Abstract
Given the profound impact of language impairment after stroke (aphasia), neuroplasticity research is garnering considerable attention as means for eventually improving aphasia treatments and how they are delivered. Functional and structural neuroimaging studies indicate that aphasia treatments can recruit both residual and new neural mechanisms to improve language function and that neuroimaging modalities may hold promise in predicting treatment outcome. In relatively small clinical trials, both non-invasive brain stimulation and behavioural manipulations targeting activation or suppression of specific cortices can improve aphasia treatment outcomes. Recent language interventions that employ principles consistent with inducing neuroplasticity also are showing improved performance for both trained and novel items and contexts. While knowledge is rapidly accumulating, larger trials emphasising how to select optimal paradigms for individualised aphasia treatment are needed. Finally, a model of how to incorporate the growing knowledge into clinical practice could help to focus future research.
INTRODUCTION
Aphasia, acquired impairment of expression and/or comprehension in spoken and written language, is associated with greater negative impact on quality of life than any other disease or medical condition, including cancer and Alzheimer’s disease,s1 and its severity predicts functional autonomy after stroke.s2 Hence, assisting persons with aphasia to recover language function is a critical research priority. Aphasia recovery is a complex process which may involve neural changes that facilitate or interfere with language outcomes. Thus, aphasia treatment should focus on restoration or reorganisation processes that bring about beneficial neural change. Understanding how neural changes underlie aphasia treatment, how to induce such neural changes and the limits of such plasticity is critical for developing effective new treatments, improving existing ones and predicting treatment response. Neuroplasticity is the term used to refer to these neural changes supporting learning, or as applied to the current topic, relearning of language elements and processes. The structural and physiological changes that constitute neuroplasticity occur at the synaptic, cellular and macrostructural levels. Practically, it is only possible to study neuroplasticity in humans at the macrostructural level; hence, this review focuses on this level of measurement. For aphasia rehabilitation, studying neuroplasticity at this level involves understanding treatment-induced changes in brain systems and how these changes impact rehabilitation outcome.
Over the past several years, important developments in neuroplasticity research include refinement of the tools to measure neural mechanisms supporting rehabilitative change and use of various methods to induce neural change. Yet, our knowledge regarding the role of neuroplasticity in aphasia treatment is nascent. This review emphasises the most important developments in the neuroplasticity literature for the last several years as applicable to treatment of stroke-induced aphasia. Our goal is to provide a unique and integrative overview that broadly covers the substantive areas of neuroplasticity relevant to aphasia treatment and that is both accessible to generalists and useful for rehabilitation specialists. In contrast to recent reviews which focus solely on treatment-induced function and structural changess3 or factors influencing neuroplasticity following intensive treatments,s4 our review of neuroplasticity in aphasia treatments encompasses a broader scope. We start by discussing neuroimaging developments that allow for measurement of structural and functional changes and prediction of outcome in aphasia treatment research, and then we turn to a variety of methods for inducing neuroplasticity during aphasia treatment. Finally, we discuss the implication of neuroplasticity research and propose a model for using research findings to inform clinical aphasia treatment in the future.
Neuroimaging and neuroplasticity
Functional and structural neuroimaging provide important information about how brain systems change as a result of aphasia therapy. These techniques provide empirical evidence to determine the degree to which different treatments rely on restorative versus reorganising processes. Imaging can also provide important clues to therapeutic processes, such as whether patients are relearning words as opposed to reactivating dormant information stores or processes or whether patterns of damage predict good or poor outcome. The information gleaned from imaging studies can help determine how to induce neuroplastic changes that optimise outcomes for current and future aphasia treatments.
Measuring neuroplasticity
Functional neuroimaging maps brain activity during a task, revealing areas engaged in language functions, which allows visualisation of language system changes from pre-treatment to post-treatment. The advantage of this method is that the brain is perturbed by a stimulus; the downside is interpreting resultant brain maps if patients are clearly struggling with the task or produce many errors. For aphasia treatment, scans most commonly map activity for a language function of interest versus some control condition. Recently, scans taken during a resting state (no task) have also been used to measure changes in functional connexions between brain regions.1 The advantage of this method is that it can be applied in theory to all patients; the downside is that one has no real handle on what cognitive processing is going on while the data are collected. Structural imaging can also identify practice-induced neuroplastic changes in both grey and white matter, particularly in longitudinal studies.s5
One important question is whether residual learning capacity is supported by brain mechanisms engaged in language-related processing pre-injury (ie, restorative mechanisms) or whether there is recruitment of mechanisms not previously involved in language processing (ie, reorganising mechanisms). A related issue is the overlap in brain structures that support word learning in healthy adults, which engages the medial temporal lobe, and word re-learning and associated neocortical plasticity in aphasia, which appears to rely on integrity and functional engagement of memory structures such as the hippocampus.2,3 The implication when patients rely on hippocampal activity is that they are re-learning as opposed to simply activating latent neural patterns maintained from premorbid encoding, implying reorganisation to cortices not previously supporting word retrieval. However, spared networks within the dominant middle cerebral artery (MCA) territory also are likely to be relevant. For example, the amount of improvement in naming ability following early intensive therapy has been shown to correlate with increased activation of the left inferior frontal gyrus (IFG),4 a structure known to be involved in language for neurologically normal persons.5s6,s7 Furthermore, recovery patterns revealed in neuroimaging studies may depend on what cognitive processes different therapies engage. For example, after training in producing specific sentence structures, aphasic patients showed increased activity in right-hemisphere structures during verb production that were different from areas neurologically normal groups activate,6 indicating reorganisation of function to new brain regions.
In the largest study to date, Fridrikssons8 identified task-dependent pre-intervention to post-intervention activity increases for picture naming in residual anterior and posterior left-hemisphere regions that were associated with positive treatment response. In contrast to these findings of increased activity, Abel et al7 demonstrated decreased activity in left-hemisphere and right-hemisphere regions that correlated with improved naming ability. This difference in therapy-driven response patterns between studies seems paradoxical. It is reasonable to expect that brain activity will increase in nodes of recovery networks that do more ‘neuronal heavy lifting’. However, activity in these nodes also may decrease later in therapy as networks become more efficient; that is, with additional practice, brain networks require less neural activity (as indicated by functional neuroimaging) to perform language functions at an equal or higher level than before practice.s9 The length of therapy trials in these studies is consistent with this suggestion (2 weeks in Fridriksson vs 4 weeks in Abel et al), but trials with repeated scanning at different stages of treatment are needed to properly understand the determinants and consequences of increased vs decreased activity. A recent study using a cued picture-naming task further illustrates the possible role of decreased activity in therapy-based improvements. A bilateral frontal network including the right anterior insula, inferior frontal and dorsal anterior cingulate cortices, and the left premotor cortex showed reduced activity for cued as opposed to uncued words from pre-treatment to post-treatment scans for trained versus untrained words, indicating increasing facilitation by speech-sound cueing as a result of treatment.8
Another key finding is that treatment-induced brain changes are not just related to language processing per se. Treatment success may require brain mechanisms involved in multiple cognitive processes, including determining the salience of stimuli, attending to them and/or regulating cognitive control.9,10 Consensus in the field is that complex cognitive processes are mediated by interacting distributed brain systems, indicating that in addition to specific brain regions, we should seek to identify therapeutic effects in network connexions.s10 For example, it was recently shown that an increase in connectivity within networks and increased segregation between networks’ activity during resting-state scans over the course of therapy was associated with greater treatment response.1,11,12 Another study demonstrated that an auditory therapy for ‘Wernicke-type’ aphasia induced changes in behaviour and altered network connectivity within the left superior temporal gyrus (STG) as well as connectivity between the left STG and left primary auditory cortex (Heschl’s gyrus (HG), figure 1).13 Different types of therapy may have differential effects on the nature and extent of neuroplasticity that occurs within these networks and may differentially engage left-hemisphere versus right-hemisphere networks. For instance, treatment of word retrieval has been associated with increased functional connectivity in left-hemisphere networks,14 whereas right-hemisphere sensorimotor networks have shown increased functional connectivity in response to an Action Observation Therapy.15
Figure 1.
