Abstract
Insulin-like growth factor binding protein-1 (IGFBP-1) belongs to the insulin-like growth factor (IGF) system, which plays an indispensable role in normal growth and development, and in the pathophysiology of various tumors. IGFBP-1 has been shown to be associated with the risk of various tumors, and has a vital function in regulating tumor behaviors such as proliferation, migration, invasion and adhesion through different molecular mechanisms. The biological actions of IGFBP-1 in cancer are found to be related to its phosphorylation state, and the IGF-dependent and -independent mechanisms. In this review, we provided an overview of IGFBP-1 in normal physiology, and its aberrantly expression and the underlying molecular mechanisms in a range of common tumors, as well as discussed the potential clinical implications of IGFBP-1 as diagnostic or prognostic biomarkers in cancer.
Keywords: Insulin-like growth factor-binding protein-1 (IGFBP-1), expression, molecular mechanisms, clinical implications, cancer
Introduction
Tumorigenesis is a highly complex process that involves a variety of genetic and epigenetic changes mediated by different signaling pathways [1], and dysregulation of the IGF system is also implicated in the pathophysiology of tumor [2]. The IGF system consists of two ligands, insulin-like growth factor 1 (IGF-1) and insulin-like growth factor 2 (IGF-2), two cell-membrane receptors, IGF-1 receptor (IGF1R) and IGF-2 receptor (IGF2R), and six IGF-binding proteins, IGFBP-1 to IGFBP-6 [3]. As a member of the IGF system, IGFBP-1 has been confirmed to be associated with the risk of various tumors though there remain some controversial results. Moreover, it has been proposed that IGFBP-1 could suppress tumorigenesis, mainly by binding to IGFs to prevent it from binding to IGF receptor and then counteract the IGF-driven tumor development. Despite the inhibitory function of IGFBP-1 in cancer development, its positive effects on proliferation, invasion and migration of tumor cells through IGF-independent pathway have also been revealed, which may result from the interaction of the Arg-Gly-Asp (RGD) motif of IGFBP-1 with α5/β1 integrin receptor [4,5].
In this article, we briefly summarized the physiological regulation and structural context of IGFBP-1 before focusing on its expression, molecular mechanisms and potential clinical implications in cancers, including liver, breast, gastrointestinal, endometrial and other cancers.
Gene organization and protein structure
IGFBP-1 gene (NCBI Dataset: NC_000007) is located in the human chromosome region 7p12.3 where it is contiguous with the IGFBP-3 gene. The chromosome DNA length of IGFBP-1 gene is 5173 bp and it contains four exons (Table 1 and Figure 1). The length of exon 1 is 514 bp containing the entire 5’ untranslated region (5’UTR) and encodes the first 91 amino acids from the N-terminal of IGFBP-1 protein. Exon 2 has a length of 170 bp and encodes most of the amino acid sequences of the central linker domain. The C-terminal of IGFBP-1 protein is encoded by exon 3 and first half of exon 4 (second half is the 3’ untranslated region (3’UTR)). The IGFBP-1 gene transcription start site is located at 165 bp upstream of the ATG translation start site, and the transcription length is 1514 bp (NCBI: NM_000596.4) [6,7]. In addition, in the proximal -300 bp of promoter regions of IGFBP-1, there are nine DNA elements shown to be of functional significance in vitro, including the TATA element, hepatic nuclear factor 1 (HNF1)-binding site, insulin response element (IRE), cAMP response element (CRE), glucocorticoid response elements GRE1 and GRE2, CAAT/enhancer-binding proteins (C/EBPs)-binding site and progesterone response elements (PREs) [7-9]. These DNA elements are responsible for basal promoter activity of IGFBP-1, as well as the stimulatory or inhibitory effects of some hormone such as glucocorticoids, progesterone and insulin to the IGFBP-1 expresssion.
Table 1.
IGFBP1 gene information
| Gene name (known as) | Position and length | Exon number | Encoding mRNA and protein | 5’UTR | CDS | 3’UTR |
|---|---|---|---|---|---|---|
| IGFBP1 (AFBP; hIGFBP-1; IBP1; IGF-BP25; PP12) | 7p12.3; 5173 bp | 4 (1..514, 2061..2230, 3445..3573, 4473..5173) | NM_000596.4, 1514 bp; NP_000587.1, 259aa | 1..165 | 166..514, 2061..2230, 3445..3573, 4473..4604 | 4605..5173 |
Figure 1.
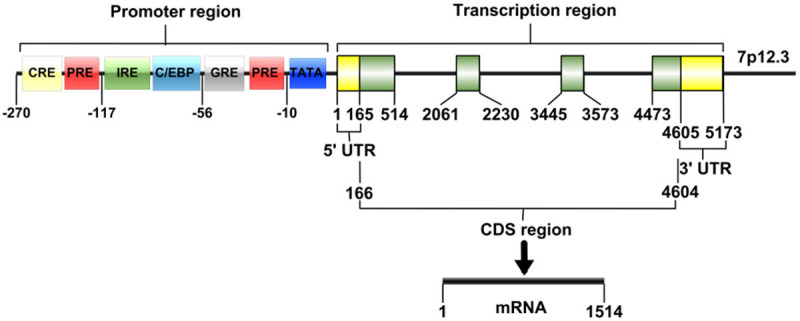
Schematic representation of the IGFBP-1 chromosomal gene and promoter region.
The biochemical characterization of IGFBP-1 protein (also known as placental protein 12) was first reported by Bohn and Kraus in the 1980s [10]. Similarly to other IGFBPs, after dissociation of the signal peptide of 25 residues, the mature IGFBP-1 protein with 234 residues can be divided into three regions (UNIPROT Dataset: P08833; NCBI Dataset: NP_000587.1): the N-terminal region, the central linker region and the C-terminal region (Figure 2). The N-terminal region contains the first 82 residues of IGFBP-1 including 12 cysteines, and the C-terminal region in residues 151 to 234 of IGFBP-1 contains 6 cysteines [11]. These 18 cysteines are important for forming a structural framework for IGFs-binding and other biological actions while surrounding amino acid residues provide functional specificity [7,12]. Additionally, the C-terminal region of IGFBP-1 contains a RGD sequence which is also presented in a group of extracellular matrix proteins such as fibronectin, laminin and collagens. The RGD domain could bind to some specific cell surface receptors, like α5/β1 integrin, to play a complex role in cell attachment and migration [5,7].
Figure 2.
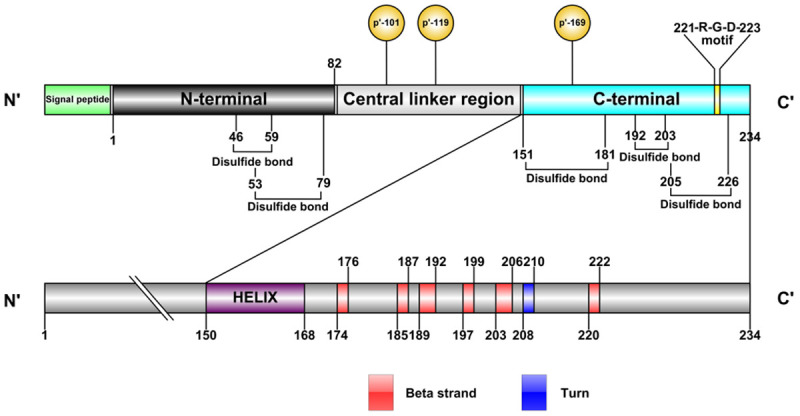
Schematic representation of the IGFBP-1 protein. After dissociation of the signal peptide, the mature IGFBP-1 protein with 234 residues can be divided into three regions: the N-terminal region, the central linker region and the C-terminal region. IGFBP-1 contains five disulfide bond to provide a structural framework for IGF binding, and the domains of C-terminal region is described detailedly with one helix, six beta strand and one turn. The Ser-101, Ser-119 and Ser-169 in IGFBP-1 are the primary phosphorylation targets, and the RGD motif is located in residues 221 to 223.
The central linker region of IGFBP-1 is the least conserved region and it has never been cited as part of the IGF-binding site [12]. However, some studies have indicated that differences in the specificity of IGFBPs binding to IGFs may not only depend on the structure of IGF itself, but also may be related to the difference of the central linker domain among IGFBPs [13,14]. The central linker region may act as a hinge modifying ligand-binding affinity and specificity, and allows the protein to fold back upon itself, so that the N-terminal region and C-terminal region can interact with the single IGF ligand [7]. The central linker region of IGFBP-1 is rich in proline (P), glutamine (E), serine (S) and threonine (T) residues which is characterized as PEST domain. The PEST domain is not found in other five IGFBPs and could increase the susceptibility of IGFBP-1 to proteolysis. Moreover, the PEST domain is usually found in rapid turnover proteins (e.g. c-fos, c-myc), and the dynamic regulation with serum IGFBP-1 levels ranges up to 10-fold or more in relation to meals, indicating that IGFBP-1 is a rapidly metabolized protein [6,7]. In addition, proteolytic cleavage and post-translational modification including glycosylation and phosphorylation always occur in this region of IGFBP-1. The most common post-translational modification of IGFBP-1 is in the form of phosphorylation of serine residues, and many phosphorylation sites on the IGFBP-1 protein sequence have been identified so far, such as Ser-20, Ser-70, Ser-73, Ser-95, Ser-98, Ser-131, Thr-132, Tyr-133, Ser-149, Thr-168, Ser-174 and Ser-217 [15-18]. But the Ser-101, Ser-119 and Ser-169 are the primary phosphorylation targets, as Ser-101 accounts for 70% of total mature IGFBP-1 phosphorylation, while Ser-169 and Ser-119 account for only 25% and 5% of phosphorylation, respectively [19].
Expression and metabolic regulation
IGFBP-1 is a secreted protein and the most predominant IGFBP in amniotic fluid normally expressed in the liver, endometrium and placenta in a tissue-specific manner [20]. IGFBP-1 expression is mainly regulated by human hormones such as growth hormone (GH), glucagon and insulin (Figure 3). Numerous studies are demonstrating the predominant regulatory effect of insulin on IGFBP-1 expression. Insulin can act through the insulin receptor to inhibit IGFBP-1 expression in hepatic cells [21], ovarian granulosa cells [22], osteoblast cells [23], kidney cells [24] and uterine decidual cells [25]. This inhibitory action occurs at the level of gene transcription [26]. Under normal conditions, the physiological concentration changes of insulin can independently regulate the secretion of IGFBP-1. Specifically, in the fasting state, IGFBP-1 level is high because of the low inhibitory effect of insulin on hepatic IGFBP-1 transcription, while carbohydrate intake leads to an increase in insulin level and a rapid decrease in post-pranational IGFBP-1 level [27,28]. Pathologically, serum IGFBP-1 level of type 2 diabetes patient is elevated approximately 2.5-fold above the normal mean expression and falls during an insulin infusion [29]. Likewise, IGFBP-1 level is increased in insulin-deficient subjects with type 1 diabetes [30]. Moreover, the inhibition of GH or glucose infusion on IGFBP-1 expression are most likely related to insulin-mediated effects. These indicate that insulin is of utmost importance in the principal mechanisms by which IGFBP-1 level is regulated.
