Abstract
Coronavirus disease 2019 (COVID‐19) is a multisystemic disease that can cause progressive lung failure, organ dysfunction, and coagulation disorder associated with high mortality and morbidity. COVID‐19 is known to either primarily cause skin symptoms or increase existing skin diseases. Human papillomavirus (HPV) is a DNA virus that can cause benign and malignant neoplasms. Mucocutaneous verruca vulgaris are common benign lesions of HPV. Here, we report a case of verruca vulgaris regressed after COVID‐19.
Keywords: COVID‐19, human papillomavirus, paradoxical immunity
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a multisystemic disease caused by the highly contagious severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus, which first appeared in Wuhan, China. COVID‐19 can cause organ dysfunction, progressive respiratory failure, and coagulation disorders which are associated with high mortality, and a wide variety of mucocutaneous manifestations as well. In the current literature, it has been stated that COVID‐19 either primarily causes skin symptoms or exacerbates existing skin disorders. 1 , 2 Here, we report a case of verruca vulgaris regressed after COVID‐19.
2. CASE PRESENTATION
A 26‐year‐old otherwise healthy female patient had multiple viral warts on both hands for 10 years without response to many regimens including single or combined use of topical salicylic acid, topical tretinoin, topical fluorouracil, electrosurgery, and cryosurgery (Figure 1A). She was admitted to the hospital on November 2020 with mild complaints of a low grade of fever, fatigue, and cough for two days. The nasopharyngeal swab sample turned out to be positive for SARS‐CoV‐2. Blood investigations including complete blood count, C‐reactive protein, erythrocyte sedimentation rate, D‐dimer, serum electrolytes, liver function tests, and renal function tests were within normal limits. The patient received a combination of favipiravir (1st day at loading dose 2 × 800 mg; 2‐5th day at maintenance dose 2 × 300 mg) and enoxaparin (40 mg/day) with the diagnosis of COVID‐19. COVID‐19–related symptoms regressed completely within seven days. It was observed that all of viral warts localized on both hands regressed spontaneously one month after the onset of COVID‐19–related symptoms (Figure 1B). The patient was followed for two months without treatment, and no recurrence was observed.
FIGURE 1.
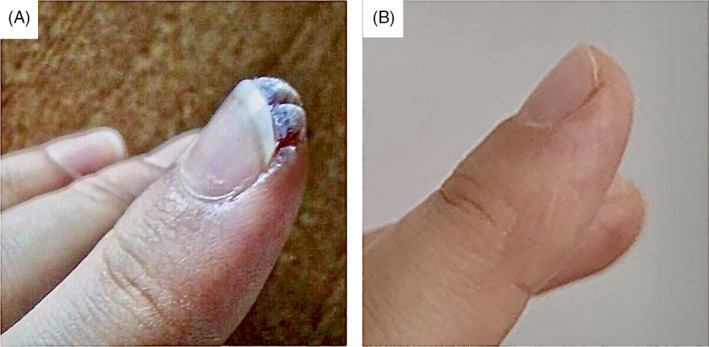
A, Periungual verruca vulgaris before COVID‐19, B, Lesions that spontaneously regress after COVID‐19
3. DISCUSSION
The immune system protects us against viruses and diseases, and produces antibodies to inactivate pathogens. Many experimental studies have been reported in the literature showing how the immune system works against SARS‐CoV‐2 infection and its mechanism. SARS‐CoV‐2 enters the lung cells and causes an excessively inflammatory form of programmed cell death called pyroptosis, triggering macrophages and monocytes from the innate immune system. Cytokines and chemokines such as IL‐1β, IL‐6, IFN‐y, MCP1, macrophage inflammatory protein 1α (MIP1α) and tumor necrosis factor (TNF), and IP‐10 secreted by these activated cells, regulate the immune responses of T and B cells from the adaptive immune system and thus suppress the viral activity. However, in some patients, dysfunctional immune system due to widespread lung inflammation may cause a cytokine storm. This situation can lead to mortality‐related complications such as progressive respiratory failure, coagulation disorders, and multi‐organ dysfunction. In conclusion, the proper functioning of the immune system and the proper level of response to the pathogen are very important for the survival of the patient in infections with high mortality and morbidity. 3 , 4
Human papillomavirus (HPV) is a DNA virus that can cause benign and malignant neoplasms. Mucocutaneous verruca vulgaris are common benign lesions of HPV. The first treatment options include topical salicylic acid, cryosurgery, topical fluorouracil, topical tretinoin, and electrosurgery. In lesions resistant to these treatments, immunotherapies such as topical imiquimod, intralesional MMR and BCG vaccine, PPD, Trichophyton antigen, Vitamin D3, zinc, levamisole, cimetidine, propolis, and HPV vaccines can be used. 5
To the best of our knowledge, no studies investigating the possible relationship between HPV and SARS‐CoV‐2 infection exist in the current literature. Vavoulidis et al, however, reported a 32‐year‐old patient followed up with a known cervical HPV infection. She was diagnosed with COVID‐19 in April 2020, completely healed, and was readmitted for regular follow‐up. The authors observed that cervical epithelium developed a lesion finally diagnosed to be cervical intraepithelial neoplasia‐1 histopathologically. They concluded that nobody may support definitely the hypothesis of a correlation between SARS‐CoV‐2 infection and the development of this lesion. The authors also suggested that this observation should motivate further investigations to uncover what exactly is happening in patients affected by COVID‐19. 6 Our report, in contrast, supports a possible negative correlation between SARS‐CoV‐2 and HPV infections. We hypothesized that excessive inflammatory response toward SARS‐CoV‐2 infection may trigger a paradoxical immune reaction resulting in regression of viral warts. Moreover, SARS‐CoV‐2 infection may have triggered viral clearance by causing a delayed hypersensitivity reaction, as observed in the treatment of intralesional MMR and BCG vaccine, PPD, and trichophyton antigens used for recalcitrant warts. From this point of view, we encourage investigations to explore whether COVID‐19 vaccines, which have entered widespread use, have a similar effect on HPV‐caused viral warts.