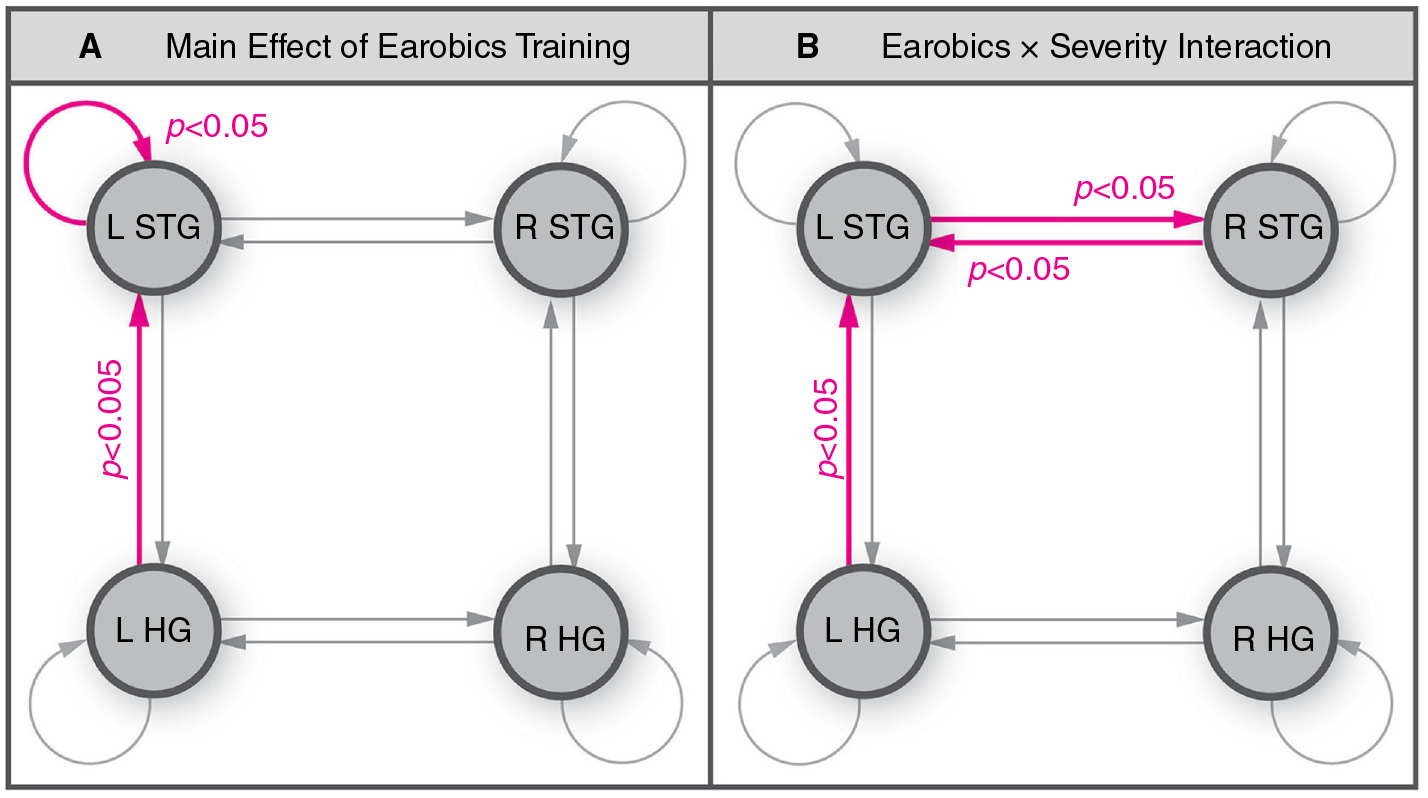
The importance of connectivity changes between elements of the language system resulting from aphasia therapy is illustrated by the work of Woodhead and colleagues.13 Specifically, effects of a phonological therapy (‘Earobics’, an e-therapy) on the connectivity within the temporal lobes of 20 patients with chronic ‘Wernicke-type’ aphasia are shown. Phonological training resulted in a small but significant improvement in patients’ speech comprehension. (A) The magnetoencephalography (MEG) connectivity analysis demonstrated that phonological training increased synaptic gain in the L STG as well as connectivity between the L STG and primary auditory cortex (HG). Pink connexions showed significantly stronger phonemic sensitivity after Earobics training (main effect of Earobics). (B) Also (not discussed in text), as opposed to increased synaptic gain in the L STG, patients with more severe speech comprehension impairments showed strengthening of bidirectional connexions between the left and right STG. L HG, left Heschl’s gyrus; L STG, left superior temporal gyrus; R HG, right Heschl’s gyrus; R STG, right superior temporal gyrus. This figure is adapted from the original figure (figure 5) in J Neurol Neurosurg Psychiatry (online first): 04 March 2017. DOI: 10.1136/jnnp-2016-3 14 621 (Link to license: http://creativecommons.org/licenses/by/4.0/” and http://jnnp.bmj.com/content/early/2017/03/04/jnnp-2016-314621.info).
Compared with functional neuroimaging studies, less research has focused on structural brain changes associated with robust and lasting changes in language function. The first study demonstrating structural brain changes associated with aphasia treatment observed an increase in the number of fibres and volume of the right arcuate fasciculus following melodic intonation therapy (MIT).s11 This is consistent with the view that MIT leverages right-hemisphere-mediated melodic intonation abilities to improve spoken language. More recent studies further support the notion that aphasia recovery relies on changes in brain structure. Allendorfer et al showed that 10 sessions of excitatory repetitive transcranial magnetic stimulation (rTMS) over the left hemisphere leads to increased fractional anisotropy (FA), a measure thought to reflect axonal density, in left frontal regions as well as the corpus callosum.16 Interestingly, decreased FA was revealed in the fusiform gyrus and left cerebellum, suggesting that the effects of rTMS were not unidirectional. These two studies did not find a linear relationship between language improvement and changes in white matter density. However, a more recent diffusion tensor imaging (DTI) study demonstrated that the extent of improvement associated with phonologically-based word retrieval treatment was linearly related to increased white matter structural integrity (FA) for the left arcuate fasciculus,17 though lower FA in the right arcuate fasciculus was associated with improved speech for MIT.18 More research is needed to determine the role of white matter changes in aphasia treatment success.
McKinnon et al19 used diffusion kurtosis imaging, which is thought to be more sensitive than DTI to microstructural changes,20 to examine white matter changes associated with aphasia treatment. The study revealed that normalisation (increase) in mean kurtosis, a measure of microstructural density, in the inferior longitudinal fasciculus was associated with decreased semantic but not phonological naming errors, suggesting that restored integrity of this structure improved semantic processing of words. This finding is consistent with the hypothesised role of the inferior longitudinal fasciculus in language processing.
