Abstract
Although inflammation and emphysema in patients with chronic obstructive pulmonary disease (COPD) can be ameliorated by antibiotics such as erythromycin, the impact of drug resistance is still controversial. We aimed to evaluate the role of F528, a new macrolide derivative without antibacterial effect, in cigarette smoke (CS)-induced pulmonary inflammation and emphysema in a mouse model, as well as in a macrophage cell line. The inflammatory cell number and cell type in the BALF were counted, and the levels of cytokines in the BALF and cultured cell medium were measured by ELISA. The degree of emphysema and apoptosis was evaluated by H&E and immunohistochemical staining, respectively. The lung function of the mice was evaluated by a small animal lung function meter. Furthermore, the expression levels of MMP-2, MMP-9, and phospho-NF-κB in the cells and lung tissue were measured by Western blot and qRT-PCR. In the BALF of the CS-induced pulmonary inflammation and emphysema model, the numbers of inflammatory cells and cytokines were significantly decreased after F528 intervention. F528 intervention also significantly protected lung function from CS-induced emphysema, while the mean lining interception (MLI) of the F528-treated CS group was significantly lower than that of the vehicle-treated CS group. In addition, F528 treatment reduced the phosphorylation of NF-κB induced by smoke, and the expression of MMP-2 and MMP-9 was also obviously decreased by F528 treatment. We therefore conclude that F528 reduces cigarette smoke-induced inflammation and emphysema in vivo and in vitro through inhibition of the activation of NF-κB.
Keywords: Emphysema, cigarette smoke, inflammation, macrolide
Introduction
Chronic obstructive pulmonary disease (COPD) has now become a global epidemic disease that is increasing in incidence worldwide [1]. COPD is currently the fourth leading cause of death worldwide and is predicted to be the third cause by 2030 [2]. Exposure to cigarette smoke is the main cause of COPD. A previous study showed that the risk of COPD for current smokers is 3.51 fold higher than that of never smokers [3]. The Global Initiative for Chronic Obstructive Lung Disease guidelines defines COPD as a disease associated with an abnormal inflammatory response of the lungs because of the inhalation of harmful particles or gases, causing airway and/or alveolar abnormalities, which subsequently further leads to the formation of emphysema [4,5].
Chronic inflammation of the airways and lung parenchyma is involved in the formation of COPD, and it increases even more during acute exacerbations [6,7]. Neutrophils, macrophages and other inflammatory cells, activated by cigarette smoke or other irritants, release reactive oxygen species and various inflammatory mediators, such as TNF-α, IL-6 and LTB4, promoting the accumulation of inflammatory cells. These inflammatory cells also produce proteinases, including matrix metalloproteinase-2 (MMP-2), MMP-9, MMP-12 and elastases, which cause emphysema through degradation of the extracellular matrix components [8-10].
Erythromycin (EM) has been reported to have an anti-inflammatory effect independent of its antibacterial activity [11]. Erythromycin and other macrolide antibiotics were initially listed as anti-inflammatory drugs in the pharmacologic therapy of COPD in GOLD 2017. It has been reported that erythromycin can reduce inflammation in the respiratory tract and improve exercise endurance in patients with COPD [12-14]. However, the long-term application of macrolides as antibiotics always has the risk of bacterial resistance and flora disorder. Therefore, the development of macrolide derivatives that have no antibacterial activity is valuable in the treatment of COPD. During the past few years, there have been a few studies on these macrolide derivatives [15-17], of which fewer have included animal experiments that have proven their effectiveness, and almost no mechanism has been verified.
In this study, we developed a new erythromycin derivative, F528, from which the antibacterial group was removed, based on the known structure of erythromycin. Here, we evaluated the efficacy of F528 on cigarette smoke-induced emphysema in mice, and erythromycin was used as a control drug. We found that both F528 and erythromycin treatment can ameliorate CS-induced inflammation, apoptosis and emphysema. Next, the mechanism by which F528 ameliorates inflammation and emphysema was explored. Our results suggested that F528 inhibits the NF-κB pathway by inhibiting the phosphorylation of NF-κB and reducing the number of inflammatory cells in the BALF, which in turn reduces the secretion of MMP-2 and MMP-9, two proteases that have been reported to be involved in the degradation of the alveolar extracellular matrix.
Materials and methods
F528
The chemical formula of F528 is proprietary and required to be confidential. The antibacterial activities of the compounds were measured as previous described [18]. For determining the cell viability, a PMA solution at 2 ng/ml was added to each well of a 48-well plate. Then, 100 μM to 1 μM erythromycin diluted in 10-fold steps was added to each well of the plate following the plate layout. After THP-1 cell suspensions at 2.5×105/mL were added, the cells were incubated at 37°C for 96 hr. The cells were then incubated with alarmar blue at 37°C for 3 more hours. Finally, the plate was read at 530 nm excitation and 590 nm emission using a PerkinElmer Victor3. The results of the antibacterial activity and cell viability tests are shown in the Figures S1 and S2.
Materials
F528 and erythromycin were from Beijing Kangdini Pharmaceutical Co., Ltd. Baisha cigarettes (tar 11 mg, nicotine 0.9 mg, flue gas carbon monoxide 12 mg) were from Hunan Zhongyan Industry Co., Ltd. Cigarette smoke extract (CSE) was generated by bubbling smoke from one cigarette at a constant rate through 10 mL DMEM, and it was finally filtered through a 0.22 μm filter to remove any bacteria and particles. H&E staining reagent was from Beijing Yili Fine Chemicals. Small animal lung function metre FlexiVent was from Scireq (Montreal, Quebec, Canada) SIBATA mouth and nose cigarette smoke tower (SG-300, Japan) was used.