Figure 3.
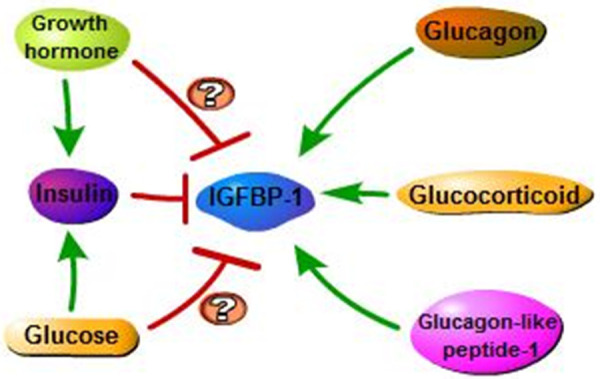
Hormonal regulation of IGFBP-1. The single green headed arrow is activation arrow and red headed arrow is inhibition arrow. The direct effect of GH and glucose on IGFBP-1 expression still remain controversial.
As for the effect of glucose on serum IGFBP-1 level, it has been demonstrated that food intake or glucose infusion can inhibit serum IGFBP-1 level in normal subjects [31,32]. But in these normal cases, the reduction of IGFBP-1 is probably related to the indirect action of insulin. Some studies also show that within physiologic concentration ranges, glucose does not have independent effects on IGFBP-1 in vitro or in vivo [33-35]. However, in the study of Snyder et al [29], fructose and triglycerides which activate glycolysis but not insulin secretion were taken to investigate whether suppression of plasma concentration of IGFBP-1 is directly controlled by glucose level. They discovered that partial inhibition of IGFBP-1 expression appears to be mediated by insulin stimulation of glucose transport or some other insulin-induced cellular metabolic processes. But they also revealed that glucose could directly inhibit the secretion of IGFBP-1 to the same extent as the insulin-mediated response [29]. Nevertheless, it still needs more evidence to support such a direct regulatory role of glucose on serum IGFBP-1 level.
Similar to the role of glucose in regulating IGFBP-1, a direct effect of GH on IGFBP-1 expression was controversial in previous studies. The possibility that the effect of GH in decreasing IGFBP-1 level is mediated by GH-induced expression of other elements like insulin, needs to be considered. Conover et al show that GH does not have independent effects on the acute regulation of serum IGFBP-1 [33], and early study has indicated that there is no temporal association of circadian patterns of GH and IGFBP-1 [36]. But the research by Norrelund et al has provided evidence for a direct suppressive effect of GH on IGFBP-1, which appears to be emerged in the presence of low or suppressed insulin levels [37].
Unlike the suppressive effects of insulin, glucose or GH on IGFBP-1, the role of glucagon in promoting IGFBP-1 expression, mediated by cathelicidin antimicrobial peptide (cAMP) or IGF-1, was well supported by Lee et al [7]. Moreover, not only does glucagon stimulate the secretion of IGFBP-1, but glucagon-like peptide-1 also exerts the same effect, both of which could increase IGFBP-1 serum level in healthy subjects and human patients [38]. It has also been confirmed that glucocorticoid could act as the major physiologic regulator of hepatic IGFBP-1 expression as insulin in vitro. Glucocorticoid has stimulatory effect while insulin is inhibitory on IGFBP-1 expression [7], but insulin may play a more predominant role in the regulation of IGFBP-1. It has been shown that glucocorticoid stimulated the expression of IGFBP-1 protein and mRNA whereas insulin suppressed basal and glucocorticoid-stimulated IGFBP-1 gene transcription in hepatocytes and osteoblasts [23,38,39].
IGF-dependent and IGF-independent actions of IGFBP-1
Generally, binding proteins are known to regulate ligand activity by extending their half-life, and IGFBP-1 is no exception. In addition to function as circulatory transport proteins for IGFs to increase their stability and modulate their tissue distribution [40], IGFBP-1 also plays an important role in the regulation of IGFs’ cellular actions [41]. Moreover, several findings have showed that the RGD domain of IGFBP-1 interaction with α5/β1 integrin could mediate cellular function in a IGF-independent way [42] (Figure 4).
Figure 4.
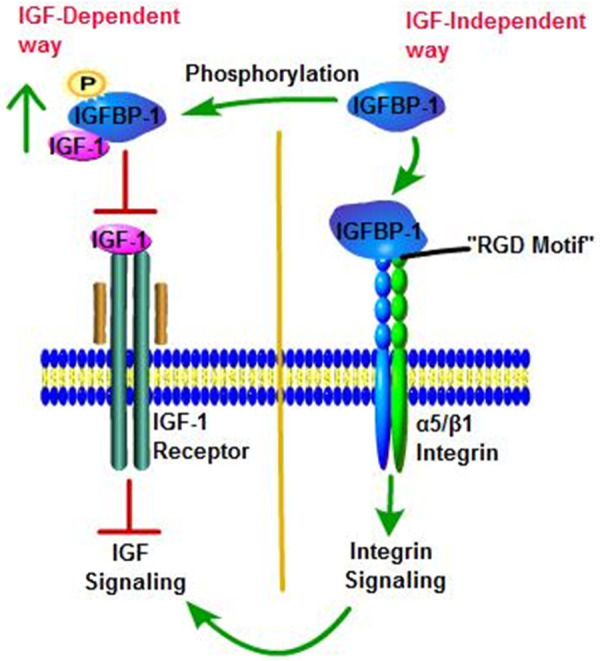
IGF-dependent and IGF-independent actions of IGFBP-1. The single green headed arrow is activation arrow and red headed arrow is inhibition arrow. (i) The phosphorylated IGFBP-1 has a much higher affinity for IGF-1; therefore, phosphorylation of IGFBP-1 would prevent IGF-1 to interact with its receptor. (ii) The RGD motif of IGFBP-1 interacting with α5/β1-integrin receptors on the cell surface is thought to be the main mechanism by which IGFBP-1 exerts IGF-independent effects, and it could lead to cross-talk with IGF signaling pathways.
It has been demonstrated that the IGF system was involved in the normal process of biological metabolism, as well as in the development and progression of various diseases including cancer [4]. For example, high serum concentration of IGF-1 is associated with increased risk of breast cancer, prostate cancer, colorectal cancer, lung cancer, glioblastoma, neuroblastoma, meningioma and rhabdomyosarcoma [3,43,44], and loss of imprinting (LOI) of IGF-2 occurs in tumors such as breast cancer, esophageal cancer, ovarian cancer, acute myeloid leukemia and Wilms’ tumor [45]. Through binding to IGFR to participate in numerous cellular actions, IGFs, a core of this system, are mostly regulated by IGFBPs such as IGFBP-1. A vast majority of in vitro and in vivo studies support the role of IGFBP-1 in modulating the acute bioavailability and insulin-like activity of IGFs on peripheral metabolism, and inhibiting IGF-stimulated cell growth and differentiative function in an IGF-dependent way. IGFBP-1 can bind to IGFs with high affinity, and then prevents the activation of IGFR signalling to provide chronic mitogenic stimuli for neoplastic transformation [6,7]. However, a few studies have described the potentiation of IGFs actions by IGFBP-1 under specific conditions [46-48]. The discrepant biological effects of IGFBP-1 are thought to be associated with its phosphorylation state. In vitro studies have demonstrated that phosphorylation of IGFBP-1 would enhance the affinity for IGF peptides, because the mutation of Ser-101, an important phosphorylation site of IGFBP-1, reduced the affinity of IGFBP-1 to IGF-1 by 6-fold [15]. In addition, through the analysis of IGFBP-1 isoforms separated by anion exchange chromatography, it was found that the isoform stimulating the actions of IGFs was nonphosphorylated while phosphorylated IGFBP-1 inhibited IGFs actions [49]. The evidence for this concept was provided by Jyung et al [50], who applied the combination of IGF-1 plus IGFBP-1 (phosphorylated and nonphosphorylated) directly to linear incisions made through dorsal rat skin and assessed breaking strength 8 days later. The results showed that nonphosphorylated IGFBP-1 had prominent potentiation for wound breaking recovery by 33 to 40% whereas the phosphorylated form of IGFBP-1 had no effect. Although other studies [51,52] have also confirmed this concept, there was a little evidence to suggest otherwise, that is nonphosphorylated IGFBP-1 could potently inhibit IGFs actions and cell growth both in vitro and in vivo, while phosphorylated IGFBP-1 from human amniotic fluid prevented IGF-1 binding to human fetal fibroblasts and enhanced IGF-1-mediated thymidine incorporation [7,53]. Therefore, the role of phosphorylation state in modulating IGFBP-1 activity is not very clear, and the mechanisms by which the nonphosphorylation IGFBP-1 enhanced the actions of IGF, more than not inhibiting it, have not been elucidated [42]. But to some extent, perhaps it could be explained from these perspectives. Firstly, phosphorylated IGFBP-1 has a higher affinity binding to IGF-1 than that of IGF-1 binding to IGF1R. Thus, compared with nonphosphorylated one, phosphorylated IGFBP-1 would be more “powerful” in preventing IGF-1 from binding to its receptor and then inhibiting its action. Secondly, the phosphorylation isoform increases IGFBP-1 resistance against protease hydrolysis, thereby enhancing its inhibitory effect on IGF-1. Conversely, nonphosphorylated IGFBP-1 prefers to form into disulfide-linked multimers and is more rapid than the phosphorylated form, and the polymerizations are shown to extenuate the inhibitory action of IGFBP-1 on IGF-1-stimulated protein synthesis [54]. Lastly, nonphosphorylated IGFBP-1 would interact with α5/β1 integrin receptor to potentiate IGFs action as an IGF-independent effect. These above suggestions are consistent with the description in the review of Jones et al [49].
From another perspective, several reports have demonstrated that even in the absence of IGFs, IGFBP-1 still can affect cellular events, suggesting that IGFBP-1 can activate other cell-surface receptors directly. Especially, it is the RGD motif in the C-terminal domain of IGFBP-1 binding to α5/β1 integrin to allow IGFBP-1 to enhance cell migration or adhesion of Chinese hamster ovary cells [55], human trophoblast cells [56], oligodendrocytes [57], smooth muscle cells [58] and tumor cells for example schwannoma [59] in a IGF-independent way. Intriguingly, other studies have revealed a opposite function of RGD motif in IGFBP-1. Irwin et al showed that IGFBP-1 bound to placental cytotrophoblast α5/β1 integrin to inhibit cytotrophoblast attachment to fibronectin, and then prevented its invasion into decidualized endometrial stromal cultures [60]. The same role is true of IGFBP-1 in breast cancer cells [61]. Therefore, it appears that IGFBP-1 plays a dual role in cell motility through IGF-independent way and the effect could be cell-specific. In addition, a recent study by Haywood et al found that IGFBP-1 enhanced insulin sensitivity and secretion with its RGD motif via integrin engagement, potentiation of focal adhesion kinase (FAK) and integrin linked kinase (ILK) [62]. It seems that integrin-FAK-ILK signaling was a core mechanism by which IGFBP-1 modulates insulin sensitivity, but it still requires further study. Meanwhile, it has been suggested that the bond of IGFBP-1 with α5/β1 integrin may enhance IGF-mediated effects. It is likely that integrin-bound IGFBP-1 on the cell surface acts as a reservoir for IGFs, thus providing a locally high concentration of IGFs to stimulate the IGF1R signaling pathway [49]. Moreover, the signal upon IGFBP-1 binding to α5/β1 integrin could interact with elements of the IGF1R signaling pathways and ultimately stimulates the actions of IGF-1.