Studies of aphasia treatment-induced structural changes in grey matter are rare. A recent longitudinal study of natural (rather than treatment-induced) recovery in chronic aphasia used voxel based morphometry (VBM) to assess changes in right-hemisphere grey-matter density across two time-points, which in turn were correlated with changes in language functions.21 Changes in naming accuracy were associated with both increased and decreased right-hemisphere grey matter density in the anterior temporal lobe and the precentral gyrus, respectively. A different, cross-sectional study also showed positive correlations between spoken word comprehension and grey-matter density from VBM in the right middle temporal gyrus and insula and between spoken word production and grey-matter density in the right supplementary motor area cortex and insula.22 These chronic aphasia studies indicate that VBM may have potential for measuring treatment-induced grey-matter changes in aphasia.
In summary, functional neuroimaging studies show differences in brain areas engaged in language processing as a result of therapy. Structural neuroimaging studies show that changes in white and possibly grey matter also occur. However, the relationship of functional and structural changes in response to treatment has yet to be determined, but is highly relevant in predicting neuroplastic changes, and is supported by structural-functional relationships determined in a large healthy cohort.s12 Whether therapies restore left perisylvian activity or reorganise activity to right-hemisphere structures seems to be treatment-dependent.s4 However, current studies are limited by small sample sizes and differences in methodologies. Replications of studies with larger samples and more consistent methodology will lend greater confidence to findings. Furthermore, as reliable evidence accumulates, the longitudinal application of neuroimaging to aphasia therapy studies, a relatively new phenomenon, will reveal overarching principles that guide development of more efficient therapies and a greater understanding of neural mechanisms that support them. For example, therapy-driven changes in language ability may correlate with changes in brain networks known to support general cognition such as the ‘cognitive-control network’. In this case, therapy trials (eg, behavioural ± non-invasive brain stimulation (NIBS)) may be directed towards optimising the role of this network in rehabilitation. This type of insight cannot be gleaned from behavioural responses alone.
Predicting aphasia treatment outcome
The location and degree of damage to language-related brain structures and the impact of that damage on functional systems will place limits on the neuroplasticity necessary for successful aphasia therapy. While functional and structural neuroimaging measures yield insights into how the brain reorganises during various treatments, using neuroimaging methods to predict treatment outcome has direct clinical implications. Specifically, regions of brain activity or damage that predict therapeutic outcome could be used as an aid in selecting treatments that are likely to succeed given a specific pattern of activity or damage. Compared with studies of remapping of brain structures and functions as a result of treatment, this is an under-studied area of research.
There are limited examples of functional neuroimaging measures at baseline that predict aphasia therapy outcome. Fridriksson et al23 showed that changes in brain activity resulting from therapy within left temporal and parietal regions predicted treatment induced naming improvements and reductions in naming errors, while baseline functional activity alone was less informative. Specifically, functional activity in the residual language network (perilesional frontal lobe) predicted post-treatment changes in semantic paraphasias but not other measures of naming improvement. One recent smaller scale study found that pre-treatment activity in the left caudate nucleus during picture naming predicted positive therapeutic success in a picture-naming treatment relying on semantic feature analysis.24 In summary, these two studies indicate areas of activity during functional neuroimaging of language have potential for predicting therapeutic outcome, but much more research is needed before such information can be applied clinically.
Voxel-based lesion symptom mapping (VLSM) is a technique used to determine whether presence vs absence of lesion at the voxel level predicts language abilities. It also can be used to determine if lesion location predicts treatment outcome for aphasia treatment. For example, speech entrainment (SE), an intervention that relies on mimicking speech in real time, may be beneficial for patients with non-fluent aphasias. Using VLSM, Fridriksson et al25 found that a positive response to SE was associated with inferior and middle frontal gyri lesions. This finding indicates that SE compensates for damage to language production mechanisms located in the IFG, provided that alternative neural pathways are still intact to support the function.
Predicting treatment outcome based on the integrity of white matter networks using DTI is another approach. In a recent study,26 diffusion imaging scans were performed prior to 30 hours of a naming therapy that involved semantic and phonemic cuing hierarchies. Not surprisingly, it was shown that a greater global language network integrity of white matter connexions led to greater treatment gains in naming, most likely because more of the original connexions were preserved and more alternate connexions were available for remapping of language function. On a more regional level, this study also showed that preserved integration of the left temporal lobe translated to increased treatment gains. Measures of integration in this study reflect the number of the network’s shortest paths that travel through selected temporal lobe nodes.
In summary, a few studies suggest the potential of functional and structural neuroimaging in predicting therapy outcome. Given the increasing availability of neuroimaging data in clinical care, it is straightforward to suggest that future clinical management of aphasia, including its rehabilitation, will rely on measures of brain damage and residual connectivity to predict long-term outcome and eventually to personalise treatment selection.
Inducing neuroplasticity to improve treatment outcomes
While neuroimaging technologies can be used to measure and predict neuroplasticity, the rise of neuroplasticity in aphasia treatment research has raised another critical question: How can we capitalise on and enhance the brain’s natural inherent plasticity that undergirds all forms of learning? Below, three ways to accomplish this goal are discussed: (1) neuromodulation using NIBS, (2) application of behavioural principles shown to stimulate neuroplasticity, and (3) neuromodulation by incorporating non-language behaviours into treatment.
NIBS to enhance treatment effects
NIBS alters neural excitability which can enhance the potential for training-induced plasticity, either by facilitating activity in recovery-relevant regions or by suppressing dysfunctional neural processes (eg, reversing maladaptive neuroplasticity). The two most frequently used NIBS techniques have been rTMS and transcranial direct current stimulation (tDCS).s13,s14 rTMS works by inducing an electrical current from changes in magnetic fields that causes neurons in target cortex to fire. Low-frequency stimulation (1 Hz) decreases and high-frequency stimulation (≥5 Hz) increases cortical excitability.s15 tDCS depolarises neurons under an anode on the scalp and hyperpolarises neurons under a cathode,s16 though effects can vary depending on current strengths17 (See figure 2 for typical tDCS protocols). There are differences between these techniques regarding focality of the stimulation, ease of application and associated costs,s18 but both can enhance the potential for adaptive neuroplasticity when administered alone (rTMS) or combined with behavioural interventions (rTMS and tDCS). Importantly, tDCS alone has not been shown to induce adaptive neuroplasticity.s14
Figure 2.
