Abstract
Objective: This study aimed to explore the value of layer-specific strain analysis by two-dimensional speckle tracking imaging (2D-STI) in the assessment of myocardial toxicity in breast cancer patients receiving anthracycline chemotherapy. Methods: Thirty-four breast cancer patients receiving anthracycline chemotherapy were prospectively enrolled. Conventional echocardiography and 2D-STI were evaluated at baseline after the third and sixth cycles of anthracycline chemotherapy. The strains of different layers of left ventricle (LV) including peak systolic longitudinal strain (endo-LS, mid-LS, epi-LS) and circumferential strain (endo-CS, mid-CS, epi-CS) were measured using EchoPAC analysis software. Peak systolic longitudinal strain (MV-LS, PM-LS, AP-LS), circumferential strain (MV-CS, PM-CS, AP-CS) and radial strain (MV-RS, PM-RS, AP-RS) were measured at mitral valve, papillary muscle and apex levels of LV respectively. Global longitudinal strain (GLS), global circumferential strain (GCS), global radial strain (GRS), and left ventricular twist (LVtw) were also analyzed. Results: There was no significant difference in the structural and functional parameters of conventional 2D echocardiography in different cycles of anthracycline chemotherapy (P>0.05); layer specific LS and CS in various cycles decreased layer by layer from inside to outside. LS and CS increased from basal segment to apical segment, while RS showed no obvious gradient characteristics; compared with baseline, GLS and LSs (endo-PM, endo-AP, mid-PM, mid-AP and epi-AP) of LV decreased significantly after the third cycle of chemotherapy (P<0.05); LSs (epi-MV and epi-AP) decreased significantly after the sixth cycle of chemotherapy (P<0.05). No significant changes were detected in layer specific CS, RS and LVtw (P>0.05). Conclusion: Layer-specific strain analysis by 2D-STI technology can quantitatively analyze global and regional functions of LV. The myocardial toxicity due to anthracycline chemotherapy can be detected by layer-specific LS of LV in early stage, which is great valuable to guiding clinical early intervention and improving prognosis.
Keywords: Speckle tracking imaging, layer-specific strain, two-dimension, breast tumor, malignant, anthracycline chemotherapy, left ventricular function
Introduction
Anthracycline drugs, as one of the most important drugs in postoperative chemotherapy for breast cancer patients, have been widely used in clinic field. However, their adverse effects, especially myocardial damage, are also worth of attention. It is necessary to control a patient’s quality of life and to prolong his or her life, but it is also accompanied by increased morbidity and mortality due to cardiac toxicity [1]. The American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (ESC) recommend echocardiography for monitoring and follow-up of cardiotoxicity [2]. The LVEF measured by conventional echocardiography is based on the study of cardiac morphology. It can detect the damage of the whole cardiac function only after the morphological changes and ventricular remodeling. Speckle Tracking Imaging (STI) technology tracks the motion trajectory of ultrasound echo spots in the myocardium, and plots the strain of the myocardium in the region of interest throughout the cardiac cycle. It reflects the real-time movement and deformation of the myocardium and can quantitatively describe the cardiac function from multiple directions in the longitudinal, radial and circumferential directions, and can more accurately analyze and study the local and overall contractile function of the myocardium. Superior to conventional echocardiographic parameters, two-dimensional speckle tracking imaging (2D-STI) can detect subtle myocardial dysfunction even when left ventricular ejection fraction (LVEF) is normal [3]. Cardiotoxicity caused by anthracyclines is dose-dependent, leads to cell apoptosis, and is therefore irreversible at the cell level. With the increasing dose of anthracycline drugs, the myocardial damage becomes more serious and irreversible [4,5]. Because of the substantial cardiac toxicity of chemotherapy, there is significant clinical interest in early detection of cardiac dysfunction. In this study, we observed the effects of anthracycline drugs on the systolic functions of different layers of the left ventricle (LV) in breast cancer patients, and aimed to explore the clinical value of this technology in the early identification of myocardial toxicity of anthracycline drugs.
Materials and methods
Study subjects
In this study, a total of 50 breast cancer patients, who were treated in Nantong Third People’s Hospital, Nantong University, were recruited from March 2018 to January 2020, of whom, 7 patients were excluded due to poor image quality, 4 patients changed treatment regimen due to serious drug side effects, 3 patients were lost to follow-up during the treatment, 2 patients did not sign the informed consent. Finally, 34 patients with complete data were included into this study, all of them were females with an average age of 51.6±9.6 (range: 31-70) years old. This study was approved by the Ethics Committee of Nantong Third People’s Hospital, Nantong University.
Inclusion criteria: (1) The patients had no history of heart disease; (2) The patients received 8 cycles of sequential chemotherapy with epirubicin, cyclophosphamide and paclitaxel; (3) The patients previously had not received chemotherapy, radiotherapy or endocrine therapy; (4) No obvious abnormality was observed in ECG before chemotherapy; (5) The ultrasound images met the requirements for 2D-STI analysis.