Cell culture experiments
RAW264.7 cells were cultured as previously described [19]. Briefly, RAW264.7 cells were seeded in a six-well plate at 2×105 cells/ml, one group was only treated with DMEM medium (Vehicle) used as the baseline, other four groups were treated with the 5% Cigarette Smoke Extract (CSE) to induce inflammatory responses. For these four CSE-treated groups, they were respectively pretreated with 50 μM F528 (F528 50 μM/CSE), 100 μM F528 (F528 100 μM/CSE), 100 μM erythromycin (EM 100 μM/CSE) or equal amount of medium (Vehicle/CSE) prior to 5% CSE stimulation. After 6 hours of CSE or Vehicle treatment, the supernatant was collected to measure the cytokine concentration and the phosphorylation of NF-κB.
Animals
All animal experiments were performed in accordance with the guidelines for the use and care of laboratory animals of the Medical Research Center of Beijing Chaoyang Hospital, Beijing. The mice were kept at a temperature of 24~26°C and a humidity of 50%~60% with a 12 light/12 dark cycle. The mice had access to clean water and pelleted mouse diet ad libitum. The experimental protocol was approved by the Medical Research Center of Beijing Chaoyang Hospital, Beijing. The animal work took place in the Medical Research Center of Beijing Chaoyang Hospital, Beijing.
Forty male C57BL/6J mice (6-8 weeks, each weighting 22~25 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. After 1 week to adapt to the new environment, the mice were used in the experiments. They were randomly divided into 4 groups (10 animals/group): one group was treated with normal air for 6 months (non-smoke group, NS6m group), and three groups were treated with cigarette smoke (CS group) for 6 months. During the last half period of CS treatment, three CS treated groups were orally administrated 0.5% sodium carboxymethyl cellulose (CS6m group), 100 mg/kg F528 (F528 100 mg/kg group), or 100 mg/kg erythromycin (EM 100 mg/kg group) daily for 3 months. F528 and erythromycin were dissolved in 0.5% sodium carboxymethyl cellulose.
Animal model for cigarette smoke
All mice except the air control group were connected to the SIBATA mouth and nose exposure tower for exposure to cigarette smoke. Cigarette smoke parameters: Cigarettes were attached to a filter, and suction was applied every 8 s, each time sucking 20 ml of smoke, setting the concentration of the air-diluted smoke to be 10%. The mice were exposed 2 times a day, for 2 hours each day, 5 days a week, for a total of 6 months. The mice in the air control group were housed in a clean environment.
Dose and specimen treatment
After exposure to cigarette smoke for 3 months, 100 mg/kg F528 and erythromycin were administered daily. The air control group and the cigarette smoke group were given an equal volume of sodium carboxymethyl cellulose. After 3 months of treatment, their lung function and BALF were assessed by tracheal intubation. The lung tissue was isolated, and the left lung was fixed in 10% formaldehyde to prepare paraffin sections for H&E staining.
Bronchial alveolar lavage fluid (BALF) classification and counting
After the lung function of the mouse was evaluated, the precooled sterile PBS lavage solution was injected into the lung twice, 0.8 ml/time. The cells were centrifuged at 1500 rpm for 10 min. The cell pellets were resuspended in 1 ml PBS. Cell counting was performed on a hemocytometer. Conventional H&E staining was performed with cytospin preparations of BAL cells, and differential cell counts were performed under the microscope.
Measurement of interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)
BALF, blood and cell supernatants were collected and centrifuged at 4°C (3000 rpm) for 5 min. TNF-α and IL-6 concentrations in the BALF, serum and cell supernatants were measured with ELISA kits from R&D according to the manufacturer’s instructions. Each sample was assayed in triplicate.
Detection of emphysema degree
After the left lung was fixed, H&E staining was performed. To evaluate the degree of alveolar wall destruction in the mice, we used Image-Pro Plus 6.0 image analysis software to measure the mean linear intercept (MLI). MLI assay: Three H&E stained sections were taken from each mouse. Five fields of view were randomly taken under low magnification (100×), avoiding blood vessels and airways. Cross lines were drawn in the center of each field of view, and the total number of alveolar spaces (NS)passing through the cross lines, was counted while measuring the total length of the cross lines (L), calculated by the formula: MLI = L/NS, to represent the average inner diameter of the alveoli.
Pulmonary function test
The mice were anaesthetized (1% sodium pentobarbital, 50 mg/kg), and the trachea was fully exposed. After tracheal intubation was performed, the mice were placed in an animal pulmonary function meter FlexiVent (Scireq, Montreal, Quebec, Canada). Both ends of the tracheal intubation were connected to flow rate and pressure sensors, and the relevant parameters were detected by using FlexiVent software.
Immunohistochemistry analysis
Immunohistochemical staining was performed according to the manufacturer’s instructions. The primary antibodies were rabbit against mouse Bcl-2 and Bax (1:50; Boster Co, Ltd., Wuhan, China) at 4°C. Three stained sections were taken from each mouse. Five images were randomly acquired under the magnification (200×) in each section. The images were analyzed by Image-Pro Plus software.