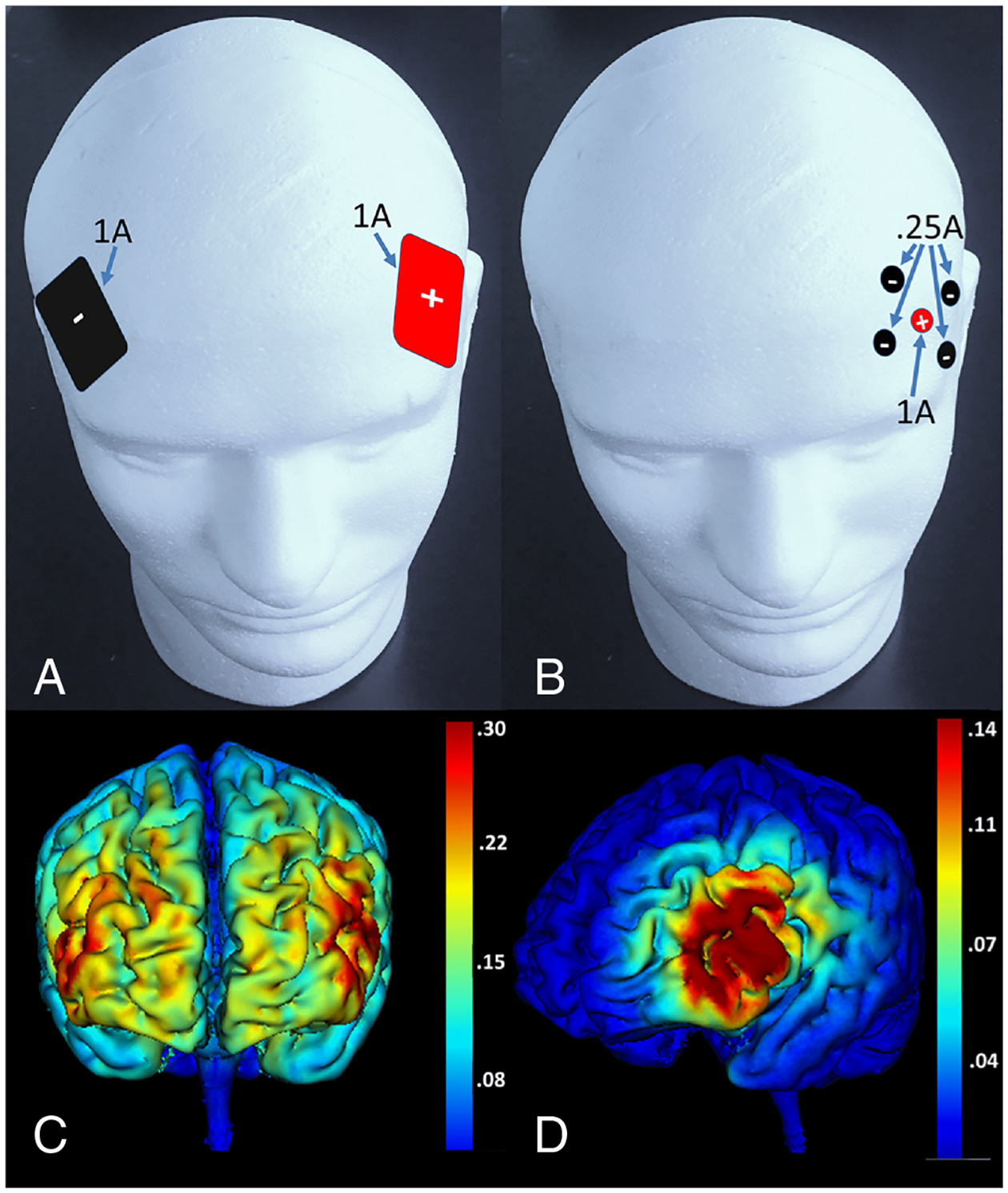
Difference in electrode placement and electrical field intensity for ‘traditional’ and high-definition tDCS at 1 MA current, illustrated in healthy brains. (A) Placement of traditional 5×7 cm electrodes approximately over inferior frontal sulcus with the anode over the left hemisphere and the cathode over the right hemisphere. To date, most studies employing tDCS in aphasia have use traditional large electrodes, though with a variety of placements. (B) Placement of one anode (approximately over left inferior frontal sulcus) and four surrounding cathodes for high-definition tDCS. Note the current distributes in approximately equal fractions over the four cathodes while it is at full strength at the anode. (C) Field intensity map showing broad distribution of current over the frontal lobes for the placement of traditional electrodes in panel A. (D) Field intensity map showing more focal stimulation in inferior and middle frontal gyri for the high- definition electrode placement in panel B. Even though high-definition tDCS produces more focal effects than traditional tDCS, it is still less focal than rTMS. It is critical to note that biophysical models have demonstrated that the induced current flow may be substantially different in healthy individuals (this figure) than in patients with stroke. Dmochowski and colleaguess25 have illustrated field maps for ‘traditional’ tDCS in several stroke brains and have provided excellent models for how high-definition electrode placement can be used to target specific cortices in these brains. (Current maps for this figure were created with HD explore software, Soterix medical, Inc., New York, NY.). tDCS = transcranial direct current stimulation.
Stimulation protocols are guided by assumptions about contributions of different brain regions to recovery, which can be derived from functional imaging.s10,s19 To date, NIBS approaches have included: (1) excitatory stimulation of spared perilesional left-hemisphere regions recruited to subserve language function after strokes19, s20; (2) inhibition of right-hemisphere regions that hinder recovery (eg, pars triangularis in the IFG)27,28; (3) combined excitatory left-hemisphere and inhibitory right-hemisphere stimulation,29,30 (4) facilitation of compensatory right-hemisphere homologues of lesioned areas by excitatory stimulations21 or (5) stimulation of non-language regions that are relevant to language production (eg, motor regions).31,32
Evidence for efficacy of these approaches to enhance aphasia treatment outcome is mainly limited to relatively small (N<60) experimental trials, but recent meta-analyses suggest that either rTMS or tDCS can enhance treatment outcome in both subacute and chronic patients.s22–s24 Qualitative appraisal of stimulation effects on naming ability in aphasia suggests that both methods result in similar add-on effects to those of behavioural treatment.s24 However, while the majority of placebo-stimulation-controlled trials reported significant enhancement of treatment outcomes across patient groups33,34 and some also generalise to everyday communication,31,33 closer inspection of individual patient data reveals substantial heterogeneity of stimulation response.s19, 27, 31 Because NIBS approaches are most efficacious when combined with behavioural treatment, some of the observed heterogeneity between studies may be attributable to variable efficacy of concurrent treatments. Predicting stimulation effects in aphasia is further complicated by variable and recovery-stage-dependent brain reorganisation after strokes10, s14 and the effects that varying lesion locations cause to current flow during tDCS.s25 Therefore, choice of optimal stimulation protocols is not straightforward.
Genetic factors may be another source affecting response to NIBS in aphasia treatment. For example, the recent discovery that the val66met brain-derived neurotrophic factor (BDNF) polymorphism affects response to tDCS but not to aphasia therapy34 indicates that behavioural interventions induce learning and neuroplasticity through different mechanisms than tDCS. If the findings regarding the effect of this BDNF polymorphism hold up to further scrutiny, then this genetic variation would be a good indicator as to whether supplementing aphasia treatment with NIBS will be productive.
Recently, there have been efforts to optimise stimulation outcomes: For example, Shah-Basak et al explored efficacy of different tDCS protocols to modulate naming ability prior to treatment. An alternative approach involves mapping of the residual language network using functional imagings19, s20 to identify stimulation sites vital to recovery.33s8, s26 This approach can induce substantial gains in language performance over that of treatment alone as demonstrated in a large randomised clinical trial (RCT, N=74)33; however, it is cost-intensive and requires substantial technological expertise. Other approaches exploit known effects of NIBS on functionally connected regions.s27 For example, stimulation of primary motor cortex or the cerebellum modulates neural processing in language regions,32s28 and motor cortex tDCS improved naming and communication ability in a placebo-tDCS-controlled RCT.s28
New NIBS techniques are also beginning to emerge in the aphasia literature like theta burst rTMS, which relies on bursts of very high frequency (eg, 50 Hz) to increase or decrease cortical excitability. Its footprint in the aphasia literature is much too small to objectively evaluate its therapeutic value, but early findings are promising.35 Likewise, high-definition tDCS (figure 2), which allows for much more focused stimulation than traditional tDCS, is showing some promise for aphasia treatment,34,36s25 but it is too early to fully evaluate its potential. As both of these techniques may be improvements over conventional forms of rTMS and tDCS, respectively, we expect to see more aphasia treatment studies employing them.