Exclusion criteria: (1) The patients had serious heart diseases such as cardiomyopathy, valvular disease and heart failure previously; (2) The patients had a history of other tumors or received anti-tumor treatment in the past; (3) The patients had received chemotherapy and adjuvant radiotherapy; (4) The patients had history of chronic diseases such as hypertension, coronary heart disease and diabetes.
Instruments and methods
Instrument
GE Vivid E9 Doppler ultrasound diagnostic instrument was equipped with M5S probe, with a frequency of 1.5-4.3 MHz. All patients underwent conventional echocardiography and 2D-STI layer-specific strain assessment in the resting stage. Images post-processing was performed by GE EchoPAC workstation, which was equipped with 2D strain analysis software.
Examination method and data analysis
All patients were evaluated by echocardiography before chemotherapy, at the ends of the third and sixth cycles of chemotherapy according to the chemotherapy protocol. Thence, the echocardiography was performed respectively at three points including baseline (before chemotherapy), cycle 3 (cumulative dose of epirubicin ≤360 mg/m2), cycle 6 (cumulative dose of epirubicin ≤480 mg/m2).
The following parameters were evaluated by conventional echocardiography: left ventricular end diastolic diameter (LVDD), left ventricular end systolic diameter (LVSD), interventricular septal thickness (IVST), left ventricular posterior wall thickness (LVPWT), early-to-late mitral inflow velocity ratio (E/A ratio) and early diastolic mitral annulus velocity (e’), and ratio of early diastolic mitral inflow velocity to early diastolic annulus velocity (E/e’). Left ventricular ejection fraction (LVEF) was measured by modified biplane Simpson method.
Parasternal short axis views at the mitral valve, papillary muscle and apex levels as well as apical 4-chamber, 2-chamber and 3-chamber views were obtained. All the images were obtained at a frame rate of 60-90 frames/s. Three consecutive cardiac cycles in sinus rhythm were digitally stored for subsequent analysis during breath-hold. Echo PAC workstation was used to analyze the peak systolic longitudinal strains of three layers of left ventricular (endo-LS, mid-LS and epi-LS), the peak systolic longitudinal strains at the mitral valve, papillary muscle and apex levels (MV-LS and PM-LS, AP-LS) and the global longitudinal strain (GLS). Peak systolic circumferential strains of three layers of left ventricle (endo-CS, mid-CS and epi-CS), systolic circumferential strain at mitral valve, papillary muscle and apex levels (MV-CS and PM-CS, AP-CS), and peak systolic radial strains at mitral valve, papillary muscle and apex levels (MV-RS, PM-RS, AP-RS) were also assessed. Global circumferential strain (GCS), global radial strain (GRS) and left ventricular twist (LVtw) were measured.
Consistency assessment
Consistency assessment was performed for 2D-STI layer-specific strain examination, 20 patients were randomly selected as the study subjects and the image acquisition and analysis were completed by two experienced doctors. The consistency assessment was divided into two types: the assessment of consistency of two repeated examinations on the same patient by the same doctor (the intra-observer consistency assessment) and the assessment of consistency of two examinations on the same patient by two doctors (inter-observer consistency assessment). GLS, GCS, GRS and LVtw were selected as consistency assessment indexes. Bland-Altman method was used for consistency assessment, and mean ± 1.96 SD was defined as the consistent interval. The good consistency is defined as that the values beyond the predefined interval were less than 10%.
Statistical method
Both the measured values of conventional echocardiography and 2D-STI layer-specific strain examination were continuous variables, which were described by mean ± standard deviation (x̅ ± sd). The comparison among the measured values at baseline and the ends of third and sixth cycles was performed by multilevel model (2 level model) with the repeated time points as the level 1 and the individual subject as the level 2. The line charts were applied to display the trends of each measurement index through the repeated measured time points, the box plots were used to show the changes in GLS and LSs from baseline to the ends of third and sixth cycles. All statistical analyses were performed using SAS version 9.4 (Statistical Analysis System Inc., USA). Differences were considered significant based on two-sided tests if P values were less than 0.05.
Results
Comparison of conventional echocardiographic parameters among three time points of chemotherapy
There were no significant differences (P>0.05) in LVDD, LVSD, IVST and LVPWT among three time points of chemotherapy. There was also no statistically significant difference in LVEF (an index for left ventricular systolic function) among three time points of chemotherapy (P>0.05). In addition, there were no significant differences in left ventricular diastolic functions such as e’, E/A and E/e’ (P>0.05; Table 1).
Table 1.