Western blot analysis
The protein for cells or the two lobes of right lungs were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to nitrocellulose membranes by electroblotting. After blocking, the membrane was incubated overnight at 4°C with primary antibodies against MMP-2 (1:500; ab7033), MMP-9 (1:1000; ab38898), NF-κB p65 (1:1000; CST 8242); phospho-NF-κB p65 (1:1000, CST 3033) while β-actin (1:1000; ab6276) was used as a loading control. The ECL was added to the membrane and film exposure was performed. The average values for each group were determined by 3 independent experiments.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the right lung using TRIzol reagent (Takara, Tokyo, Japan), and cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-Rad, USA). The primers are listed in Table S1. The procedures were performed in accordance with the manufacturers’ instructions. MMP-2, MMP-9 and β-actin were quantified by iTaqTM universal SYBR Green Supermix (Bio-Rad, USA) on a CFX Connect (Bio-Rad, USA). Each experiment was repeated independently three times.
Statistical analysis
All values are expressed as the mean ± standard error of the mean (SEM). A one-way analysis of variance (ANOVA) followed by Dunnett’s test was used to analyze the differences between groups. Statistical analysis was performed by using the GraphPad Prism software. P<0.05 was considered statistically significant.
Results
F528 does not possess antibacterial activity and cytotoxicity
It has been reported that erythromycin can reduce inflammation and improve exercise endurance in patients with COPD, but long-term-use of erythromycin significantly increases the risk of bacterial drug resistance and flora disorders. Therefore, we decided to develop an erythromycin derivative that does not have antibacterial activity without affecting its other function. Based on this consideration, we developed an erythromycin derivative, F528.
First, we tested the antibacterial activities of F528 in vitro, and erythromycin was used as a control. Unlike erythromycin, with dose-dependent inhibition of bacterial growth from 10 μM, F528 treatment did not show any significant change in OD600, suggesting that F528 did not have antibacterial activity at concentrations less than 200 μM (Figure S1A and S1B). The LD50 of F528 is 112 μM, and its cytotoxicity was less than that of erythromycin at 100 μM (Figure S2).
F528 prevented the development of smoke-induced emphysema
Next, we addressed whether F528 could prevent emphysema as erythromycin did in a mouse model. C57/BL6 mice were exposed to cigarette smoke (CS) or non-smoke (NS) for 6 months. During the last three months of CS/NS exposure, CS-exposed mice were orally administered either F528 or erythromycin at a dose of 100 mg/kg once a day. As shown in Figure 1A and 1B, CS exposure obviously resulted in a broken alveolar wall and an irregularly enlarged alveolar space. However, the phenotype of CS-induced pulmonary emphysema could be significantly rescued by oral administration of either F528 or erythromycin at a dose of 100 mg/kg for 3 months. The enlargement of the airspaces was quantified by the MLI and the results are presented in Table S2 and Figure 1C.
Figure 1.
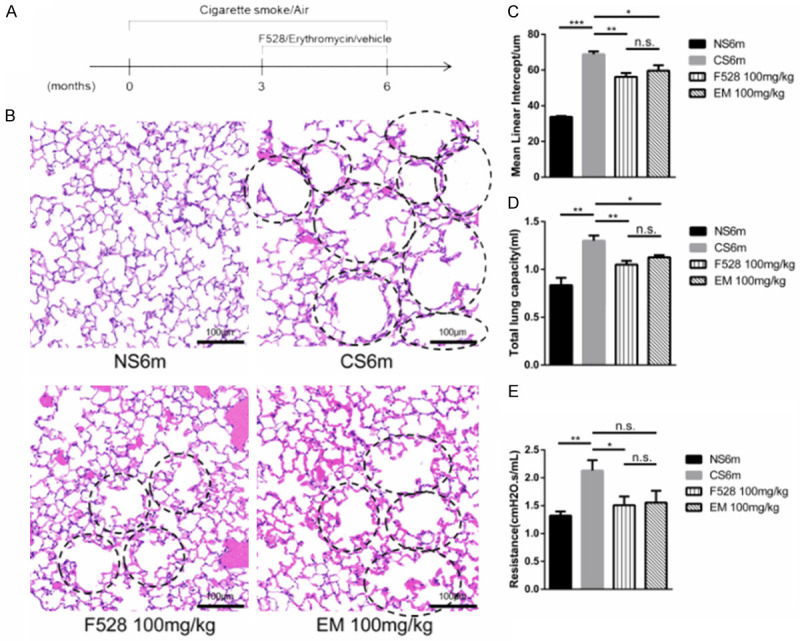
F528 treatment reduced the development of emphysema in mice. A. The different treatment for mice in four groups were performed at indicated time points. B. Representative HE photomicrographs of lung sections from mice treated with F528 and erythromycin. The circles represent the part of the enlarged and broken alveoli. Scale bar: 100 μm. C. Morphometric measurements of mean linear intercept (MLI) (μm). D, E. Total lung capacity and lung resistance assessment. n=6 mice in each group. Data are represented as mean ± SEM. NS6m: Non-smoke group; CS6m: Cigarette smoke group for 6 months; F528 100 mg/kg: Cigarette smoke 6 months + F528 100 mg/kg group; EM 100 mg/kg: Cigarette smoke 6 months + Erythromycin 100 mg/kg. *P<0.05, **P<0.01, ***P<0.001, n.s., not significant.