IGFBP-1 and cancers
With other molecule elements of various signal pathways, the role of IGFBP-1 in cancer cells has been explored both in vitro and in vivo assays (Table 2 and Figure 5). In this section, the aberrantly expression of IGFBP-1 in cancers and the underlying molecular mechanisms would be illustrated detailedly, as well as its potential clinical implication in a range of common tumors (Table 3).
Table 2.
Potential pathway of IGFBP-1 in multiple tumor types
| Tumor types | Potential pathway | Description | Refs |
|---|---|---|---|
| Liver Cancer | P38 MAPK/IGFBP-1/FOXO3a | Forming a feedback regulation loop with FOXO3a on p38 MAPK leading to an reciprocal pathway | [64] |
| HIF-2α/IGFBP-1/IGFs/IGF1R | Upregulated by HIF-2α to inhibit the actions of the IGFs/IGF1R system | [72] | |
| HBx/IGFBP-1/IGF-1/IGFR | Inhibited by HBx to enhanced IGF-1-dependent and -independent effects | [73] | |
| mTOR/CSNK-2β/IGFBP-1/IGF-1/IGF1R | Enhanced by CSNK-2β activation mediated by mTOR inhibition to decrease IGF-1-induced IGF1R autophosphorylation | [75] | |
| PXR/HNF4α | Derepressed by PXR via inhibiting the HNF4α gene | [77] | |
| Breast Cancer | α5/β1 integrin/FAK | Interacting with α5/β1 integrin and induce FAK dephosphorylation | [93] |
| α5/β1 integrin and IGF-1/IGF1R/IRS-2 | Interacting with α5/β1 integrin to enhance IRS-2 phosphorylation mediated by IGF-1/IGF1R | [61] | |
| GPER1/PKA/CREB/IGFBP-1/IGF1R/AKT, ERK1/2 | Facilitating phosphorylation of IGF1R, AKT and ERK1/2 in a GPER1/PKA/CREB-dependent pathway | [95] | |
| IGF1R/ERK1/2 or ERα/FoxO1 and CREB | Induced via the cooperation between CREB and FoxO1 mediated by IGF1R dependent ERK1/2 or ERα pathway | [96] | |
| Endometrial Cancer | FOXO1A and PGR | Enhanced by FOXO1A alone or with PGR in a co-operative way | [117] |
| Glioblastoma | P53/IGFBP-1/ERK1/2/ZIC2, NR2F1 | Enhanced by P53 and activating ERK1/2, ZIC2 and NR2F1 to form a self-activating pathway | [127] |
| SYK/PI3K/NFκB/MCSF | Induced by the SYK/PI3K/NFκB/MCSF mechanism and promotes angiogenesis | [128] | |
| Schwannoma | α5/β1 integrin/Src/FAK and PTEN/AKT | Interacting with α5/β1 integrin to stimulate Src/FAK signaling and inhibiting PTEN to increase AKT activity | [59] |
Figure 5.
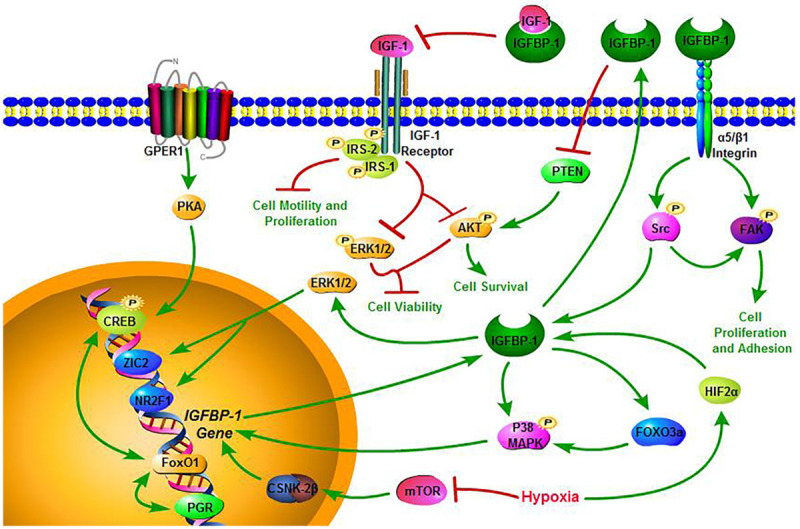
Schematic diagram of the potential pathway of IGFBP-1 in the present review. The single and double green headed arrow is activation arrow and red headed arrow is inhibition arrow. IGFBP-1 governs a carcinogenic network involved in the proliferation, adhesion, motility, viability of tumor cells.
Table 3.
Potential clinical implication of IGFBP-1 in multiple tumor types
| Tumor type | Sample type | Detection Method | Tumor cases, n | Controls, n | Description | Refs |
|---|---|---|---|---|---|---|
| Liver Cancer | Blood | ELISA | 42 | 47 | Significantly increased in liver cancer | [65] |
| Serum | ELISA | 24 | - | Elevated IGFBP-1 level correlated with improved PFS and OS | [66] | |
| Tissue | IHC | 90 | 90 | Down-regulated in HCC tissues and correlated with poor survival | [70] | |
| Blood | Q-PCR | 25 | - | Lower IGFBP-1 level correlated with shortened time to progression | [71] | |
| Breast Cancer | Blood | ELISA | 512 | - | Lower IGFBP-1 level associated with distant recurrence | [81] |
| Blood | Immunoradi-ometric Assay; ELISA; Genotyping | - | - | No important association with breast cancer risk | [83-89] | |
| Gastrointestinal Cancers | Tissue (gastric cancer) | IHC | 219 | - | Higher IGFBP-1 level associated with haematogenous metastasis and poor survival | [103] |
| Serum (gastric cancer) | ELISA | 207 | 333 | Elevated in the serum; AUC: 0.906, 73.43% sensitivity, 91.29% specificity | [104] | |
| Serum (esophageal squamous cell carcinoma) | ELISA | 287 | 333 | Elevated in the serum; AUC: 0.901, 70.38% sensitivity, 91.29% specificity | [104] | |
| Serum (esophagogastric junction adenocarcinom) | ELISA | 237 | 333 | Elevated in the serum; AUC: 0.938, 77.22% sensitivity, 91.29% specificity | [104] | |
| Blood (pancreatic cancer) | ELISA | 144 | 429 | Low IGFBP-1 level significantly predicted an increased risk of pancreatic cancer | [105] | |
| Endometrial Cancer | Serum, Tissue | IHC, ELISA, Q-PCR | 34 | 34 | Significantly increased in endometrial cancer | [113] |
| Blood | Genotyping | 692 | 1723 | No significant association with endometrial cancer risk | [115] | |
| Other Cancers | Plasma (Ovarian Cancer) | Genotyping | 1173 | 1201 | Significantly associated with ovarian cancer risk | [120] |
| Plasma (Prostate Cancer) | ELISA | 957 | 1021 | Higher IGFBP-1 level associated with lower risk of prostate cancer | [123] | |
| Plasma (Nasopharyngeal Carcinoma) | ELISA | 142 | 128 | Higher IGFBP-1 level significantly correlated with poor RFS and OS | [126] |
Liver cancer
Hepatocellular carcinoma (HCC) is one of the primary liver cancers and accounts for 70% to 90% of all primary liver cancers worldwide. It is also the third most frequent cause of cancer-related mortality [63,64]. As an important plasma biomarker, the clinical implication of serum IGFBP-1 in HCC patients has been explored. Hwang et al found that the serum IGFBP-1 level of HCC patients was significantly higher than that of normal control group [65]. In a phase II study of cixutumumab, a monoclonal antibody of high specificity that binds to IGF1R in advanced HCC, elevated IGFBP-1 level was correlated with improved progression-free survival (PFS) (1.2 [95% CI 1-1.4]; P=0.009) and overall survival (OS) (1.2 [95% CI 1.1-1.4]; P=0.003) of 24 patients [66]. On the other hand, the expression of IGFBP-1 in HCC tissue specimens remains controversial. A small study showed that higher level of IGFBP-1 mRNA was detected in at least four of five primary HCCs compared to the matched non-tumor liver tissues [67], while Zhou et al reported that using IGF signaling antibody array technology of high sensitivity and specificity, there was no difference in IGFBP-1 expression between HCC and adjacent normal tissues [68]. Conversely, another research demonstrated that IGFBP-1 mRNA expression was significantly downregulated in HCC tissues [69], which was in agreement with the study of Dai et al [70]. The discrepancy between these studies is probably due to the variance in sample size (lacking a large number of samples) and the method of detection. In addition, compared with patients of high IGFBP-1 expression, a poor prognosis was associated with low IGFBP-1 expression, so IGFBP-1 may serve as an important indicator for the prognosis of HCC [70]. After selective internal radiation therapy, a mainstay of treating hepatocellular carcinoma, it has also illustrated a significant association of IGFBP-1 mRNA expression with shortened time to progression (ROC: 0.8; 95% CI: 0.44-0.98; P=0.03) [71].