Comparison of conventional echocardiographic parameters among three time points of chemotherapy (x̅ ± sd)
| Time | LVDD (mm) | LVSD (mm) | IVST (mm) | LVPWT (mm) | LVEF (%) | E/A | e’ (mm/s) | E/e’ |
|---|---|---|---|---|---|---|---|---|
| Baseline | 43.7±3.5 | 27.8±2.5 | 8.4±0.8 | 8.2±0.8 | 65.9±3.3 | 1.1±0.3 | 8.3±2.4 | 10.1±3.1 |
| Cycle 3 (AD≤360 mg/m2) | 43.8±4.0 | 28.6±2.7 | 8.3±0.9 | 8.3±0.7 | 64.3±3.4 | 1.0±0.3 | 7.7±2.5 | 10.4±3.2 |
| Cycle 6 (AD≤480 mg/m2) | 43.8±3.6 | 28.4±3.2 | 8.4±0.7 | 8.4±0.6 | 64.5±4.1 | 1.0±0.4 | 7.6±2.1 | 10.6±2.6 |
| P values | 0.9939 | 0.2453 | 0.6612 | 0.3195 | 0.1006 | 0.1619 | 0.0508 | 0.5793 |
Note: AD: Accumulated dose; P values were based on multilevel models with the repeated time points as the level 1 and the individual subject as the level 2. LVDD: left ventricular end diastolic diameter; LVSD: left ventricular end systolic diameter; IVST: interventricular septal thickness; LVPWT: left ventricular posterior wall thickness; E/A ratio: early-to-late mitral inflow velocity ratio; e’: early diastolic mitral annulus velocity; E/e’: ratio of early diastolic mitral inflow velocity to early diastolic annulus velocity; LVEF: left ventricular ejection fraction.
Comparison of layer specific strain among different segments in three time points of chemotherapy
In different chemotherapy cycles, layer specific LS and CS showed gradient characteristics (Figures 1 and 2). The layer specific LS and CS decreased from the endo-myocardium, mid-myocardium to the epi-myocardium in baseline, the third cycle and the sixth cycle (Figure 3A and 3B). Conversely, the segmental LS and CS increased from basal segment, middle segment to apical segment at baseline and the ends of third and sixth cycles (Figure 3C and 3D). However, RS had no obvious gradient characteristic (Figure 3E).
Figure 1.
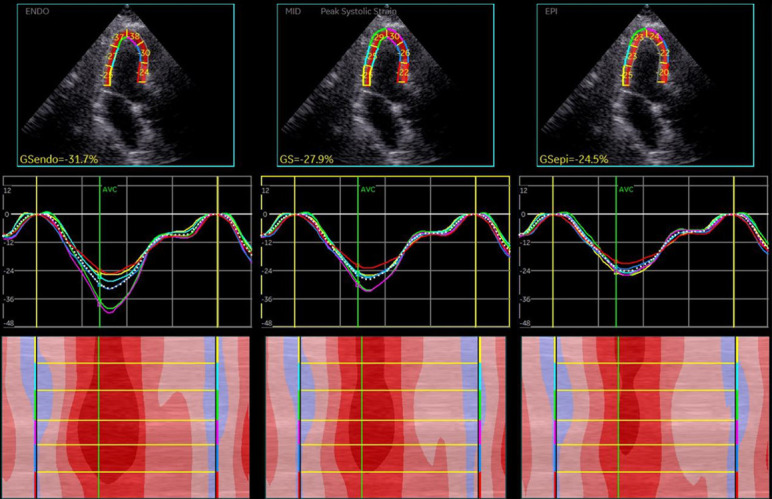
Longitudinal layer-specific strain of left ventricular myocardium (apical 3 chamber view): endo > mid > epi.
Figure 2.
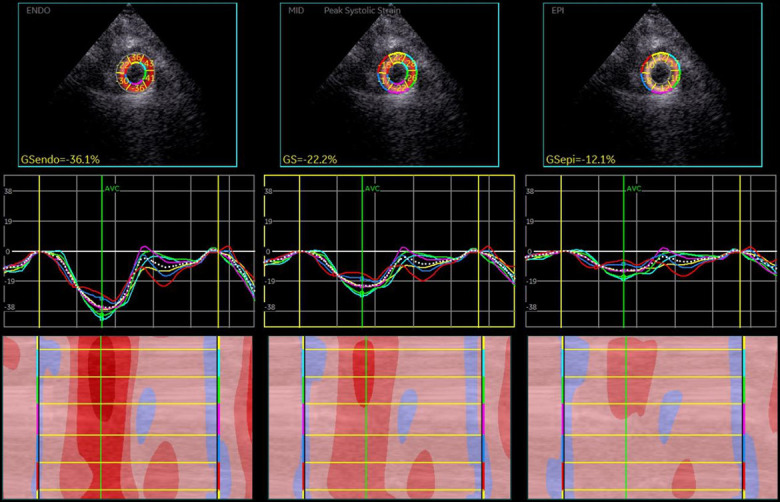
Circumferential layer-specific strain of left ventricular myocardium (parasternal short view at apical level): endo > mid > epi.