Pulmonary function is an important indicator for evaluating emphysema. CS exposure for 6 months significantly enhanced the total lung capacity and lung resistance compared to those of NS-exposed mice (Figure 1D and 1E). Oral administration of 100 mg/kg F528 as well as 100 mg/kg erythromycin for 3 months significantly prevented the increase in total lung capacity and lung resistance as compared to the CS group, except that the lung resistance of the 100 mg/kg erythromycin group showed no significant difference. Meanwhile, there was no significant difference in alveolar morphometric assessment and pulmonary function tests between 100 mg/kg F528 and erythromycin.
F528 treatment inhibited smoke-induced lung inflammation
We next determined the role of F528 in lung inflammation. CS exposure triggered a significant increase in the total cell number of inflammatory cells, neutrophils and macrophages in the BALF. The administration of F528 at 100 mg/kg resulted in a significant reduction of total inflammatory cells, neutrophils and macrophages in the BALF (Figure 2A-C). The pro-inflammatory cytokines, IL-6 and TNF-α measured in the BALF and serum were higher after 6 months of CS exposure compared with the non-smoke mice, and the mice treated with 100 mg/kg of F528 had less IL-6 and TNF-α in their BALF and serum (Figure 2D-G). While 100 mg/kg of erythromycin also decreased the total number of inflammatory cells, neutrophils and macrophages in the BALF, as well as IL-6 and TNF-α, the effect of F528 was almost similar to that of erythromycin at the same dose.
Figure 2.
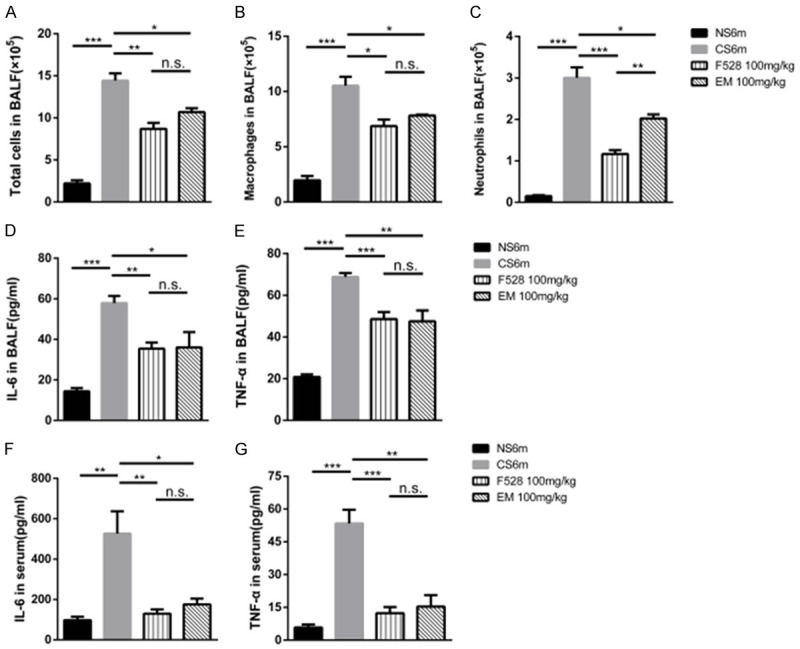
F528 treatment attenuated smoke-induced lung inflammation in mice. A-C. Total number, the number of neutrophils and macrophages in BALF were counted under the microscope. D, E. The levels of IL-6 and TNF-α in BALF were determined by ELISA. F, G. The levels of IL-6 and TNF-α in serum were measured by ELISA. n=6 mice in each group. Data are represented as mean ± SEM. NS6m: Non-smoke group; CS6m: Cigarette smoke group for 6 months; F528 100 mg/kg: Cigarette smoke 6 months + F528 100 mg/kg group; EM 100 mg/kg: Cigarette smoke 6 months + Erythromycin 100 mg/kg. *P<0.05, **P<0.01, ***P<0.001, n.s., not significant.
F528 reduced smoke-induced cytokine production in RAW264.7 cells
We next investigated whether F528 regulated the production of pro-inflammatory cytokines in vitro. RAW264.7 cells were treated with either F528 or erythromycin for 1 hour before CSE stimulation, and the cytokines were quantified in 6-hour conditioned media. As shown in Figure 3A and 3B, both F528 and erythromycin at 100 mg/kg clearly reduced the CSE-induced production of IL-6 and TNF-α, but F528 was more effective at same dose.
Figure 3.

F528 treatment decreased the levels of IL-6 and TNF-α in RAW264.7 cells after CSE stimulation. F528 50 µM, 100 µM and erythromycin 100 µM were respectively given to RAW264.7 cells 1 h prior to 5% CSE stimulation for 6 h. The supernatant was taken and the content of (A) IL-6 and (B) TNF-α was measured by ELISA. Data are represented as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, n.s., not significant.
F528 ameliorated the apoptosis in the lung of smoke-induced emphysema
To test the effect of F528 on the pathologic apoptosis in the emphysema, the protein expression of the apoptosis genes, such as Bax and Bcl-2, was evaluated by immumohistochemical staining. As shown in Figure 4A-C, a marked elevation of Bax and Bcl-2 expression was observed in the CS6m group as compared to NS6m control group, and the protein expression of Bax and Bcl-2 were significantly decreased by F528 100 mg/kg and EM 100 mg/kg treatment as compared with the CS6m group.
Figure 4.