The function of IGFBP-1 that inhibits the invasion and metastasis of HCC cells via downregulation of matrix metalloproteinase 9 (MMP-9) expression has been proven. Furthermore, in the human liver non-tumor cell line HL-7702, HCC cell lines HuH-7, HepG2, SMMC-7721 and MHCC97-H, the expression of IGFBP-1 was slowly decreased with gradually increased invasion of the above cell lines [70]. In Yang et al’s study about the molecular mechanisms of ursolic acid (UA) anti-tumor effects in HCC, it has shown that UA induced the expression of IGFBP-1 and forkhead box O3 (FOXO3a) through p38 mitogen activated kinase-like protein (MAPK) to inhibit the proliferation of HCC cells. But in this process, intriguingly, the overexpression of FOXO3a by the induction of UA promoted phosphorylation of p38 MAPK, of which was not observed in cells with endogenous IGFBP-1 gene silenced. However, the exogenous expressed IGFBP-1 could restore the role of UA in enhancing the phosphorylation of p38 MAPK and the expression of FOXO3a. Collectively, there is a feedback regulation loop of IGFBP-1 and FOXO3a on p38 MAPK leading to an interactive pathway and promoting the effect of UA on inhibiting HCC cell proliferation [64]. Obviously, IGFBP-1 plays a crucial role in this reciprocal pathway. Another study by Geis et al has confirmed that the IGFBP-1/IGF system was involved in the differentiation of hypoxia-inducible factor (HIF)-2α-dependent stem cells into lymphatic structures. HIF-2α negatively affected lymphangiogenesis in HCC by upregulating IGFBP-1 expression which could decrease the bioavailability of IGFs and inhibit the actions of the IGFs/IGF1R signaling, thereby attenuating lymphangiogenesis. But the exact molecular mechanism of this action has not been demonstrated, and the researchers failed to support the correlation of IGF-1 with vascular endothelial growth factor (VEGF) regulation which was associated with lymphangiogenic. Nonetheless, they postulated that the regulation of lymphangiogenesis by mechanistic target of rapamycin kinase (mTOR)-dependent and Ras/MAPK-mediated VEGF receptor 3 (VEGFR3) expression which could be activated by IGF/IGF1R [72], and this postulation still needs to be confirmed experimentally. Similarly, the IGFBP-1/IGF system was also substantiated in involving in the mechanisms causing HBV-related HCC. The study demonstrated that Hepatitis B virus (HBV) via HBV X protein inhibited the expression and secretion of IGFBP-1, and enhanced IGF-1-dependent and -independent pro-survival and anti-apoptotic effects in the HBV-replicating HepG2 cells model. Moreover, such effect not only contributes to the development of HBV-mediated HCC, but may also be associated with other HBV-associated liver malignancies [73]. As for the effect of hypoxia on IGFBP-1 in HepG2 cells, it is mainly related to the phosphorylation state of IGFBP-1. Tsunawaki et al demonstrated that hypoxia increased the phosphorylated isoform of IGFBP-1 and then reduced the stimulation effect of IGF-1 on cell proliferation, presumably by inhibiting the binding of IGF-1 to IGF1R due to the higher affinity of phosphorylated IGFBP-1 binding to IGF-1 than that of IGF-1 to IGF1R [74]. Furthermore, the molecular mechanisms linking hypoxia to IGFBP-1 phosphorylation have been established to some extent. It has showed that hypoxia potentiated casein kinase 2β (CSNK-2β) activation mediated by mTOR inhibition, and thus enhanced the secretion/phosphorylation of IGFBP-1 to decrease IGF-1-induced IGF1R autophosphorylation in HepG2 cells [75,76]. This mechanistic link between mTOR and IGF-1 signaling via CSNK-2β was strongly supported by the research of Singal et al, which has used diverse complementary methods to observe the close intracellular localization and interactions between CSNK-2β and IGFBP-1 as well as mTOR and CSNK-2β, respectively [17]. Intriguingly, the action of IGFBP-1 in the research of Kodama et al seems to be different compared to those above studies. In this research, after treatment with the pregnane X receptor (PXR) activator rifampicin in the HepG2-derived ShP51 cells which stably expresses PXR, PXR was observed to indirectly derepress the IGFBP-1 gene by inhibiting the hepatocyte nuclear factor (HNF) 4α gene, thereby facilitating the epithelial-mesenchymal transition (EMT)-like morphological changes and migration of ShP51 cells [77]. It is likely that the effects of IGFBP-1 on inducing morphological changes and migration of ShP51 cells were not associated with IGF/IGFR system, because fetal bovine serum was removed in the cell morphology and migration assays. Thus, the experimental conditions were equivalent to the absence of IGF activity. As previously mentioned, the RGD motif of IGFBP-1 via binding to α5/β1 integrin could increase cell migration or adhesion as an IGF-independent action. It could be hypothesized that the morphological changes and migration of ShP51 cells are induced by IGFBP-1 binding to α5/β1 integrin. But it still needs to be verified experimentally.
Breast cancer
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related deaths among females worldwide [63], and approximately 75% of breast tumors are driven by estrogen receptor (ER) mediated transcriptional activity [78]. According to Clemmons et al, they identified the type of IGFBPs secreted by seven separated breast tumor cell lines containing three ER negative cell lines and four ER positive, and concluded that ER negative cell lines secreted IGFBP-1 while ER positive did not [79]. In contrast, Pekonen et al were unable to discover the relationship between ER content and IGFBP-1 expression by analyzing 47 breast cancers tissue specimens. The disparity between the two studies may due to the greater heterogeneity in tumor cells than that in cell lines. However, Pekonen et al yet found another manifestation, that is the IGFBPs expression including IGFBP-1, was significantly higher in breast cancer tissues than that in adjacent normal tissues, suggesting that the malignant transformation of breast tissue was related to the increment of IGFBPs [80]. Additionally, low level of circulating IGFBP-1 was associated with an increased risk of distant recurrence and death in breast cancer [81], as well as with progesterone receptor (PGR) negativity, an adverse prognostic factor [82]. But the prognostic effect of IGFBP-1 was not an independent action. It appears to be related to the inhibition effect of insulin (a prognostically important factor) on IGFBP-1 expression. By the way, high level of IGFBP-1 was significantly associated with ER positivity in this study [81]. Regarding the relationship between IGFBP-1 and the risk of breast cancer, many studies showed that no matter from circulating IGFBP-1 or haplotype tagging single nucleotide polymorphisms and haplotypes in IGFBP-1 gene, IGFBP-1 has no important association with breast cancer risk in either pre- or postmenopausal women [83-89].
Several studies have demonstrated that either endogenous or exogenous IGFBP-1 inhibited the IGF-mediated cell growth of human MCF-7 breast cancer cells [90-92]. Among these studies, the research by Figueroa et al has shown that when compared with the control group, IGFBP-1 at a concentration of 80 nM completely inhibited the growth of MCF-7 cell stimulated by IGF-1, 5% charcoal stripped serum (CSS) or estrogen, as well as completely eliminated ligand-dependent IGF1R phosphorylation. Moreover, there is an interesting result indicating that the inhibitory effect of recombinant IGFBP-1 on MCF-7 cells growth stimulated by both estrogen and 5% CSS was not through abolishing IGF1R phosphorylation, as the treatment of estrogen or 5% CSS were unable to induce the phosphorylation of IGF1R [90]. Therefore, it reminds us that recombinant IGFBP-1 may achieve its inhibition on MCF-7 cells growth via various mechanisms, not just abrogating IGF1R phosphorylation. It could be hypothesized that recombinant IGFBP-1 may regulate other growth factors existing in serum, or IGF-2, as IGFBP-1 also has high affinity for IGF-2. When IGF-2 binds to IGF2R to exert the stimulatory action on cell proliferation independent IGF1R activation, it also could be inhibited by IGFBP-1. But there might invovle another mechanism presented in the study of Perks et al, which has showed that IGFBP-1 could bind to α5/β1 integrin via its RGD motif to induce FAK dephosphorylation and cell detachment from the matrix and cell apoptosis as an IGF-independent action. Moreover, it is essential to note that Hs578T cell line lacks a functional IGF1R and could not respond to IGFs. Thus, the effect of IGFBP-1 on this cell line could be fully considered as an IGF-independent action [93]. Besides, the correlation of RGD motif interacted with IGFBP-1’s IGF-dependent effects has been revealed by Zhang et al, and they found that the bond of RGD motif of IGFBP-1 with α5/β1 integrin disrupted the interaction between fibronectin and α5/β1 integrin which was required for cell migration. Interestingly, the RGD peptide was also able to reduce IGF-1-stimulated motility, suggesting that there was an interaction between α5/β1 integrin and IGF1R signaling pathway. When the insulin receptor substrate-2 (IRS-2) phosphorylation was induced by IGF-1, it would also be activated by α5/β1 integrin binding to fibronectin at the same time, which means that the activation of both IGF1R and α5/β1 integrin were required to phosphorylate IRS-2 and initiate cell motility. Ultimately, via the neutralization of IGF-1 and interaction with α5/β1 integrin blocking fibronectin to bind to it, recombinant human IGFBP-1 inhibited the IRS-2 phosphorylation and cell motility of MDA-231BO cell [61]. Tamoxifen (Tam), as a selective ER modulator and an adjuvant hormone therapy, is commonly prescribed to ERα-positive breast cancer patients. Early study has shown that tamoxifen could inhibit plasma IGF-1 and increase IGFBP-1 level by other independent mechanisms, rather than those known to regulate plasma IGF-1 and IGFBP-1 level primarily by GH or insulin [94]. The research by Vaziri-Gohar et al revealed the mechanism how tamoxifen or its active metabolite, 4-hydroxytamoxifen (4-OHT), increased the extracellular expression of IGFBP-1 in MCF-7 cells. 4-OHT activated G protein-coupled estrogen receptor 1 (GPER1) and its downstream kinase, protein kinase A (PKA) to induce the phosphorylation of cAMP-response element-binding protein (CREB) and then enhanced the transcription of IGFBP-1. But the binding of CREB to IGFBP-1 promoter still require experimental proof in breast cancer cells. Furthermore, the extracellular IGFBP-1 accumulation by 4-OHT could diminish MCF-7 cell viability throughinhibition of IGF-1-dependent cell signaling including dephosphorylation of IGF1R, serine/threonine kinase (AKT) and extracellular signal-regulated kinase 1/2 (ERK1/2) [95]. Vaziri-Gohar et al also confirmed that IGFBP-1 transcription level was increased by Tam treatment in MCF-7 cells. In addition, it has also illustrated that IGF1R and FoxO1 (a member of the forkhead box class O family of transcription factors) play a significant role in Tam-induced breast cancer cell death, and IGF1R would activate FoxO1 to induce IGFBP-1 transcription to exert an action in breast cancer cells. Furthermore, another paper has showed that ERK1/2 destabilized FoxO1 to decreased its protein levels. Thus, it is likely that there was an association between decreased FoxO1 protein level and increased ERK1/2 activity after IGF1R knockdown, and it may need to fully confirmed. The study also proposed an IGF1R-dependent p-ERα/PR/FoxO1/IGFBP-1 pathway which may be another potential mechanism by IGF1R mediating FoxO1 and IGFBP-1 protein level in breast cancer cell. In addition, Vaziri-Gohar et al indicated that CREB and FoxO1 were cooperated to induce IGFBP-1 transcription in Tam-treated breast cancer cells, and finally resulted in Tam-induced breast cancer cell death [96].
Gastrointestinal cancers
Gastrointestinal cancers, mainly including esophageal, gastric, colorectal, and pancreatic cancer, are the most common tumors with high mortality rate in the world, and pose a serious threat to human health [97]. Previously, two prospective observational studies have showed that increased serum IGFBP-1 level was statistically significant inversely associated with risk of colorectal or colon cancer in women [98,99]. However, other studies yet described a distinct different result, of which showed that plasma IGFBP-1 had no clear association with risk of colorectal cancer [100,101]. The contradiction between these studies may be due to the gender of study population or statistical analysis method. In a panel of human gastric cancer cell lines, the mRNA and protein levels of IGFBP-1 were not detected in any cell lines [102], but higher IGFBP-1 expression in gastric cancer tissue was significantly associated with hematogenous metastasis and poor survival in patients who underwent gastrectomy [103]. Serum IGFBP-1 has been proven to have high diagnostic accuracy to distinguish gastric cancer (AUC: 0.906, 73.43% sensitivity, 91.29% specificity), esophageal squamous cell carcinoma (AUC: 0.901, 70.38% sensitivity, 91.29% specificity) and esophagogastric junction adenocarcinoma (AUC: 0.938, 77.22% sensitivity, 91.29% specificity) from normal controls, even in the early stage of these cancers [104]. Additionally, low plasma level of IGFBP-1 could significantly predicted the increased risk of pancreatic cancer independent of other known or suspected risk factors like plasma IGF-1, C-peptide and IGFBP-3 [105]. Meanwhile, a meta-analysis study of genetic variations explored the IGFBP-IGF-IGFR axis polymorphisms, which indicated that IGFBP-1 genetic polymorphisms may increase the risk of esophageal cancer [106].