In sum, preliminary evidence primarily from relatively small, methodologically heterogeneous trials suggests that both rTMS and tDCS are promising adjuvant approaches to enhance aphasia treatment outcomes. However, little is known about long-term effects, optimal stimulation parameters (eg, duration, frequency or intensity) and montages for individual patients, effect of additional variables (eg, genotype34) and whether positive effects from laboratory settings translate into improved everyday communication. Moreover, only a handful of studies investigated the neural mechanisms by which NIBS modulates behavioural performance. For example, Harvey et al37 recently demonstrated that long-lasting improvement of naming ability after 10 days of 1 Hz rTMS were associated with shift in IFG activity from anterior to posterior regions and also activity increases in the left hemisphere. However, how NIBS interacts with the reorganised language network and with the processes engaged during treatment needs to be investigated more systematically to enhance effectiveness of future NIBS trials.s13 An important consideration in this regard is that neuroplasticity is sometimes maladaptive and can interfere with function.S29 Indeed, it has been proposed that the use of NIBS in aphasia rehabilitation can help to overcome maladaptive plasticity emerging in the course of recovery.s30 Yet, the possibility should not be ignored that NIBS might fail or even induce maladaptive plasticity when there is a mismatch between the where NIBS is applied and where the best opportunities for stimulating recovery lie. Hence, more research regarding predicting variable outcomes for specific NIBS and aphasia treatment combinations would be helpful in selecting the best treatments for individual patients.
Principles for inducing beneficial neuroplasticity through aphasia treatment
Animal model studies of rehabilitation after brain injury have identified principles that can facilitate remapping of brain functions, creation of new connexions between neurons and/or engagement of alternative pathways to re-establish function.s31, s32 There are striking parallels between these principles and the mechanisms undergirding normal learning and development.s31 Furthermore, application of principles learnt in basic neuroscience research to aphasia rehabilitation has been widely acknowledgeds33 to assist in understanding how treatments that aim to restore and/or reorganise language functions work. Of the neuroplasticity principles that Kleim and Joness31 suggested to guide rehabilitation, the following discussion covers those receiving substantial attention in the recent aphasia treatment literature. These include the following: (1) improvement in behaviour is use dependent, (2) sufficient repetition is necessary, (3) intensity of treatment matters and (4) transference (or generalisation) can occur.
For example, use-dependent treatment approaches rely on the principles that neural circuits not actively used for extended periods degrade and that plasticity can be induced through training.s31 Because constraint-induced language therapy (CILT) forces patients to rely on verbal language to accomplish goals in various language-action games, it is an example of a use-dependent treatement.s31 Although systematic reviews34 showed that early CILT studies yielded positive outcomes compared with alternative interventions, more recent comparative trials have demonstrated that language modality and patient characteristics can determine outcome. For example, Wilssens et al38 showed that semantic treatment improved comprehension and CILT improved language production, whereas Rose and colleagues39,40 demonstrated that a multi-modality aphasia treatment which engages gesture, drawing, and writing to facilitate verbal production yielded better outcomes for individuals with moderate aphasia and CILT yielded better outcomes for mild aphasia. Three recent clinical trials comparing CILT and conventional treatment in acute and subacute aphasias41–43 showed significant improvements for both treatments. Collectively, these studies demonstrate the need for more research to understand variables (ie, severity, stage of recovery) that influence use-dependent learning. Additionally, recent research has called into question44 the assumption that the neuroplasticity principle of intensity is a fundamental component of CILT. Thus, further studies that systematically manipulate neuroplasticity principles such as intensity and repetition are needed to understand the active treatment components of CILT and their effect on brain structure and function.
Greater repetition of behaviours and higher intensity of treatment schedules have been shown to induce neuroplasticity in animal model rehabilitation studies.s31 This observation suggests that invoking long-term changes following aphasia treatment require sufficient repetition within sessions (saturated practice) and intensive opportunities to produce target language behaviours over time. One recent study employing cued picture naming demonstrated that saturated practice (400 exposures per session) can lead to word retrieval improvement after only 3 hours of training.45 However, a comparative study using a repetition-based treatment demonstrated a mixed pattern of results between patients who received 160 exposures/session and 40 exposures/session.46 Thus, more research is necessary to define ‘sufficient repetition’ and to understand how treatment and patient-related variables determine which repetitive practice conditions induce lasting behavioural and brain changes.
A number of factors contribute to the cumulative intensity (total amount) of any given treatment, including dose, dose frequency (distribution of sessions over time; massed practice=high-dose frequency, distributed practice=low-dose frequency), session duration (number of hours per session) and intervention duration (length of intervention over time).s35 A complex relationship exists between factors that contribute to cumulative intensity and treatment outcome in aphasia. There is evidence that higher-dose frequency of therapy in early stages of aphasia rehabilitation improves treatment outcomes.s36 Similarly, a recent large-scale study in chronic aphasia demonstrated that massed practice (10 hours per week) was associated with significant improvements in language measures compared with deferred treatment.47 However, a comparative study of highly intensive (4 hours/day) versus moderately intensive (2 hours/day) treatment over two 2-week intervals demonstrated that only longer intervention duration (not longer session duration) improved treatment outcomes in chronic aphasia, suggesting there may be a threshold at which greater session duration no longer yields additional benefit.48 Recent investigations of dose frequency (massed vs distributed practice) also demonstrated that treatment schedule may influence outcomes. For example, Dignam and colleagues showed that distributed practice (6 hours/week over 8 weeks) compared with massed practice (16 hours/week over 3 weeks) yielded larger immediate acquisition and retention of gains,49 whereas Martins and colleagues demonstrated no difference between massed practice (10 hours/week over 10 weeks) versus distributed practice (2 hours/week over 50 weeks) for acquisition and retention.50 A review of comparative studies reveals that the advantages afforded by massed versus distributed practice may equalise when cumulative intensity is >50 hours total.s37 These findings have important clinical implications as distributed practice schedules, which seem to yield enhanced long-term benefit, may be implemented more easily in most clinical settings than massed schedules. More research is necessary to understand the interaction between amount and distribution of therapy across different stages of recovery and different treatment types, as well as the mechanisms that support response to different treatment schedules.
A highly desirable outcome in aphasia treatment is to transfer gains from trained language behaviours to other language behaviours, tasks or contexts (ie, generalisation). Since clinicians cannot train every word patients may use or every context in which they use words, generalisation to untrained items and contexts is highly desirable. Treatment studies have demonstrated that generalisation across behaviours increases when there is a hierarchical relationship between trained and untrained targets (eg, within category generalisation from abstract words to concrete words,51 when more complex words or sentences are selected for treatment of word finding or syntax, respectively6,52 and when trained words or sentences engage common linguistic rules or principles.s38, s39 Furthermore, generalisation across tasks occurs when the tasks share psycholinguistic mechanisms (eg, training novel phoneme sequences to strengthen the phonological system can generalise to improvements in word retrieval53 and reading.)54 Improvements in discourse, which are important for generalisation across contexts, have been found in approaches that treat longer utterances, such as CILT55 and verb network strengthening treatment.56 However, the variables that influence different types of generalisation and the underlying mechanisms required for generalisation to occur remain incompletely understood. Given the importance of generalisation for improving everyday communicative function, determining what neuroplasticity principles induce generalisation should be a priority.
In brief, neuroplasticity principles derived from animal research have been applied to language rehabilitation with promising results. However, the success of treatments applying such principles depends on multiple factors, including type or severity of aphasia, the language skill that is targeted in treatment and stage of recovery. The aim to transfer training effects to untrained items and contexts requires consideration of what linguistic mechanisms are addressed. An additional concern is that principles derived from motor rehabilitation in animals may not ultimately encompass, or be fully consistent with, the principles needed to optimise rehabilitation of the uniquely human function of language.