F528 attenuated the expression of Bax and Bcl-2 protein in mice. (A) Representative immunostaining of Bax and Bcl-2 proteins in the lung of mice in the Non-smoke group (a), CS6m group: Cigarette smoke group for 6 months (b); F528 100 mg/kg: Cigarette smoke 6 months + F528 100 mg/kg group (c); EM 100 mg/kg: Cigarette smoke 6 months + Erythromycin 100 mg/kg (d). Original magnifications, (a-d), 200X. Scale bar: 100 μm. (B, C) Semi-quantitative analysis of the optical density of Bax and Bcl-2. *P<0.05, **P<0.01, ***P<0.001, n.s., not significant.
F528 attenuated the activation of NF-κB expression of MMP-2 and MMP-9 in RAW264.7 cells
The NF-κB signaling pathway, strongly associated with inflammation, is closely related to cigarette smoke exposure in human and mice. We next investigated the effect of F528 on the activation of NF-κB signaling. NF-κB was phosphorylated upon CSE stimulation in RAW264.7 cells. F528 markedly inhibited the CSE-induced activation of NF-κB (Figure 5A and 5C).
Figure 5.
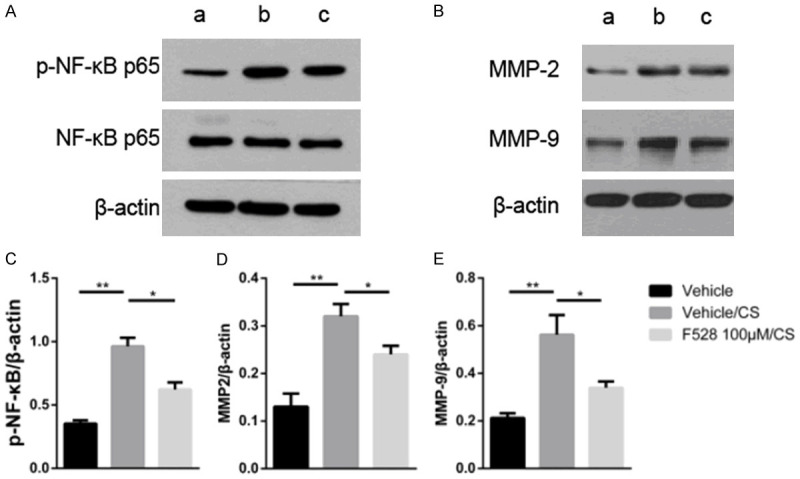
F528 reduced the expression of phospho-NFκB, MMPs in RAW 264.7 cells. (A) The protein expression of phospho-NF-κB and total NF-κB in RAW264.7 cells after the CSE stimulation. (B) The protein expression of MMP-2 and MMP-9 in RAW264.7 cells after the CSE stimulation. (C-E) Quantification of the bands shown in (A and B). Data are represented as mean ± SEM. a: Vehicle; b: Vehicle/CS; c: F528 100 μM/CS. *P<0.05, **P<0.01, ***P<0.001, n.s., not significant.
Matrix metalloproteinases (MMPs) have been linked to the development and progression of smoke-induced emphysema in the destruction of elastin, the aberrant remodeling of damaged alveoli, and the amplification of inflammatory responses [20], and they are regulated by the NF-κB signaling pathway. As shown in Figure 5B, 5D and 5E, F528 remarkably inhibited the CSE-induced production of MMP-2 and MMP-9. This result suggested that F528 possibly ameliorated the inflammation and emphysema by regulating the secretion of MMPs through the NF-κB signaling pathway.
F528 reduced smoke-induced NF-κB activity and the expression of smoke-induced MMPs in the lung tissue
Based on the findings that F528 reduced the activation of NF-κB and the expression of MMPs in vitro, we then validated them in mice. Compared with the CS group, 100 mg/kg of F528 significantly decreased the expression of phospho-NF-κB p65 (Figure 6A and 6C). Furthermore, the protein (Figure 6B, 6D and 6E) and mRNA (Figure 6F and 6G) levels of MMP-2 and MMP-9 were markedly reduced by 100 mg/kg of F528 treatment relative to the CS group. Thus, this demonstrated that CS-induced NF-κB activation and the expression of MMPs can be significantly inhibited by treatment with F528 in mice.
Figure 6.
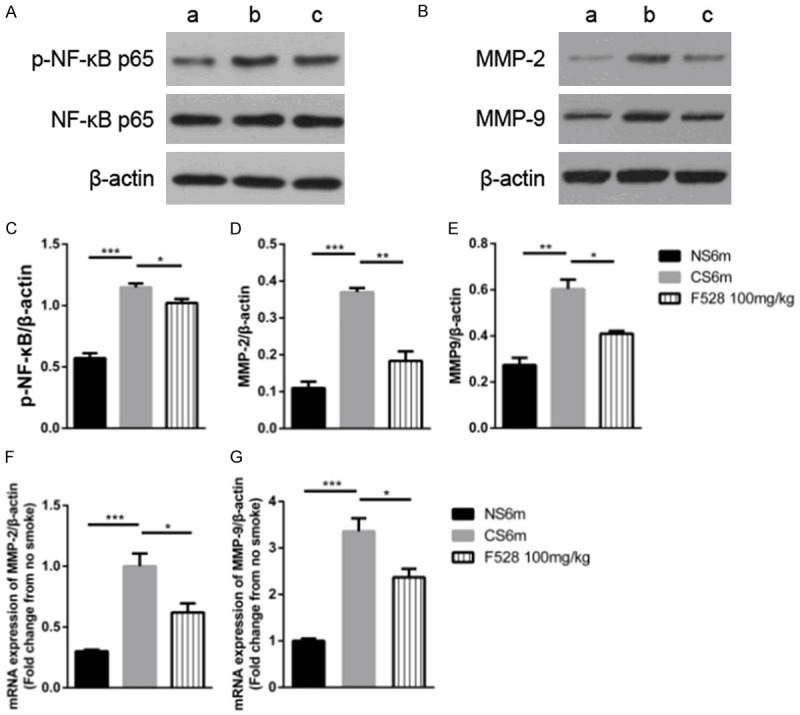
F528 treatment reduced the expression of phospho-NF-κB, MMPs in lung tissue. (A) The protein expression of phospho-NF-κB in lung tissue after 6 months of cigarette smoke. (B) The protein expression of MMP-2 and MMP-9 in lung tissue after 6 months of cigarette smoke. (C-E) Quantification of the bands shown in (A and B). (F, G) The mRNA level of MMP-2 and MMP-9 in lung tissue after 6 months of cigarette smoke. Data are represented as mean ± SEM. a: NS6m (Non-smoke group); b: CS6m (Cigarette smoke 6 months group); c: F528 100 mg/kg (Cigarette smoke 6 months + F528 100 mg/kg group). *P<0.05, **P<0.01, ***P<0.001, n.s., not significant.