Although many studies have demonstrated the association between IGFBP-1 expression and gastrointestinal cancers, the cellular actions of IGFBP-1 in these cancers have not been fully revealed. Luo et al found that the expression and secretion of IGFBP-1 were upregulated in gastric cancer cell lines with H. pylori 26695 infection, as well as the increased MMP-9 expression and intensive cell migration. Intriguingly, they also demonstrated that overexpression of IGFBP-1 could inhibit the expression of MMP-9 protein and the migration of BGC-823 cells with or without H. pylori 26695 infection. Collectively, it could be concluded that IGFBP-1 may effect BGC-823 cells migration through regulating MMP-9 expression [107]. But some problems of how the upregulated IGFBP-1 interacted with increased MMP-9 expression and cell migration in gastric cancer cell lines with H. pylori 26695 infection remained to be addressed. Similarly, in another study of Kim et al to explore the role of aldehyde dehydrogenase 1A1 (ALDH1A1) and IGFBP-1 in liver metastasis from colorectal cancer, the overexpression of ALDH1A1 and IGFBP-1 also significantly decreased cell proliferation and invasiveness of SW480 cell line (cloned from primary CRC) in the in vitro assays. However, in vivo experiment yet showed liver metastasis in mice transplanted with IGFBP-1-overexpressing SW480 cells, which seemed to contradict with in vitro results [108]. These above studies suggested that IGFBP-1 may play a dual role as tumor suppressor and metastasis enhancer, just like the c-Myc [109] or transforming growth factor-β (TGF-β) [110].
Endometrial cancer
Endometrial cancer (EC) is the sixth most common cancer in the world and the 14th leading cause of female cancer mortality in 2018 [111]. A previous research showed that the relative level mRNA of IGFBP-1 in endometrial adenocarcinoma tissues was either undetectable or minimal [112]. In a cross-sectional study, IGFBP-1 gene expression was significantly increased in the endometrium of EC women compared to that of controls [113]. Plasma level of IGFBP-1 was inversely associated with endometrial cancer risk. It was diminished and lost statistical significance when adjusted for confounders such as BMI or C-peptide, but it still remained statistically significant in the group of postmenopausal women [114]. Additionally, in a prospective study, there was no significant association between the haplotype-tagging single nucleotide polymorphisms of IGFBP-1 gene and endometrial cancer risk among Caucasian women [115].
The effect of FOXO1A (also known as FOXO1) on IGFBP-1 gene has been described in breast cancer [96] and endometrial stromal cells [116], as well as in the IGFBP-1 transcription of endometrial cancer cells [117]. Kim et al demonstrated that silenced FOXO1A expression would lead to the decreased IGFBP-1 expression in HEC-1B cells (a human endometrial adenocarcinoma cell line). Moreover, the transfection reporter experiment revealed that IGFBP-1 promoter activity would be enhanced by FOXO1A alone or co-operation with another transcription factor PGR [117]. In addition, the effects of metformin and/or PPP (an IGF1R inhibitor) in endometrial cancer cells have been investigated [118]. Metformin could inhibit the proliferation and enhance the apoptosis rate of endometrial cancer cells. Meanwhile, metformin was also found to increase IGFBP-1 and downregulate IGF1R mRNA and protein expression, but compound C as an inhibitor of adenosine monophosphate protein kinase (AMPK) could reverse this effect, suggesting that activation of AMPK by metformin to inhibit cancer cell growth may be blocked by compound C [119]. And IGFBP-1 would serve as downstream of AMPK signal pathway to exert such antitumor action possibly.
Other cancers
The expression and potential clinical implication of IGFBP-1 has also been explored in many other tumors. A statistically significant association existed between three single nucleotide polymorphisins (rs10228265, rs4988515 and rs2270628) in IGFBP-1/3 with ovarian cancer risk [120]. The reduced expression of IGFBP-1 gene was associated with the development of hydatidiform mole to gestational trophoblastic neoplasia [121]. The expression of IGFBP-1 was more specifical than that of HNF-1β in ovarian clear cell adenocarcinoma (CCA) and IGFBP-1 may serve as a biomarker for CCA [122]. Higher prediagnostic fasting IGFBP-1 level was independently associated with lower risk of prostate cancer even after adjusting for IGF-1 or C-peptide [123]. Level of serum IGFBP-1 was significantly higher in oral cancer patients than that in controls [124]. There was no significant association between prediagnostic serum level of IGFBP-1 and lung cancer risk in women [125]. Serum IGFBP-1 level and the serum IGFBP-1/IGF-1 ratio were significantly higher in nasopharyngeal carcinoma patients compared to those in healthy control individuals, and were significantly correlated with poor relapse-free survival (RFS) and OS. The IGFBP-1/IGF-1 ratio was also revealed by multivariate analysis to be an independent risk factor for poor RFS and OS of nasopharyngeal carcinoma patients [126].
IGFBP-1 expression was generally low in glioblastoma cells but highly upregulated in RG7388 (a mouse double minute 2 inhibitor)-resistant glioblastoma cells. The IGFBP-1 protein expression in RG7388 resistant glioblastoma cells was enhanced by activation of the p53 pathway, and then led to inspiration of ERK1/2 signaling and increment of two transcription factors Zic family member 2 (ZIC2) and nuclear receptor subfamily 2 group F member 1 (NR2F1) which might further enhance the IGFBP-1 expression through binding the sites at IGFBP-1 gene promotor. The proliferation and invasion induced by this self-activating pathway could be inhibited by trametinib (the MEK inhibitor) treatment or IGFBP-1 knockdown [127]. A recent study on tumor-interstitial interactions in glioblastoma showed that the increment in IGFBP-1 expression of microglial cells induced by glioma-secreted macrophage colony-stimulating factor (MCSF) was an important mediator in facilitating tumor angiogenesis [128]. In addition, IGFBP-1 was overexpressed and released from both human primary schwannoma cells and tissues. Furthermore, it was found that the bond of IGFBP-1 with α5/β1 integrin could result in the activation of Src followed by FAK phosphorylation and autophosphorylation, and then potentiate the proliferation and cell-matrix adhesion of schwannoma cells. Meanwhile, the activated Src contributed to the production and release of IGFBP-1 in return, and accumulated IGFBP-1 was observed to decrease the expression of phosphatase and tensin homolog (PTEN) resulting in the increased AKT activity and inducing survival of schwannoma cells [59].
Conclusions
The expression of IGFBP-1 is associated with the risk of various tumors though there remain some controversial results, and it may be a diagnostic or prognostic marker for some tumors. Moreover, there are many in vitro and in vivo studies supporting a dual role of IGFBP-1 in tumor behaviors such as proliferation, migration, invasion and adhesion through IGF-dependent and IGF-independent mechanisms, suggesting that the role of IGFBP-1 is cell-specific dependent on the kind of target cell. Last but not the least, the comprehensive understanding of the signaling pathways by IGFBP-1 to exert its effects on tumor development is necessary for us to figure out more specific clinical treatment to the suitable patients, and the IGF signaling inhibitor would be a prospective treatment in the future.
Acknowledgements
This work was supported by funding from the Natural Science Foundation of China [81972801 to Y-HP]; Guangdong Basic and Applied Basic Research Foundation [2018A030307079 to Y-HP, 2019A1515011873 to Y-WX]; the Medical Project of Science and Technology Planning of Shantou (200605115266724 to Y-WX); the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant [2020LKSFG01B to Y-WX] and Science and Technology Special Fund of Guangdong Province of China [190829105556145].
Disclosure of conflict of interest
None.
References
- 1.Hanahan D. Rethinking the war on cancer. Lancet. 2014;383:558–563. doi: 10.1016/S0140-6736(13)62226-6. [DOI] [PubMed] [Google Scholar]
- 2.Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005;16:421–439. doi: 10.1016/j.cytogfr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Fürstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 4.Bach LA. IGF-binding proteins. J Mol Endocrinol. 2018;61:T11–T28. doi: 10.1530/JME-17-0254. [DOI] [PubMed] [Google Scholar]
- 5.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329–341. [Google Scholar]
- 6.Hoeflich A, Russo VC. Physiology and pathophysiology of IGFBP-1 and IGFBP-2 - consensus and dissent on metabolic control and malignant potential. Best Pract Res Clin Endocrinol Metab. 2015;29:685–700. doi: 10.1016/j.beem.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med. 1997;216:319–357. doi: 10.3181/00379727-216-44182. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh AK, Lacson R, Liu P, Cichy SB, Danilkovich A, Guo S, Unterman TG. A nucleoprotein complex containing CCAAT/enhancer-binding protein beta interacts with an insulin response sequence in the insulin-like growth factor-binding protein-1 gene and contributes to insulin-regulated gene expression. J Biol Chem. 2001;276:8507–8515. doi: 10.1074/jbc.M008541200. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Mazella J, Suwanichkul A, Powell DR, Tseng L. Activation of the insulin-like growth factor binding protein-1 promoter by progesterone receptor in decidualized human endometrial stromal cells. Mol Cell Endocrinol. 1999;153:11–17. doi: 10.1016/s0303-7207(99)00096-9. [DOI] [PubMed] [Google Scholar]
- 10.Bohn H, Kraus W, Winckler W. New soluble placental tissue proteins: their isolation, characterization, localization and quantification. Placenta Suppl. 1982;4:67–81. [PubMed] [Google Scholar]
- 11.Sala A, Capaldi S, Campagnoli M, Faggion B, Labò S, Perduca M, Romano A, Carrizo ME, Valli M, Visai L, Minchiotti L, Galliano M, Monaco HL. Structure and properties of the C-terminal domain of insulin-like growth factor-binding protein-1 isolated from human amniotic fluid. J Biol Chem. 2005;280:29812–29819. doi: 10.1074/jbc.M504304200. [DOI] [PubMed] [Google Scholar]