Non-language behaviours for therapeutic neuromodulation
The idea that non-language behaviours can be used to modulate neural activity for aphasia treatment has also been investigated. As mentioned earlier, use of rhythm and melody in MIT leverages structural changes in right-hemisphere pathways to facilitate word production in Broca’s aphasia.s11 Inevitably, investigators have developed other strategies to target specific brain regions for modulation using non-language behaviours.
For example, intention treatment, which uses a left-hand movement to initiate word finding efforts, increases right relative to left frontal activity, demonstrating reorganisation of word retrieval for picture naming and category member generation.57 Simple practice in word retrieval without the hand movement does not produce this re-organisation. In the relatively fluent patients in this study, the impact of this manipulation on right posterior perisylvian activity correlated with treatment gains. The most desirable outcome of this treatment was that it led to significantly greater improvement on (generalisation to) untrained category-member generation items57 and on word-finding during narrative production.58 Another recent example of neuromodulation with non-language behaviours capitalised on the observation that mirror neurons, which are activated during observation of others’ behaviour, are located in the IFG and other areas that process language in the dominant hemisphere.59 In this study, patients with non-fluent aphasia watched videos involving manual manipulation of objects or static videos of objects. Naming performance was significantly greater for objects learnt while observing object manipulation during word-finding attempts compared with static videos. There was evidence of generalisation to naming of untrained objects and untrained language tasks. Limited evidence indicated that watching the object manipulation videos engaged mirror neuron systems more than watching static object videos.59
To summarise, initial evidence indicates that specific brain regions or systems can be targeted for neuromodulation using non-linguistic behavioural strategies to enhance therapeutic outcome. The idea that this type of modulation leads to generalisation to untrained items and contexts is supported by the two studies, and because generalisation is highly desirable in rehabilitation, whether these and similar strategies facilitate generalisation deserves continued attention.
CONCLUSIONS
The variability in findings using functional and structural neuroimaging does not allow for generalisable conclusions about what changes in neural systems lead to optimal treatment outcomes. While differences in methodologies between studies may account for some of the diversity in findings between studies, it is necessary to consider whether a monolithic pathway to optimise aphasia treatment outcomes ever will emerge. Given the likelihood that patients with different symptom and lesion patterns may require engagement of different mechanisms, future studies should endeavour to identify factors that can predict which treatments are likely to produce clinically significant outcomes for patients with common symptom or lesion patterns. Regarding the latter, structural and functional imaging studies at pre-treatment demonstrate some promise for predicting aphasia treatment outcome. However, this is an under-explored research area. Given the potential to improve treatment outcomes when viable predictions are used for individualised treatment selection, this area deserves greater attention.
Research suggests that it is possible to enhance neuroplasticity during aphasia treatment through the use of NIBS or through linguistic or non-linguist treatment strategies targeting specific cortices and/or processes. Although NIBS studies in aphasia generally have been small with variable findings, one recent large randomised, sham-controlled clinical trial indicates that active tDCS supplements the effects of aphasia treatment compared with sham.33 Neural activity evoked by linguistic and non-linguistic behavioural strategies also may have the potential to evoke long-term relearning and brain-system reorganisation during aphasia treatment.
The potential contribution of aphasia type, lesion parameters and other patient characteristics to outcome for any particular treatment has been a consistent theme throughout this review. This observation indicates that greater research attention should be given to what patient characteristics predict success of specific treatments, which can vary considerably by treatment. Emphasis on prediction will enable individualisation of treatment approaches. Figure 3 provides one vision for future clinical application in which a large database correlating structural and functional neuroimaging features to outcomes of specific treatments is used to facilitate initial selection of the treatment option likely to produce the best outcome for a specific patient.60 Subsequently, changes in neural substrates and intermediate treatment outcomes are monitored so that adjustments to the treatment plan can be made. This type of vision for integrating and applying accumulating knowledge could be used to guide future research and help bridge the gap between research and clinical practice.
Figure 3.
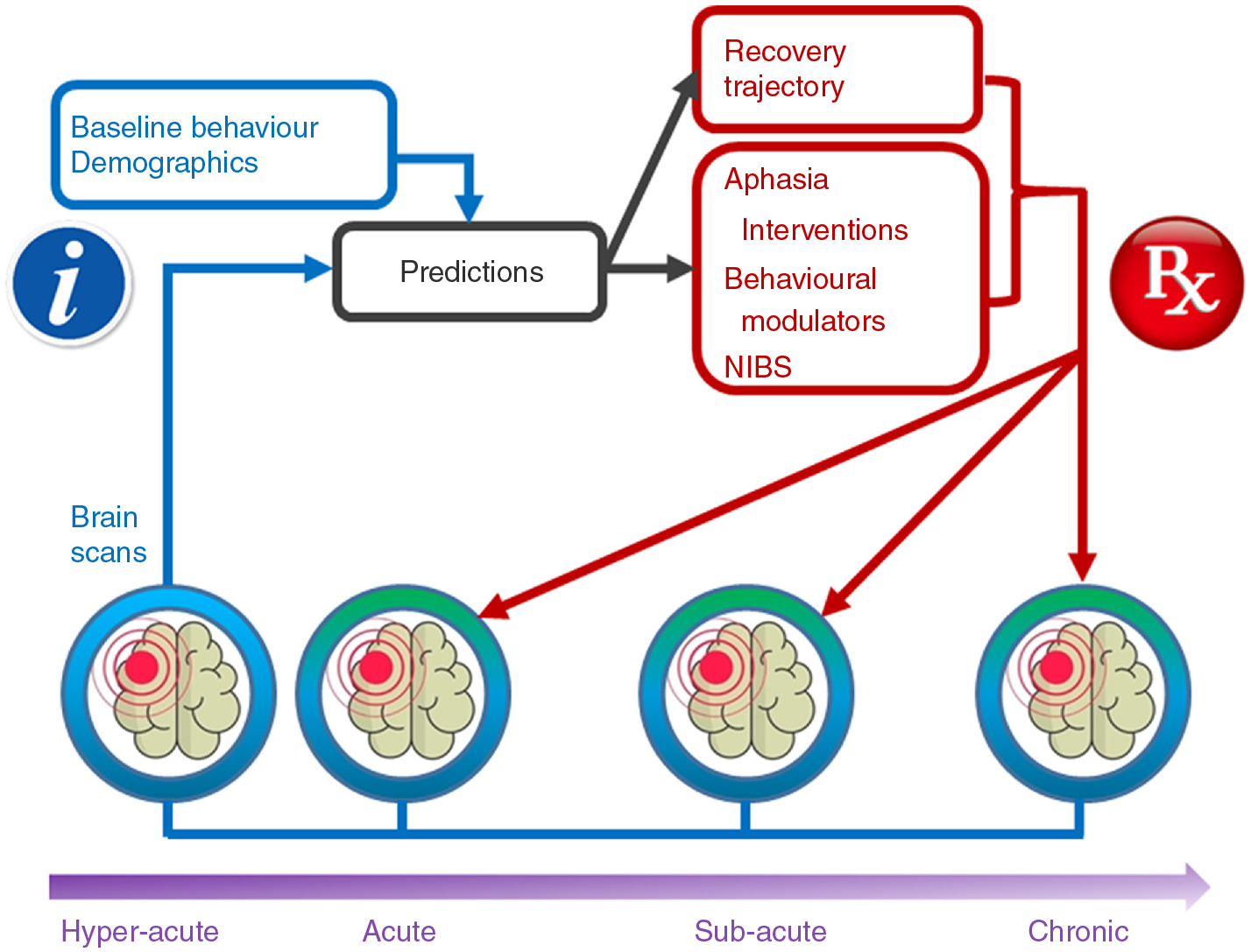
Schematic diagram showing how neuroimaging data eventually could be used clinically for aphasia rehabilitation. The white ‘I’ on a blue field represents information, which refers to baseline behaviour, demographics and brain scans (blue outlines and arrows). Brain scans may include structural and/or functional MRI scans, DTI (which provides information about white matter integrity), or CT scans. When this information is fed into an algorithm relying on a large database, predictions (black boxes and arrows) can be made regarding recovery trajectory and response to various treatments (red boxes and arrows). From these recovery and treatment predictions, the best treatment and dose (ie, number of hours to achieve a quantifiable treatment outcome) can be prescribed (represented by the white RX on a red background) for the recovery stage of the patient. Treatments might include application of aphasia interventions (which directly address language functions), other behavioural modulators (such as those described in the texts11, 57,59), or NIBS (such as repetitive transcranial magnetic stimulation and transcranial direct current stimulation) to facilitate neuroplasticity. Prescribed treatments will lead to neuroplastic changes that can be monitored using functional or structural brain scans (signified by the green colour above the scan). These measures of neural response to treatment, along with measures of behavioural response, can be used for prediction and prescription of subsequent treatment as the patient progresses through the treatment regimen and various recovery stages. Thus, this kind of algorithm can personalise treatment selection, using neuroimaging to predict best treatments and measure treatment response to interventions involving aphasia therapies accompanied by NIBS and behavioural modulators of neuroplasticity, as algorithmically prescribed. Some groups currently are amassing large-scale databases,s40, 60 which might contribute to the kind of aphasia treatment selection envisioned here. Development of more effective treatments through an understanding of how to evoke the necessary neuroplasticity and determining which patients benefit from the treatments that we now have and are developing will greatly expand the benefits of aphasia therapy. DTI = diffusion tensor imaging; NIBS = non-invasive brain stimulation.