Discussion
Macrolides have been used as one of the key anti-inflammatory drugs in the treatment of COPD in GOLD 2017 [21]. However, long-term use of these macrolides leads to the bacterial resistance [22-24]. Therefore, a macrolide derivative without antibacterial effect, such as F528 may be a long-sought-after drug for the future treatment of chronic obstructive pulmonary disease.
Studies of the effects of the amelioration of inflammation and emphysema of macrolides have been reported during past decades [25-27]. A few non-antibiotic macrolide derivatives have been developed to reduce mucus secretion and oxidative stress in the airway [28,29], but few studies have been reported on the use of these drugs to treat chronic respiratory diseases.
In this study, smoke-induced mouse emphysema was used as a model of COPD. Compared with the pathological morphology, lung function, and inflammatory cytokines, we found that F528 can remarkably reduce the inflammation and emphysema in vitro and in vivo. Meanwhile, F528’s amelioration of inflammation was better than that of erythromycin at the same dose in vitro. It can also reduce the pathological apoptosis indicated by the expression of Bax and Bcl-2. Therefore, F528 has anti-inflammatory and therapeutic effects on cigarette- smoke-induced inflammation, apoptosis and emphysema, while it has no antibacterial effect.
NF-κB, a transcription factor that regulates the expression of various inflammatory genes, is closely associated with the pathogenesis of COPD [30-32]. Inflammation, innate immunity and repair processes are closely related to the activation of NF-κB. As previously described [33,34], the NF-κB signaling pathway can be regulated by macrolides. MMPs, such as MMP-2 and MMP-9, are some of the target genes of NF-κB, and the imbalance of matrix metalloproteases and tissue inhibitors of MMPs plays an important role in the pathogenesis of COPD. The expression of MMP-2 and MMP-9 becomes excessive and they cannot be neutralized by their inhibitors, which results in the degradation of elastin in the lung parenchyma, leading to the destruction of the lung parenchyma in COPD [35]. Therefore, we investigated the effect of F528 on the NF-κB signaling pathway, as well as the expression of MMPs.
In this study, we showed that F528 suppressed the activation of NF-κB and the expression of MMP-2 and MMP-9 in mice. It could directly reduce the phosphorylation of NF-κB and the expression of MMPs in CSE-stimulated macrophages. Hence, we suggest that F528 reduces the degree of inflammation and emphysema by inhibiting of the activation of the NF-κB signaling pathway in inflammatory cells (Figure 7).
Figure 7.
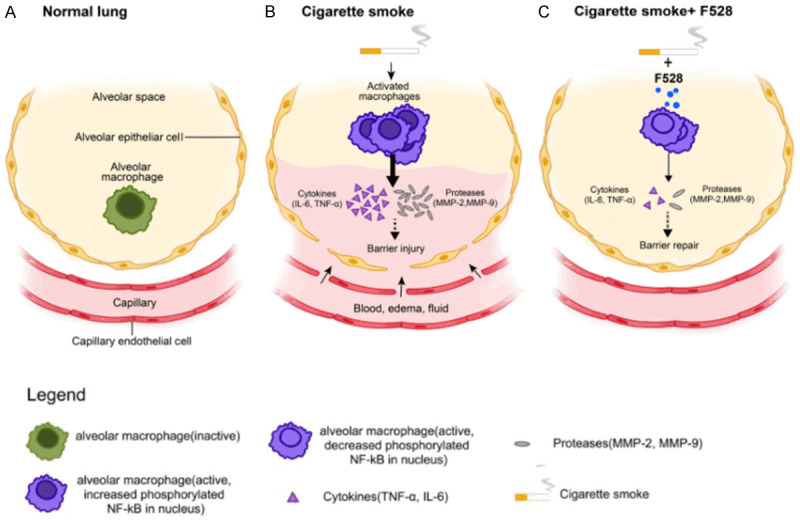
F528 treatment ameliorated smoke-induced inflammation and emphysema through NF-κB signaling pathway. Cigarette smoke can induce the inflammation by the phosphorylation of the NF-κB signaling pathway of macrophages and the expression of pro-inflammatory cytokines and matrix metallopeptidases, leading to the destruction of the alveolar structure and the development of lung emphysema. F528 ameliorates the inflammation and emphysema by inhibition of the activation of the NF-κB signaling pathway in macrophages and attenuates CS-induced recruitment of pro-inflammatory cytokines and proteinases, such IL-6, TNF-α and MMPs.