- 12.Sitar T, Popowicz GM, Siwanowicz I, Huber R, Holak TA. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci U S A. 2006;103:13028–13033. doi: 10.1073/pnas.0605652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bach LA, Hsieh S, Sakano K, Fujiwara H, Perdue JF, Rechler MM. Binding of mutants of human insulin-like growth factor II to insulin-like growth factor binding proteins 1-6. J Biol Chem. 1993;268:9246–9254. [PubMed] [Google Scholar]
- 14.Oh Y, Müller HL, Lee DY, Fielder PJ, Rosenfeld RG. Characterization of the affinities of insulin-like growth factor (IGF)-binding proteins 1-4 for IGF-I, IGF-II, IGF-I/insulin hybrid, and IGF-I analogs. Endocrinology. 1993;132:1337–1344. doi: 10.1210/endo.132.3.7679979. [DOI] [PubMed] [Google Scholar]
- 15.Jones JI, D’Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc Natl Acad Sci U S A. 1991;88:7481–7485. doi: 10.1073/pnas.88.17.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tagliabracci VS, Wiley SE, Guo X, Kinch LN, Durrant E, Wen J, Xiao J, Cui J, Nguyen KB, Engel JL, Coon JJ, Grishin N, Pinna LA, Pagliarini DJ, Dixon JE. A single kinase generates the majority of the secreted phosphoproteome. Cell. 2015;161:1619–1632. doi: 10.1016/j.cell.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singal SS, Nygard K, Dhruv MR, Biggar K, Shehab MA, Li SS, Jansson T, Gupta MB. Co-localization of insulin-like growth factor binding protein-1, casein kinase-2β, and mechanistic target of rapamycin in human hepatocellular carcinoma cells as demonstrated by dual immunofluorescence and in situ proximity ligation assay. Am J Pathol. 2018;188:111–124. doi: 10.1016/j.ajpath.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolcini L, Sala A, Campagnoli M, Labò S, Valli M, Visai L, Minchiotti L, Monaco HL, Galliano M. Identification of the amniotic fluid insulin-like growth factor binding protein-1 phosphorylation sites and propensity to proteolysis of the isoforms. FEBS J. 2009;276:6033–6046. doi: 10.1111/j.1742-4658.2009.07318.x. [DOI] [PubMed] [Google Scholar]
- 19.Gupta MB. The role and regulation of IGFBP-1 phosphorylation in fetal growth restriction. J Cell Commun Signal. 2015;9:111–123. doi: 10.1007/s12079-015-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unterman TG, Oehler DT, Murphy LJ, Lacson RG. Multihormonal regulation of insulin-like growth factor-binding protein-1 in rat H4IIE hepatoma cells: the dominant role of insulin. Endocrinology. 1991;128:2693–2701. doi: 10.1210/endo-128-6-2693. [DOI] [PubMed] [Google Scholar]
- 22.Poretsky L, Chandrasekher YA, Bai C, Liu HC, Rosenwaks Z, Giudice L. Insulin receptor mediates inhibitory effect of insulin, but not of insulin-like growth factor (IGF)-I, on IGF binding protein 1 (IGFBP-1) production in human granulosa cells. J Clin Endocrinol Metab. 1996;81:493–496. doi: 10.1210/jcem.81.2.8636256. [DOI] [PubMed] [Google Scholar]
- 23.Conover CA, Lee PD, Riggs BL, Powell DR. Insulin-like growth factor-binding protein-1 expression in cultured human bone cells: regulation by insulin and glucocorticoid. Endocrinology. 1996;137:3295–3301. doi: 10.1210/endo.137.8.8754754. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman CR, Catanese VM. Pre- and post-translational regulation of renal insulin-like growth factor binding protein-1 in insulin-deficient diabetes. J Investig Med. 1995;43:178–186. [PubMed] [Google Scholar]
- 25.Tseng L ZH, Mazella J, Bell SC. Differential regulation of IGFBP-1 and prolactin by insulin, IGF-I and relaxin in progestin primed human endometrial stromal cells. 73rd annual meeting of the Endocrine Society. 1991 Abstract 1062. [Google Scholar]
- 26.Suwanickul A, Morris SL, Powell DR. Identification of an insulin-responsive element in the promoter of the human gene for insulin-like growth factor binding protein-1. J Biol Chem. 1993;268:17063–17068. [PubMed] [Google Scholar]
- 27.Wheatcroft SB, Kearney MT. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends Endocrinol Metab. 2009;20:153–162. doi: 10.1016/j.tem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Yki-Järvinen H, Mäkimattila S, Utriainen T, Rutanen EM. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab. 1995;80:3227–3232. doi: 10.1210/jcem.80.11.7593430. [DOI] [PubMed] [Google Scholar]
- 29.Snyder DK, Clemmons DR. Insulin-dependent regulation of insulin-like growth factor-binding protein-1. J Clin Endocrinol Metab. 1990;71:1632–1636. doi: 10.1210/jcem-71-6-1632. [DOI] [PubMed] [Google Scholar]
- 30.Lewitt MS, Dent MS, Hall K. The insulin-like growth factor system in obesity, insulin resistance and type 2 diabetes mellitus. J Clin Med. 2014;3:1561–1574. doi: 10.3390/jcm3041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busby WH, Snyder DK, Clemmons DR. Radioimmunoassay of a 26,000-dalton plasma insulin-like growth factor-binding protein: control by nutritional variables. J Clin Endocrinol Metab. 1988;67:1225–1230. doi: 10.1210/jcem-67-6-1225. [DOI] [PubMed] [Google Scholar]
- 32.Suikkari AM, Koivisto VA, Rutanen EM, Yki-Järvinen H, Karonen SL, Seppälä M. Insulin regulates the serum levels of low molecular weight insulin-like growth factor-binding protein. J Clin Endocrinol Metab. 1988;66:266–272. doi: 10.1210/jcem-66-2-266. [DOI] [PubMed] [Google Scholar]
- 33.Conover CA, Butler PC, Wang M, Rizza RA, Lee PD. Lack of growth hormone effect on insulin-associated suppression of insulinlike growth factor binding protein 1 in humans. Diabetes. 1990;39:1251–1256. doi: 10.2337/diab.39.10.1251. [DOI] [PubMed] [Google Scholar]
- 34.Cotterill AM, Holly JM, Amiel S, Wass JA. Suppression of endogenous insulin secretion regulates the rapid rise of insulin-like growth factor binding protein (IGFBP)-1 levels following acute hypoglycaemia. Clin Endocrinol (Oxf) 1993;38:633–639. doi: 10.1111/j.1365-2265.1993.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee PD, Jensen MD, Divertie GD, Heiling VJ, Katz HH, Conover CA. Insulin-like growth factor-binding protein-1 response to insulin during suppression of endogenous insulin secretion. Metabolism. 1993;42:409–414. doi: 10.1016/0026-0495(93)90095-6. [DOI] [PubMed] [Google Scholar]
- 36.Baxter RC, Cowell CT. Diurnal rhythm of growth hormone-independent binding protein for insulin-like growth factors in human plasma. J Clin Endocrinol Metab. 1987;65:432–440. doi: 10.1210/jcem-65-3-432. [DOI] [PubMed] [Google Scholar]
- 37.Nørrelund H, Fisker S, Vahl N, Børglum J, Richelsen B, Christiansen JS, Jørgensen JO. Evidence supporting a direct suppressive effect of growth hormone on serum IGFBP-1 levels. Experimental studies in normal, obese and GH-deficient adults. Growth Horm IGF Res. 1999;9:52–60. doi: 10.1054/ghir.1998.0087. [DOI] [PubMed] [Google Scholar]
- 38.Hilding A, Brismar K, Thorén M, Hall K. Glucagon stimulates insulin-like growth factor binding protein-1 secretion in healthy subjects, patients with pituitary insufficiency, and patients with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1142–1147. doi: 10.1210/jcem.77.5.7521339. [DOI] [PubMed] [Google Scholar]
- 39.Suwanichkul A, Allander SV, Morris SL, Powell DR. Glucocorticoids and insulin regulate expression of the human gene for insulin-like growth factor-binding protein-1 through proximal promoter elements. J Biol Chem. 1994;269:30835–30841. [PubMed] [Google Scholar]
- 40.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 41.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 42.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 43.Samani AA, Brodt P. The receptor for the type I insulin-like growth factor and its ligands regulate multiple cellular functions that impact on metastasis. Surg Oncol Clin N Am. 2001;10:289–312. viii. [PubMed] [Google Scholar]
- 44.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 45.Livingstone C. IGF2 and cancer. Endocr Relat Cancer. 2013;20:R321–339. doi: 10.1530/ERC-13-0231. [DOI] [PubMed] [Google Scholar]
- 46.Kratz G, Lake M, Ljungström K, Forsberg G, Haegerstrand A, Gidlund M. Effect of recombinant IGF binding protein-1 on primary cultures of human keratinocytes and fibroblasts: selective enhancement of IGF-1 but not IGF-2-induced cell proliferation. Exp Cell Res. 1992;202:381–385. doi: 10.1016/0014-4827(92)90089-q. [DOI] [PubMed] [Google Scholar]
- 47.Elgin RG, Busby WH Jr, Clemmons DR. An insulin-like growth factor (IGF) binding protein enhances the biologic response to IGF-I. Proc Natl Acad Sci U S A. 1987;84:3254–3258. doi: 10.1073/pnas.84.10.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clemmons DR, Gardner LI. A factor contained in plasma is required for IGF binding protein-1 to potentiate the effect of IGF-I on smooth muscle cell DNA synthesis. J Cell Physiol. 1990;145:129–135. doi: 10.1002/jcp.1041450118. [DOI] [PubMed] [Google Scholar]
- 49.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 50.Jyung RW, Mustoe JA, Busby WH, Clemmons DR. Increased wound-breaking strength induced by insulin-like growth factor I in combination with insulin-like growth factor binding protein-1. Surgery. 1994;115:233–239. [PubMed] [Google Scholar]
- 51.Yu J, Iwashita M, Kudo Y, Takeda Y. Phosphorylated insulin-like growth factor (IGF)-binding protein-1 (IGFBP-1) inhibits while non-phosphorylated IGFBP-1 stimulates IGF-I-induced amino acid uptake by cultured trophoblast cells. Growth Horm IGF Res. 1998;8:65–70. doi: 10.1016/s1096-6374(98)80323-7. [DOI] [PubMed] [Google Scholar]
- 52.Frost RA, Tseng L. Insulin-like growth factor-binding protein-1 is phosphorylated by cultured human endometrial stromal cells and multiple protein kinases in vitro. J Biol Chem. 1991;266:18082–18088. [PubMed] [Google Scholar]
- 53.Koistinen R, Angervo M, Leinonen P, Hakala T, Seppälä M. Phosphorylation of insulin-like growth factor-binding protein-1 increases in human amniotic fluid and decidua from early to late pregnancy. Clin Chim Acta. 1993;215:189–199. doi: 10.1016/0009-8981(93)90125-n. [DOI] [PubMed] [Google Scholar]
