 13. Heilingloh CS, Aufderhorst UW, Schipper L, et al. Susceptibility of SARS‐CoV‐2 to UV irradiation. Am J Infect Control. 2020;48(10):1273‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J, Zheng X, Tong Q, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J Med Virol. 2020;92(5):491‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crowe JE Jr, Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell‐mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol. 2001;167(7):3910‐3918. [DOI] [PubMed] [Google Scholar]
- 16. Braun J, Loyal L, Frentsch M, et al. SARS‐CoV‐2‐reactive T cells in healthy donors and patients with COVID‐19. Nature. 2020;587:270‐274. 10.1038/s41586-020-2598-9 [DOI] [PubMed] [Google Scholar]
- 17. Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID‐19. Nature. 2020;584(7821):463‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rojas M, Rodríguez Y, Monsalve DM, et al. Convalescent plasma in Covid‐19: possible mechanisms of action. Autoimmun Rev. 2020;19(7):102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaiswal V, Nasa P, Raouf M, et al. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID‐19—An exploratory study. Int J Infect Dis. 2021;102:332‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kronbichler A, Gauckler P, Windpessl M, et al. COVID‐19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020;16(7):365‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haynes R. Randomised evaluation of COVID‐19 therapy (RECOVERY). 2020; https://clinicaltrials.gov/ct2/show/NCT04381936. Accessed November 26, 2020.