This study provides new evidence of the effectiveness of a macrolide derivative without antibacterial activity for the treatment of COPD patients. Additionally, it will be interesting to investigate how F528 modifies the NF-κB signaling pathway so that a specific target can be selected for future drug development. Future analysis in clinical trials of this drug for COPD patients could reveal the effect of the amelioration of inflammation and emphysema in these patients.
The results of this study do not confirm a direct relationship between the alleviation of inflammation and emphysema and the inhibition of NF-κB activation by F528. In addition, there may be other signaling pathways involved in this process.
In conclusion, F528 ameliorates the inflammation and emphysema in vivo and in vitro by inhibiting of the activation of NF-κB, which provides novel insight into the treatment of chronic obstructive pulmonary disease. However, there is still much in-depth exploration necessary, laying the foundation for the development of better anti-inflammatory drugs, and providing strong evidence for the clinical application of targeted prevention and treatment of chronic inflammatory diseases. Thus, F528 might be expected to be a novel treatment option for patients with COPD.
Acknowledgements
This paper was funded by CAMS Innovation Fund for Medical Sciences (CIFMS, No. 2018-I2M-1-001) and Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2019PT320021). The authors are grateful for the donation of F528 and erythromycin from Beijing Kangdini Pharmaceutical Co., Ltd. We also thank Dianhua Jiang, MD, Ph.D, of Cedars Sinai Medical Center for critical reading of the manuscript, and Ping Xin (Department of Respiratory and Critical Care Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China) for assistance in performing the BALF classification and counting.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 2.Brandsma CA, de Vries M, Costa R, Woldhuis RR, Konigshoff M, Timens W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur Respir Rev. 2017;26:170073. doi: 10.1183/16000617.0073-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan KY, Li X, Chen W, Song P, Wong NWK, Poon AN, Jian W, Soyiri IN, Cousens S, Adeloye D, Sheikh A, Campbell H, Rudan I Global Health Epidemiology Research Group (GHERG) Prevalence of chronic obstructive pulmonary disease (COPD) in China in 1990 and 2010. J Glob Health. 2017;7:020704. doi: 10.7189/jogh.07.020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Stockley RA, Halpin DMG, Celli BR, Singh D. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med. 2019;199:1195–1204. doi: 10.1164/rccm.201810-1860SO. [DOI] [PubMed] [Google Scholar]
- 6.Aleva FE, Voets L, Simons SO, de Mast Q, van der Ven A, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: a systematic review and meta-analysis. Chest. 2017;151:544–554. doi: 10.1016/j.chest.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond) 2017;131:1541–1558. doi: 10.1042/CS20160487. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Lomas DA. Does protease-antiprotease imbalance explain chronic obstructive pulmonary disease? Ann Am Thorac Soc. 2016;13(Suppl 2):S130–137. doi: 10.1513/AnnalsATS.201504-196KV. [DOI] [PubMed] [Google Scholar]
- 10.Ostridge K, Williams N, Kim V, Bennett M, Harden S, Welch L, Bourne S, Coombs NA, Elkington PT, Staples KJ, Wilkinson TM. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax. 2016;71:126–132. doi: 10.1136/thoraxjnl-2015-207428. [DOI] [PubMed] [Google Scholar]
- 11.Arredondo A, Blanc V, Mor C, Nart J, Leon R. Azithromycin and erythromycin susceptibility and macrolide resistance genes in prevotella from patients with periodontal disease. Oral Dis. 2019;25:860–867. doi: 10.1111/odi.13043. [DOI] [PubMed] [Google Scholar]
- 12.Huckle AW, Fairclough LC, Todd I. Prophylactic antibiotic use in COPD and the potential anti-inflammatory activities of antibiotics. Respir Care. 2018;63:609–619. doi: 10.4187/respcare.05943. [DOI] [PubMed] [Google Scholar]
- 13.Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–1147. doi: 10.1164/rccm.200801-145OC. [DOI] [PubMed] [Google Scholar]
- 14.Tan C, Huang H, Zhang J, He Z, Zhong X, Bai J. Effects of low-dose and long-term treatment with erythromycin on interleukin-17 and interleukin-23 in peripheral blood and induced sputum in patients with stable chronic obstructive pulmonary disease. Mediators Inflamm. 2016;2016:4173962. doi: 10.1155/2016/4173962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasetty G, Bhongir RKV, Papareddy P, Herwald H, Egesten A. The nonantibiotic macrolide EM703 improves survival in a model of quinolone-treated pseudomonas aeruginosa airway infection. Antimicrob Agents Chemother. 2017;61:e02761–16. doi: 10.1128/AAC.02761-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara A, Shima H, Sueki A, Hirose T, Matsui H, Nakano H, Hanaki H, Akagawa KS, Omura S, Sunazuka T. Non-antibiotic 12-membered macrolides: design, synthesis and biological evaluation in a cigarette-smoking model. J Antibiot (Tokyo) 2016;69:319–326. doi: 10.1038/ja.2015.91. [DOI] [PubMed] [Google Scholar]