- 54.Sakai K, Busby WH Jr, Clarke JB, Clemmons DR. Tissue transglutaminase facilitates the polymerization of insulin-like growth factor-binding protein-1 (IGFBP-1) and leads to loss of IGFBP-1’s ability to inhibit insulin-like growth factor-I-stimulated protein synthesis. J Biol Chem. 2001;276:8740–8745. doi: 10.1074/jbc.M008359200. [DOI] [PubMed] [Google Scholar]
- 55.Jones JI, Gockerman A, Busby WH Jr, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci U S A. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gleeson LM, Chakraborty C, McKinnon T, Lala PK. Insulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through alpha 5 beta 1 integrin via mitogen-activated protein Kinase pathway. J Clin Endocrinol Metab. 2001;86:2484–2493. doi: 10.1210/jcem.86.6.7532. [DOI] [PubMed] [Google Scholar]
- 57.Chesik D, De Keyser J, Bron R, Fuhler GM. Insulin-like growth factor binding protein-1 activates integrin-mediated intracellular signaling and migration in oligodendrocytes. J Neurochem. 2010;113:1319–1330. doi: 10.1111/j.1471-4159.2010.06703.x. [DOI] [PubMed] [Google Scholar]
- 58.Gockerman A, Prevette T, Jones JI, Clemmons DR. Insulin-like growth factor (IGF)-binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinology. 1995;136:4168–4173. doi: 10.1210/endo.136.10.7545099. [DOI] [PubMed] [Google Scholar]
- 59.Ammoun S, Schmid MC, Zhou L, Ristic N, Ercolano E, Hilton DA, Perks CM, Hanemann CO. Insulin-like growth factor-binding protein-1 (IGFBP-1) regulates human schwannoma proliferation, adhesion and survival. Oncogene. 2012;31:1710–1722. doi: 10.1038/onc.2011.357. [DOI] [PubMed] [Google Scholar]
- 60.Irwin JC, Giudice LC. Insulin-like growth factor binding protein-1 binds to placental cytotrophoblast alpha5beta1 integrin and inhibits cytotrophoblast invasion into decidualized endometrial stromal cultures. Growth Horm IGF Res. 1998;8:21–31. doi: 10.1016/s1096-6374(98)80318-3. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Yee D. Insulin-like growth factor binding protein-1 (IGFBP-1) inhibits breast cancer cell motility. Cancer Res. 2002;62:4369–4375. [PubMed] [Google Scholar]
- 62.Haywood NJ, Cordell PA, Tang KY, Makova N, Yuldasheva NY, Imrie H, Viswambharan H, Bruns AF, Cubbon RM, Kearney MT, Wheatcroft SB. Insulin-like growth factor binding protein 1 could improve glucose regulation and insulin sensitivity through its RGD domain. Diabetes. 2017;66:287–299. doi: 10.2337/db16-0997. [DOI] [PubMed] [Google Scholar]
- 63.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 64.Yang LJ, Tang Q, Wu J, Chen Y, Zheng F, Dai Z, Hann SS. Inter-regulation of IGFBP1 and FOXO3a unveils novel mechanism in ursolic acid-inhibited growth of hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2016;35:59. doi: 10.1186/s13046-016-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang DL, Huang SP, Lan WS, Lee PD. Elevated insulin, proinsulin and insulin-like growth factor-binding protein-1 in liver disease. Growth Horm IGF Res. 2003;13:316–321. doi: 10.1016/s1096-6374(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 66.Abou-Alfa GK, Capanu M, O’Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, Kalin M, Katz S, Abad L, Reidy-Lagunes DL, Kelsen DP, Chen HX, Saltz LB. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60:319–324. doi: 10.1016/j.jhep.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, Horiuchi S, Goseki N, Matsubara O, Takenaka K, Shichita M, Tanaka K, Shuda M, Yamamoto M. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990–4996. [PubMed] [Google Scholar]
- 68.Zhou Q, Mao YQ, Jiang WD, Chen YR, Huang RY, Zhou XB, Wang YF, Shi Z, Wang ZS, Huang RP. Development of IGF signaling antibody arrays for the identification of hepatocellular carcinoma biomarkers. PLoS One. 2012;7:e46851. doi: 10.1371/journal.pone.0046851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong Y, Cui L, Minuk GY. The expression of insulin-like growth factor binding proteins in human hepatocellular carcinoma. Mol Cell Biochem. 2000;207:101–104. doi: 10.1023/a:1007010818094. [DOI] [PubMed] [Google Scholar]
- 70.Dai B, Ruan B, Wu J, Wang J, Shang R, Sun W, Li X, Dou K, Wang D, Li Y. Insulin-like growth factor binding protein-1 inhibits cancer cell invasion and is associated with poor prognosis in hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:5645–5654. [PMC free article] [PubMed] [Google Scholar]
- 71.Nel I, Baba HA, Weber F, Sitek B, Eisenacher M, Meyer HE, Schlaak JF, Hoffmann AC. IGFBP1 in epithelial circulating tumor cells as a potential response marker to selective internal radiation therapy in hepatocellular carcinoma. Biomark Med. 2014;8:687–698. doi: 10.2217/bmm.14.23. [DOI] [PubMed] [Google Scholar]
- 72.Geis T, Popp R, Hu J, Fleming I, Henke N, Dehne N, Brüne B. HIF-2α attenuates lymphangiogenesis by up-regulating IGFBP1 in hepatocellular carcinoma. Biol Cell. 2015;107:175–188. doi: 10.1111/boc.201400079. [DOI] [PubMed] [Google Scholar]
- 73.Nielsen KO, Mirza AH, Kaur S, Jacobsen KS, Winther TN, Glebe D, Pociot F, Hogh B, Størling J. Hepatitis B virus suppresses the secretion of insulin-like growth factor binding protein 1 to facilitate anti-apoptotic IGF-1 effects in HepG2 cells. Exp Cell Res. 2018;370:399–408. doi: 10.1016/j.yexcr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Tsunawaki T, Sakai K, Momomura M, Wachi Y, Matsuzawa Y, Iwashita M. Hypoxia alters phosphorylation status of insulin-like growth factor (IGF)-binding protein-1 and attenuates biological activities of IGF-I in HepG2 cell cultures. J Obstet Gynaecol Res. 2013;39:1367–1373. doi: 10.1111/jog.12078. [DOI] [PubMed] [Google Scholar]
- 75.Abu Shehab M, Damerill I, Shen T, Rosario FJ, Nijland M, Nathanielsz PW, Kamat A, Jansson T, Gupta MB. Liver mTOR controls IGF-I bioavailability by regulation of protein kinase CK2 and IGFBP-1 phosphorylation in fetal growth restriction. Endocrinology. 2014;155:1327–1339. doi: 10.1210/en.2013-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Damerill I, Biggar KK, Abu Shehab M, Li SS, Jansson T, Gupta MB. Hypoxia increases IGFBP-1 phosphorylation mediated by mTOR inhibition. Mol Endocrinol. 2016;30:201–216. doi: 10.1210/me.2015-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kodama S, Yamazaki Y, Negishi M. Pregnane X receptor represses HNF4α gene to induce insulin-like growth factor-binding protein IGFBP1 that alters morphology of and migrates HepG2 cells. Mol Pharmacol. 2015;88:746–757. doi: 10.1124/mol.115.099341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siersbæk R, Kumar S, Carroll JS. Signaling pathways and steroid receptors modulating estrogen receptor α function in breast cancer. Genes Dev. 2018;32:1141–1154. doi: 10.1101/gad.316646.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clemmons DR, Camacho-Hubner C, Coronado E, Osborne CK. Insulin-like growth factor binding protein secretion by breast carcinoma cell lines: correlation with estrogen receptor status. Endocrinology. 1990;127:2679–2686. doi: 10.1210/endo-127-6-2679. [DOI] [PubMed] [Google Scholar]
- 80.Pekonen F, Nyman T, Ilvesmäki V, Partanen S. Insulin-like growth factor binding proteins in human breast cancer tissue. Cancer Res. 1992;52:5204–5207. [PubMed] [Google Scholar]
- 81.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Hartwick W, Hoffma B, Hood N. Insulin-like growth factor binding proteins 1 and 3 and breast cancer outcomes. Breast Cancer Res Treat. 2002;74:65–76. doi: 10.1023/a:1016075709022. [DOI] [PubMed] [Google Scholar]
- 82.Ng EH, Ji CY, Tan PH, Lin V, Soo KC, Lee KO. Altered serum levels of insulin-like growth-factor binding proteins in breast cancer patients. Ann Surg Oncol. 1998;5:194–201. doi: 10.1007/BF02303854. [DOI] [PubMed] [Google Scholar]
- 83.Schernhammer ES, Holly JM, Hunter DJ, Pollak MN, Hankinson SE. Insulin-like growth factor-I, its binding proteins (IGFBP-1 and IGFBP-3), and growth hormone and breast cancer risk in The Nurses Health Study II. Endocr Relat Cancer. 2006;13:583–592. doi: 10.1677/erc.1.01149. [DOI] [PubMed] [Google Scholar]
- 84.Schernhammer ES, Holly JM, Pollak MN, Hankinson SE. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:699–704. doi: 10.1158/1055-9965.EPI-04-0561. [DOI] [PubMed] [Google Scholar]
- 85.Krajcik RA, Borofsky ND, Massardo S, Orentreich N. Insulin-like growth factor I (IGF-I), IGF-binding proteins, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1566–1573. [PubMed] [Google Scholar]
- 86.Keinan-Boker L, Bueno De Mesquita HB, Kaaks R, Van Gils CH, Van Noord PA, Rinaldi S, Riboli E, Seidell JC, Grobbee DE, Peeters PH. Circulating levels of insulin-like growth factor I, its binding proteins-1, -2, -3, C-peptide and risk of postmenopausal breast cancer. Int J Cancer. 2003;106:90–95. doi: 10.1002/ijc.11193. [DOI] [PubMed] [Google Scholar]
- 87.Kaaks R, Lundin E, Rinaldi S, Manjer J, Biessy C, Söderberg S, Lenner P, Janzon L, Riboli E, Berglund G, Hallmans G. Prospective study of IGF-I, IGF-binding proteins, and breast cancer risk, in northern and southern Sweden. Cancer Causes Control. 2002;13:307–316. doi: 10.1023/a:1015270324325. [DOI] [PubMed] [Google Scholar]
- 88.Patel AV, Cheng I, Canzian F, Le Marchand L, Thun MJ, Berg CD, Buring J, Calle EE, Chanock S, Clavel-Chapelon F, Cox DG, Dorronsoro M, Dossus L, Haiman CA, Hankinson SE, Henderson BE, Hoover R, Hunter DJ, Kaaks R, Kolonel LN, Kraft P, Linseisen J, Lund E, Manjer J, McCarty C, Peeters PH, Pike MC, Pollak M, Riboli E, Stram DO, Tjonneland A, Travis RC, Trichopoulos D, Tumino R, Yeager M, Ziegler RG, Feigelson HS. IGF-1, IGFBP-1, and IGFBP-3 polymorphisms predict circulating IGF levels but not breast cancer risk: findings from the Breast and Prostate Cancer Cohort Consortium (BPC3) PLoS One. 2008;3:e2578. doi: 10.1371/journal.pone.0002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng I, Penney KL, Stram DO, Le Marchand L, Giorgi E, Haiman CA, Kolonel LN, Pike M, Hirschhorn J, Henderson BE, Freedman ML. Haplotype-based association studies of IGFBP1 and IGFBP3 with prostate and breast cancer risk: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:1993–1997. doi: 10.1158/1055-9965.EPI-06-0361. [DOI] [PubMed] [Google Scholar]
- 90.Figueroa JA, Sharma J, Jackson JG, McDermott MJ, Hilsenbeck SG, Yee D. Recombinant insulin-like growth factor binding protein-1 inhibits IGF-I, serum, and estrogen-dependent growth of MCF-7 human breast cancer cells. J Cell Physiol. 1993;157:229–236. doi: 10.1002/jcp.1041570204. [DOI] [PubMed] [Google Scholar]