In the present case, it seems unlikely that the regression of the warts could be related to favipiravir and enoxaparin administered for the treatment of COVID‐19. Favipiravir is a new generation viral RNA‐dependent RNA polymerase inhibitor and is not expected to show antiviral effects on DNA viruses such as HPV. 7 Enoxaparin is a low molecular weight heparin used in the prophylaxis of thrombovascular complications associated with COVID‐19. In a preprint in‐vitro study using a pseudotyped model of spike glycoprotein, it has been suggested that enoxaparin may exert an antiviral effect by blocking the entry of SARS‐CoV‐2. 8 Spike glycoprotein has been demonstrated to bind to heparan sulfate, which is found on the surface of all mammalian cells. 8 Considering that HPV also interacts with the cell surface via interaction of the major capsid protein with heparan sulfate proteoglycans, enoxaparin may show antiviral activity on HPV with a similar mechanism. 9 This potential antiviral activity of enoxaparin, however, is limited to the cell entry stage of the virus, and it seems unlikely that it has an effect on an active viral infection.
On the basis of our observation, we conclude that in‐depth studies investigating the possible virologic and immunologic relationships between COVID‐19, COVID‐19–related treatments, and HPV may offer a new perspective in dealing with viral pathogens.
AUTHOR CONTRIBUTION
A.D. involved in literature searching, designing, and writing the manuscript. A.D., H.E., Ö.F.E., G.U.D., and M.U. involved in substantial contributions to conception and design, interpretation of data. A.D., Ö.F.E., Ü.T., and T.L. involved in editing, revising and final approval of the manuscript.
CONSENT TO THE PUBLICATION
The patient in this manuscript has given written informed consent to the publication of her case details.
4. ETHICS STATEMENT
Written informed consent was obtained from the patient for publication of this case report.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Elmas ÖF, Demirbaş A, Özyurt K, Atasoy M, Türsen Ü. Cutaneous manifestations of COVID‐19: A review of the published literature. Dermatol Ther. 2020;33(4):e13696. 10.1111/dth.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Türsen Ü, Türsen B, Lotti T. Coronavirus‐days ın dermatology. Dermatol Ther. 2020;33(4):e13438. 10.1111/dth.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Demirbaş A, Elmas ÖF, Türsen Ü, Atasoy M, Lotti T. Superficial thrombophlebitis in a patient with COVID 19: heparin treatment after evaluation of D‐Dimer. Dermatol Ther. 2020;33(4):e13768. 10.1111/dth.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thappa DM, Chiramel MJ. Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016;7(5):364‐370. 10.4103/2229-5178.190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vavoulidis E, Margioula‐Siarkou C, Petousis S, Dinas K. SARS‐CoV‐2 infection and impact on female genital tract: an untested hypothesis. Med Hypotheses. 2020;144:110162. 10.1016/j.mehy.2020.110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zarandi PK, Zinatizadeh MR, Zinatizadeh M, Yousefi MH, Rezaei N. SARS‐CoV‐2: from the pathogenesis to potential anti‐viral treatments. Biomed Pharmacother. 2021;137:111352. 10.1016/j.biopha.2021.111352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tandon R, Sharp JS, Zhang F, et al. Effective inhibition of SARS‐CoV‐2 entry by heparin and enoxaparin derivatives. bioRxiv [Preprint]. 2020. 10.1101/2020.06.08.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horvath CA, Boulet GA, Renoux VM, Delvenne PO, Bogers JP. Mechanisms of cell entry by human papillomaviruses: an overview. Virol J. 2010;7:11. 10.1186/1743-422X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