- 17.Tojima I, Shimizu S, Ogawa T, Kouzaki H, Omura S, Sunazuka T, Shimizu T. Anti-inflammatory effects of a novel non-antibiotic macrolide, EM900, on mucus secretion of airway epithelium. Auris Nasus Larynx. 2015;42:332–336. doi: 10.1016/j.anl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Mencarelli A, Distrutti E, Renga B, Cipriani S, Palladino G, Booth C, Tudor G, Guse JH, Hahn U, Burnet M, Fiorucci S. Development of non-antibiotic macrolide that corrects inflammation-driven immune dysfunction in models of inflammatory bowel diseases and arthritis. Eur J Pharmacol. 2011;665:29–39. doi: 10.1016/j.ejphar.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Teng J, Li Y, Yu W, Zhao Y, Hu X, Tao NP, Wang M. Naringenin, a common flavanone, inhibits the formation of AGEs in bread and attenuates AGEs-induced oxidative stress and inflammation in RAW264.7 cells. Food Chem. 2018;269:35–42. doi: 10.1016/j.foodchem.2018.06.126. [DOI] [PubMed] [Google Scholar]
- 20.Gharib SA, Manicone AM, Parks WC. Matrix metalloproteinases in emphysema. Matrix Biol. 2018;73:34–51. doi: 10.1016/j.matbio.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, Lopez Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agusti A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 22.Dinos GP. The macrolide antibiotic renaissance. Br J Pharmacol. 2017;174:2967–2983. doi: 10.1111/bph.13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jhun BW, Kim SY, Moon SM, Jeon K, Kwon OJ, Huh HJ, Ki CS, Lee NY, Shin SJ, Daley CL, Koh WJ. Development of macrolide resistance and reinfection in refractory mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2018;198:1322–1330. doi: 10.1164/rccm.201802-0321OC. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder MR, Stephens DS. Macrolide resistance in streptococcus pneumoniae. Front Cell Infect Microbiol. 2016;6:98. doi: 10.3389/fcimb.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang S, Ma T, Zhang H, Zhang J, Zhong X, Tan C, Qiu Y, Zeng W, Feng X. Erythromycin prevents elastin peptide-induced emphysema and modulates CD4(+)T cell responses in mice. Int J Chron Obstruct Pulmon Dis. 2019;14:2697–2709. doi: 10.2147/COPD.S222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan YF, Huang ZH, Jing K, Li J, Wang Y, Xu CQ, Chen JH, Zheng YL. Azithromycin attenuates pulmonary inflammation and emphysema in smoking-induced COPD model in rats. Respir Care. 2015;60:128–134. doi: 10.4187/respcare.03344. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Gu D, Hou G. Erythromycin attenuates metalloprotease/anti-metalloprotease imbalance in cigarette smoke-induced emphysema in rats via the mitogen-activated protein kinase/nuclear factor-kappaB activation pathway. Mol Med Rep. 2017;15:2983–2990. doi: 10.3892/mmr.2017.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosnar M, Kragol G, Kostrun S, Vujasinovic I, Bosnjak B, Bencetic Mihaljevic V, Marusic Istuk Z, Kapic S, Hrvacic B, Brajsa K, Tavcar B, Jelic D, Glojnaric I, Verbanac D, Culic O, Padovan J, Alihodzic S, Erakovic Haber V, Spaventi R. N’-substituted-2’-O,3’-N-carbonimidoyl bridged macrolides: novel anti-inflammatory macrolides without antimicrobial activity. J Med Chem. 2012;55:6111–6123. doi: 10.1021/jm300356u. [DOI] [PubMed] [Google Scholar]
- 29.Li YJ, Shimizu T, Hirata Y, Inagaki H, Takizawa H, Azuma A, Kawada T, Sugawara I, Kudoh S, Sunazuka T, Omura S. EM, EM703 inhibit NF-κB activation induced by oxidative stress from diesel exhaust particle in human bronchial epithelial cells: importance in IL-8 transcription. Pulm Pharmacol Ther. 2013;26:318–324. doi: 10.1016/j.pupt.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell S, Vargas J, Hoffmann A. Signaling via the NFkappaB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulero MC, Huxford T, Ghosh G. NF-κB, IκB, and IKK: Integral Components of Immune System Signaling. In: Jin T, Yin Q, editors. Structural Immunology. Singapore: Springer Singapore; 2019. pp. 207–226. [Google Scholar]
- 32.Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 33.Koon HW, Wang J, Mussatto CC, Ortiz C, Lee EC, Tran DH, Chen X, Kelly CP, Pothoulakis C. Fidaxomicin and OP-1118 inhibit clostridium difficile toxin A- and B-mediated inflammatory responses via inhibition of NF-kappaB activity. Antimicrob Agents Chemother. 2018;62:e01513–17. doi: 10.1128/AAC.01513-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma N, Deng TT, Wang Q, Luo ZL, Zhu CF, Qiu JF, Tang XJ, Huang M, Bai J, He ZY, Zhong XN, Li MH. Erythromycin regulates cigarette smoke-induced proinflammatory mediator release through sirtuin 1-nuclear factor kappaB axis in macrophages and mice lungs. Pathobiology. 2019;86:237–247. doi: 10.1159/000500628. [DOI] [PubMed] [Google Scholar]
- 35.Navratilova Z, Kolek V, Petrek M. Matrix metalloproteinases and their inhibitors in chronic obstructive pulmonary disease. Arch Immunol Ther Exp (Warsz) 2016;64:177–193. doi: 10.1007/s00005-015-0375-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