- 91.McGuire WL Jr, Jackson JG, Figueroa JA, Shimasaki S, Powell DR, Yee D. Regulation of insulin-like growth factor-binding protein (IGFBP) expression by breast cancer cells: use of IGFBP-1 as an inhibitor of insulin-like growth factor action. J Natl Cancer Inst. 1992;84:1336–1341. doi: 10.1093/jnci/84.17.1336. [DOI] [PubMed] [Google Scholar]
- 92.Yee D, Jackson JG, Kozelsky TW, Figueroa JA. Insulin-like growth factor binding protein 1 expression inhibits insulin-like growth factor I action in MCF-7 breast cancer cells. Cell Growth Differ. 1994;5:73–77. [PubMed] [Google Scholar]
- 93.Perks CM, Newcomb PV, Norman MR, Holly JM. Effect of insulin-like growth factor binding protein-1 on integrin signalling and the induction of apoptosis in human breast cancer cells. J Mol Endocrinol. 1999;22:141–150. doi: 10.1677/jme.0.0220141. [DOI] [PubMed] [Google Scholar]
- 94.Lønning PE, Hall K, Aakvaag A, Lien EA. Influence of tamoxifen on plasma levels of insulin-like growth factor I and insulin-like growth factor binding protein I in breast cancer patients. Cancer Res. 1992;52:4719–4723. [PubMed] [Google Scholar]
- 95.Vaziri-Gohar A, Houston KD. GPER1-mediated IGFBP-1 induction modulates IGF-1-dependent signaling in tamoxifen-treated breast cancer cells. Mol Cell Endocrinol. 2016;422:160–171. doi: 10.1016/j.mce.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaziri-Gohar A, Zheng Y, Houston KD. IGF-1 receptor modulates FoxO1-mediated tamoxifen response in breast cancer cells. Mol Cancer Res. 2017;15:489–497. doi: 10.1158/1541-7786.MCR-16-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herszényi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249–258. [PubMed] [Google Scholar]
- 98.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14:850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 99.Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 100.Jenab M, Riboli E, Cleveland RJ, Norat T, Rinaldi S, Nieters A, Biessy C, Tjønneland A, Olsen A, Overvad K, Grønbaek H, Clavel-Chapelon F, Boutron-Ruault MC, Linseisen J, Boeing H, Pischon T, Trichopoulos D, Oikonomou E, Trichopoulou A, Panico S, Vineis P, Berrino F, Tumino R, Masala G, Peters PH, van Gils CH, Bueno-de-Mesquita HB, Ocké MC, Lund E, Mendez MA, Tormo MJ, Barricarte A, Martínez-García C, Dorronsoro M, Quirós JR, Hallmans G, Palmqvist R, Berglund G, Manjer J, Key T, Allen NE, Bingham S, Khaw KT, Cust A, Kaaks R. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121:368–376. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 101.Palmqvist R, Stattin P, Rinaldi S, Biessy C, Stenling R, Riboli E, Hallmans G, Kaaks R. Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int J Cancer. 2003;107:89–93. doi: 10.1002/ijc.11362. [DOI] [PubMed] [Google Scholar]
- 102.Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257–2263. doi: 10.1016/s0959-8049(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 103.Sato Y, Inokuchi M, Takagi Y, Kojima K. IGFBP1 is a predictive factor for haematogenous metastasis in patients with gastric cancer. Anticancer Res. 2019;39:2829–2837. doi: 10.21873/anticanres.13411. [DOI] [PubMed] [Google Scholar]
- 104.Xu YW, Chen H, Hong CQ, Chu LY, Yang SH, Huang LS, Guo H, Chen LY, Liu CT, Huang XY, Lin LH, Chen SL, Wu ZY, Peng YH, Xu LY, Li EM. Serum IGFBP-1 as a potential biomarker for diagnosis of early-stage upper gastrointestinal tumour. EBioMedicine. 2020;51:102566. doi: 10.1016/j.ebiom.2019.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolpin BM, Michaud DS, Giovannucci EL, Schernhammer ES, Stampfer MJ, Manson JE, Cochrane BB, Rohan TE, Ma J, Pollak MN, Fuchs CS. Circulating insulin-like growth factor binding protein-1 and the risk of pancreatic cancer. Cancer Res. 2007;67:7923–7928. doi: 10.1158/0008-5472.CAN-07-0373. [DOI] [PubMed] [Google Scholar]
- 106.Huang XP, Zhou WH, Zhang YF. Genetic variations in the IGF-IGFR-IGFBP axis confer susceptibility to lung and esophageal cancer. Genet Mol Res. 2014;13:2107–2119. doi: 10.4238/2014.January.24.17. [DOI] [PubMed] [Google Scholar]
- 107.Luo C, Sun F, Zhu H, Ni Y, Fang J, Liu Y, Shao S, Shen H, Hu J. Insulin-like growth factor binding protein-1 (IGFBP-1) upregulated by Helicobacter pylori and is associated with gastric cancer cells migration. Pathol Res Pract. 2017;213:1029–1036. doi: 10.1016/j.prp.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Kim JC, Ha YJ, Tak KH, Roh SA, Kim CW, Kim TW, Kim SK, Kim SY, Cho DH, Kim YS. Complex behavior of ALDH1A1 and IGFBP1 in liver metastasis from a colorectal cancer. PLoS One. 2016;11:e0155160. doi: 10.1371/journal.pone.0155160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang C, Tai Y, Lisanti MP, Liao DJ. c-Myc induction of programmed cell death may contribute to carcinogenesis: a perspective inspired by several concepts of chemical carcinogenesis. Cancer Biol Ther. 2011;11:615–626. doi: 10.4161/cbt.11.7.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Calon A, Tauriello DV, Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer Biol. 2014;25:15–22. doi: 10.1016/j.semcancer.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 111.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 112.Rutanen EM, Nyman T, Lehtovirta P, Ammälä M, Pekonen F. Suppressed expression of insulin-like growth factor binding protein-1 mRNA in the endometrium: a molecular mechanism associating endometrial cancer with its risk factors. Int J Cancer. 1994;59:307–312. doi: 10.1002/ijc.2910590303. [DOI] [PubMed] [Google Scholar]
- 113.Shafiee MN, Seedhouse C, Mongan N, Chapman C, Deen S, Abu J, Atiomo W. Up-regulation of genes involved in the insulin signalling pathway (IGF1, PTEN and IGFBP1) in the endometrium may link polycystic ovarian syndrome and endometrial cancer. Mol Cell Endocrinol. 2016;424:94–101. doi: 10.1016/j.mce.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 114.Lukanova A, Zeleniuch-Jacquotte A, Lundin E, Micheli A, Arslan AA, Rinaldi S, Muti P, Lenner P, Koenig KL, Biessy C, Krogh V, Riboli E, Shore RE, Stattin P, Berrino F, Hallmans G, Toniolo P, Kaaks R. Prediagnostic levels of C-peptide, IGF-I, IGFBP-1, -2 and -3 and risk of endometrial cancer. Int J Cancer. 2004;108:262–268. doi: 10.1002/ijc.11544. [DOI] [PubMed] [Google Scholar]
- 115.McGrath M, Lee IM, Buring J, De Vivo I. Common genetic variation within IGFI, IGFII, IGFBP-1, and IGFBP-3 and endometrial cancer risk. Gynecol Oncol. 2011;120:174–178. doi: 10.1016/j.ygyno.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG. Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod. 2003;68:24–30. doi: 10.1095/biolreprod.102.009316. [DOI] [PubMed] [Google Scholar]
- 117.Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod. 2005;73:833–839. doi: 10.1095/biolreprod.105.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie Y, Wang JL, Ji M, Yuan ZF, Peng Z, Zhang Y, Wen JG, Shi HR. Regulation of insulin-like growth factor signaling by metformin in endometrial cancer cells. Oncol Lett. 2014;8:1993–1999. doi: 10.3892/ol.2014.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y, Liao QP. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011;126:113–120. doi: 10.1016/j.jsbmb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 120.Terry KL, Tworoger SS, Gates MA, Cramer DW, Hankinson SE. Common genetic variation in IGF1, IGFBP1 and IGFBP3 and ovarian cancer risk. Carcinogenesis. 2009;30:2042–2046. doi: 10.1093/carcin/bgp257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng HC, Tsao SW, Ngan HY, Xue WC, Chiu PM, Cheung AN. Differential expression of insulin-like growth factor binding protein 1 and ferritin light polypeptide in gestational trophoblastic neoplasia: combined cDNA suppression subtractive hybridization and microarray study. Cancer. 2005;104:2409–2416. doi: 10.1002/cncr.21483. [DOI] [PubMed] [Google Scholar]
- 122.Sugita S, Morishita Y, Kano J, Furuya S, Shiba-Ishii A, Noguchi M. IGFBP-1 is expressed specifically in ovarian clear cell adenocarcinoma. Histopathology. 2011;58:729–738. doi: 10.1111/j.1365-2559.2011.03817.x. [DOI] [PubMed] [Google Scholar]
- 123.Cao Y, Nimptsch K, Shui IM, Platz EA, Wu K, Pollak MN, Kenfield SA, Stampfer MJ, Giovannucci EL. Prediagnostic plasma IGFBP-1, IGF-1 and risk of prostate cancer. Int J Cancer. 2015;136:2418–2426. doi: 10.1002/ijc.29295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brady G, O’Regan E, Miller I, Ogungbowale A, Kapas S, Crean SJ. Serum levels of insulin-like growth factors (IGFs) and their binding proteins (IGFBPs), -1, -2, -3, in oral cancer. Int J Oral Maxillofac Surg. 2007;36:259–262. doi: 10.1016/j.ijom.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 125.Lukanova A, Toniolo P, Akhmedkhanov A, Biessy C, Haley NJ, Shore RE, Riboli E, Rinaldi S, Kaaks R. A prospective study of insulin-like growth factor-I, IGF-binding proteins-1, -2 and -3 and lung cancer risk in women. Int J Cancer. 2001;92:888–892. doi: 10.1002/ijc.1265. [DOI] [PubMed] [Google Scholar]
- 126.Feng X, Lin J, Xing S, Liu W, Zhang G. Higher IGFBP-1 to IGF-1 serum ratio predicts unfavourable survival in patients with nasopharyngeal carcinoma. BMC Cancer. 2017;17:90. doi: 10.1186/s12885-017-3068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wick W, Berberich A, Kessler T, Thomé CM, Pusch S, Hielscher T, Sahm F, Oezen I, Schmitt LM, Ciprut S, Hucke N, Ruebmann P, Fischer M, Lemke D, Breckwoldt MO, von Deimling A, Bendszus M, Platten M. Targeting resistance against the MDM2 inhibitor RG7388 in glioblastoma cells by the MEK inhibitor trametinib. Clin Cancer Res. 2019;25:253–265. doi: 10.1158/1078-0432.CCR-18-1580. [DOI] [PubMed] [Google Scholar]
- 128.Nijaguna MB, Patil V, Urbach S, Shwetha SD, Sravani K, Hegde AS, Chandramouli BA, Arivazhagan A, Marin P, Santosh V, Somasundaram K. Glioblastoma-derived macrophage colony-stimulating factor (MCSF) induces microglial release of insulin-like growth factor-binding protein 1 (IGFBP1) to promote angiogenesis. J Biol Chem. 2015;290:23401–23415. doi: 10.1074/jbc.M115.664037. [DOI] [PMC free article] [PubMed] [Google Scholar]