Additional References, marked with an ‘s’ in citations and enumerated separately, can be found in the online supplementary material.
Supplementary Material
Funding
BC received funding from the Veterans Affairs Rehabilitation Research & Development Service (USA) grant B6364-L; DAC received funding from National Health and Medical Research Council (Australia) Grant APP1104194 and University of Queensland Vice Chancellor’s Fellowship; JF received funds from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (USA) grant P50 DC014664; APL received funds from a National Institute for Health Research (UK) Research Professorship RP-2015-06-012; MM received funding from Australian Research Council Future Fellowship FT120100608 and National Health and Medical Research Council (Australia) Grant Number 1085272; AR received funding from the Veterans Affairs Rehabilitation Research & Development Service (USA) grant C2238-P. LCK received funding from the Veterans Affairs Rehabilitation Research & Development Service (USA) grant IK1 RX002629. The views presented in this work do not necessarily represent the views of the United States Government or the Department of Veterans Affairs.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/jnnp-2018-319649).
Competing interests None declared.
REFERENCES
- 1.Siegel JS, Seitzman BA, Ramsey LE, et al. Re-emergence of modular brain networks in stroke recovery. Cortex 2018;101:44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meinzer M, Mohammadi S, Kugel H, et al. Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. NeuroImage 2010;53:283–90. [DOI] [PubMed] [Google Scholar]
- 3.Menke R, Meinzer M, Kugel H, et al. Imaging short- and long-term training success in chronic aphasia. BMC Neuroscience 2009;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattioli F, Ambrosi C, Mascaro L, et al. Early aphasia rehabilitation is associated with functional reactivation of the left inferior frontal gyrus. Stroke 2014;45:545–52. [DOI] [PubMed] [Google Scholar]
- 5.Regel S, Kotz SA, Henseler I, et al. Left inferior frontal gyrus mediates morphosyntax: Erp evidence from verb processing in left-hemisphere damaged patients. Cortex 2017;86:156–71. [DOI] [PubMed] [Google Scholar]
- 6.Thompson CK, Riley EA, den Ouden D-B, et al. Training verb argument structure production in agrammatic aphasia: behavioral and neural recovery patterns. Cortex 2013;49:2358–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abel S, Weiller C, Huber W, et al. Therapy-induced brain reorganization patterns in aphasia. Brain 2015;138:1097–112. [DOI] [PubMed] [Google Scholar]
- 8.Nardo D, Holland R, Leff AP, et al. Less is more: neural mechanisms underlying anomia treatment in chronic aphasic patients. Brain 2017;140:3039–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brownsett SLE, Warren JE, Geranmayeh F, et al. Cognitive control and its impact on recovery from aphasic stroke. Brain 2014;137:242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geranmayeh F, Leech R, Wise RJS. Network dysfunction predicts speech production after left hemisphere stroke. Neurology 2016;86:1296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan ES, Small SL. Increased modularity of resting state networks supports improved narrative production in aphasia recovery. Brain Connectivity 2016;6:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan ES, Small SL. Changes in dynamic resting state network connectivity following aphasia therapy. Brain Imaging Behav 2018;12:1141–9. [DOI] [PubMed] [Google Scholar]
- 13.Woodhead ZVJ, Crinion J, Teki S, et al. Auditory training changes temporal lobe connectivity in ‘Wernicke’s aphasia’: a randomised trial. J Neurol Neurosurg Psychiatry 2017;88:586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandberg CW, Bohland JW, Kiran S. Changes in functional connectivity related to direct training and generalization effects of a word finding treatment in chronic aphasia. Brain and Language 2015;150:103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gili T, Fiori V, De Pasquale G, et al. Right sensory-motor functional networks subserve action observation therapy in aphasia. Brain Imaging Behav 2017;11:1397–411. [DOI] [PubMed] [Google Scholar]
- 16.Allendorfer JB, Storrs JM, Szaflarski JP. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci 2012;30:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Hees S, McMahon K, Angwin A, et al. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil Neural Repair 2014;28:325–34. [DOI] [PubMed] [Google Scholar]
- 18.Wan CY, Zheng X, Marchina S, et al. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain Lang 2014;136:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinnon ET, Fridriksson J, Glenn GR, et al. Structural plasticity of the ventral stream and aphasia recovery. Ann Neurol 2017;82:147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glenn GR, Kuo L-W, Chao Y-P, et al. Mapping the orientation of white matter fiber bundles: a comparative study of diffusion tensor imaging, diffusional Kurtosis imaging, and diffusion spectrum imaging. AJNR Am J Neuroradiol 2016;37:1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hope TMH, Leff AP, Prejawa S, et al. Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain 2017;140:1718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukic S, Barbieri E, Wang X, et al. Right hemisphere grey matter volume and language functions in stroke aphasia. Neural Plast 2017;2017:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridriksson J, Richardson JD, Fillmore P, et al. Left hemisphere plasticity and aphasia recovery. NeuroImage 2012;60:854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Hees S, McMahon K, Angwin A, et al. Neural activity associated with semantic versus phonological anomia treatments in aphasia. Brain and Language 2014;129:47–57. [DOI] [PubMed] [Google Scholar]
- 25.Fridriksson J, Basilakos A, Hickok G, et al. Speech entrainment compensates for Broca’s area damage. Cortex 2015;69:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonilha L, Gleichgerrcht E, Nesland T, et al. Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabil Neural Repair 2016;30:266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiel A, Hartmann A, Rubi-Fessen I, et al. Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke 2013;44:2240–6. [DOI] [PubMed] [Google Scholar]
- 28.Rubi-Fessen I, Hartmann A, Huber W, et al. Add-on effects of repetitive transcranial magnetic stimulation on Subacute aphasia therapy: enhanced improvement of functional communication and basic linguistic skills. A randomized controlled study. Arch Phys Med Rehabil 2015;96:1935–44. [DOI] [PubMed] [Google Scholar]
- 29.Khedr EM, Abo El-Fetoh N, Ali AM, et al. Dual-hemisphere repetitive transcranial magnetic stimulation for rehabilitation of poststroke aphasia: a randomized, double-blind clinical trial. Neurorehabil Neural Repair 2014;28:740–50. [DOI] [PubMed] [Google Scholar]
- 30.Marangolo P, Fiori V, Cipollari S, et al. Bihemispheric stimulation over left and right inferior frontal region enhances recovery from apraxia of speech in chronic aphasia. Eur J Neurosci 2013;38:3370–7. [DOI] [PubMed] [Google Scholar]
- 31.Meinzer M, Darkow R, Lindenberg R, et al. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain 2016;139:1152–63. [DOI] [PubMed] [Google Scholar]
- 32.Darkow R, Martin A, Würtz A, et al. Transcranial direct current stimulation effects on neural processing in post-stroke aphasia. Human Brain Mapping 2017;38:1518–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fridriksson J, Rorden C, Elm J, et al. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke. JAMA Neurol 2018;75:1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fridriksson J, Basilakos A, Stark BC, et al. Transcranial direct current stimulation to treat aphasia: longitudinal analysis of a randomized controlled trial. Brain Stimul 2018. epub ahead of print;1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffis JC, Nenert R, Allendorfer JB, et al. Interhemispheric plasticity following intermittent theta burst stimulation in chronic poststroke aphasia. Neural Plast 2016;2016:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson J, Datta A, Dmochowski J, et al. Feasibility of using high-definition transcranial direct current stimulation (HD-tDCS) to enhance treatment outcomes in persons with aphasia. NeuroRehabilitation 2015;36:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey DY, Podell J, Turkeltaub PE, et al. Functional reorganization of right prefrontal cortex underlies sustained naming improvements in chronic aphasia via repetitive transcranial magnetic stimulation. Cogn Behav Neurol 2017;30:133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilssens I, Vandenborre D, van Dun K, et al. Constraint-induced aphasia therapy versus intensive semantic treatment in fluent aphasia. Am J Speech Lang Pathol 2015;24:281–94. [DOI] [PubMed] [Google Scholar]
- 39.Attard MC, Rose ML, Lanyon L. The comparative effects of multi-modality aphasia therapy and Constraint-Induced aphasia Therapy-Plus for severe chronic Broca’s aphasia: an in-depth pilot study. Aphasiology 2013;27:80–111. [Google Scholar]
- 40.Rose ML, Mok Z, Carragher M, et al. Comparing multi-modality and constraint-induced treatment for aphasia: a preliminary investigation of generalisation to discourse. Aphasiology 2016;30:678–98. [Google Scholar]
- 41.Woldag H, Voigt N, Bley M, et al. Constraint-Induced aphasia therapy in the acute stage: what is the key factor for efficacy? A randomized controlled study. Neurorehabil Neural Repair 2017;31:72–80. [DOI] [PubMed] [Google Scholar]
- 42.Ciccone N, West D, Cream A, et al. Constraint-induced aphasia therapy (CIAT): a randomised controlled trial in very early stroke rehabilitation. Aphasiology 2016;30:566–84. [Google Scholar]
- 43.Sickert A, Anders L-C, Münte TF, et al. Constraint-induced aphasia therapy following sub-acute stroke: a single-blind, randomised clinical trial of a modified therapy schedule. J Neurol Neurosurg Psychiatry 2014;85:51–5. [DOI] [PubMed] [Google Scholar]
- 44.Mozeiko J, Coelho CA, Myers EB. The role of intensity in constraint-induced language therapy for people with chronic aphasia. Aphasiology 2016;30:339–63. [Google Scholar]
- 45.Harnish SM, Morgan J, Lundine JP, et al. Dosing of a cued picture-naming treatment for anomia. Am J Speech Lang Pathol 2014;23:S285–99. [DOI] [PubMed] [Google Scholar]
- 46.Off CA, Griffin JR, Spencer KA, et al. The impact of dose on naming accuracy with persons with aphasia. Aphasiology 2016;30:983–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breitenstein C, Grewe T, Flöel A, et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet 2017;389:1528–38. [DOI] [PubMed] [Google Scholar]
- 48.Stahl B, Mohr B, Büscher V, et al. Efficacy of intensive aphasia therapy in patients with chronic stroke: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2018;89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dignam JK, Copland DA, McKinnon E, et al. Intensive versus distributed aphasia therapy: a nonrandomized, parallel-group, dosage-controlled study. Stroke 2015;46:2206–11. [DOI] [PubMed] [Google Scholar]
- 50.Martins IP, Leal G, Fonseca I, et al. A randomized, rater-blinded, parallel trial of intensive speech therapy in sub-acute post-stroke aphasia: the SP-I-R-IT study. Int J Lang Commun Disord 2013;48:421–31. [DOI] [PubMed] [Google Scholar]
- 51.Sandberg C, Kiran S. How justice can affect jury: training Abstract words promotes generalisation to concrete words in patients with aphasia. Neuropsychol Rehabil 2014;24:738–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riley EA, Thompson CK. Training pseudoword reading in acquired dyslexia: a phonological complexity approach. Aphasiology 2015;29:129–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kendall DL, Oelke M, Brookshire CE, et al. The influence of Phonomotor treatment on word retrieval abilities in 26 individuals with chronic aphasia: an open trial. J Speech Lang Hear Res 2015;58:798–812. [DOI] [PubMed] [Google Scholar]
- 54.Brookshire CE, Conway T, Pompon RH, et al. Effects of intensive phonomotor treatment on reading in eight individuals with aphasia and phonological alexia. Am J Speech Lang Pathol 2014;23:S300–11. [DOI] [PubMed] [Google Scholar]
- 55.Stahl B, Mohr B, Dreyer FR, et al. Using language for social interaction: communication mechanisms promote recovery from chronic non-fluent aphasia. Cortex 2016;85:90–9. [DOI] [PubMed] [Google Scholar]
- 56.Edmonds LA, Mammino K, Ojeda J. Effect of Verb network strengthening treatment (VNeST) in persons with aphasia: extension and replication of previous findings. Am J Speech Lang Pathol 2014;23:S312–29. [DOI] [PubMed] [Google Scholar]
- 57.Benjamin ML, Towler S, Garcia A, et al. A behavioral manipulation engages right frontal cortex during aphasia therapy. Neurorehabil Neural Repair 2014;28:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altmann LJP, Hazamy AA, Carvajal PJ, et al. Delayed stimulus-specific improvements in discourse following anomia treatment using an intentional gesture. J Speech Lang Hear Res 2014;57:439–54.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Ye Q, Ji X, et al. Mirror neuron system based therapy for aphasia rehabilitation. Frontiers in Psychology 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seghier ML, Patel E, Prejawa S, et al. The PLORAS database: a data Repository for predicting language outcome and recovery after stroke. NeuroImage 2016;124:1208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