Figure 3.
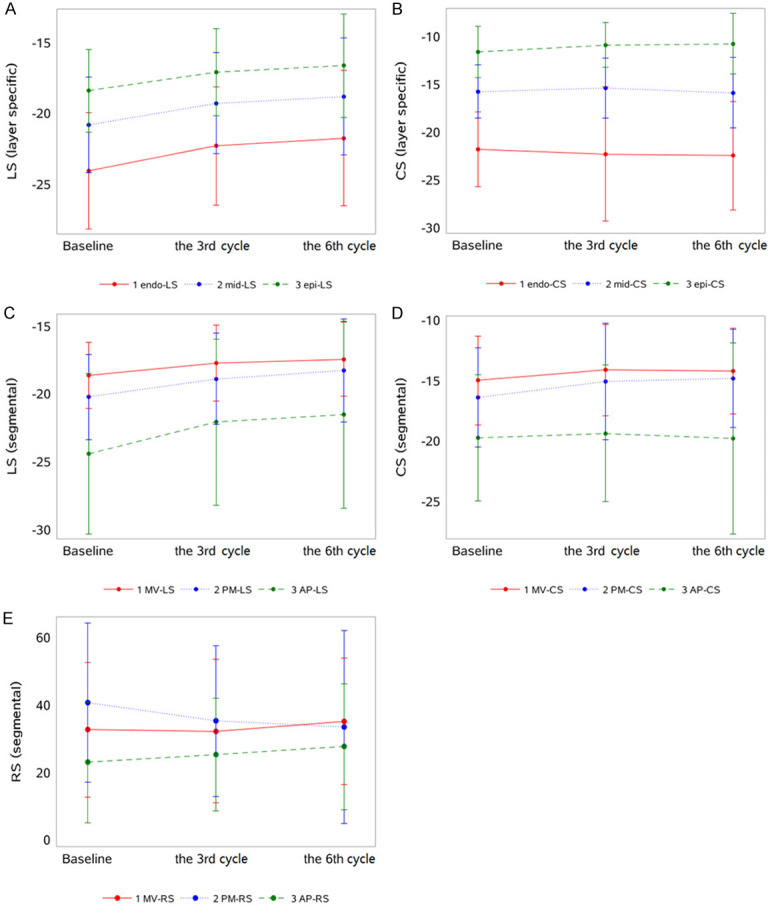
Line charts for the trends of LS, CS in different layers and LS, CS, RS in different segments within three time points of chemotherapy. A and B: Line charts for the trends of LS, CS in different layers; C-E: LS, CS, RS in different segments within three time points of chemotherapy.
Comparison of layer specific strain among different segments at three time points of chemotherapy
Compared to layer specific strains at baseline, longitudinal layer-specific strain of left ventricle showed a downward trend along with increased chemotherapy cycles (Figure 4).
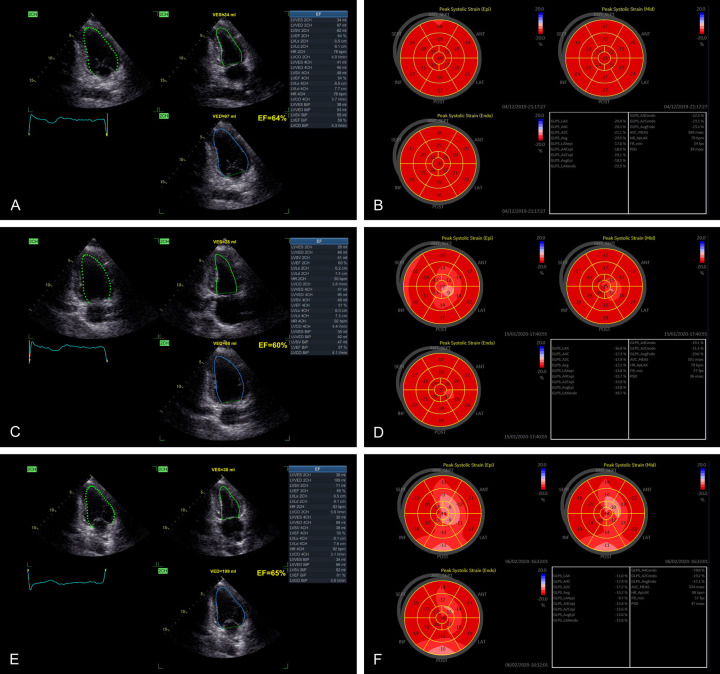
Figure 4.
LVEF measured by conventional echocardiography and longitudinal layer-specific strain (LS) measured by 2D-STI in different chemotherapy cycles in the same breast cancer patient. A, C, E: LVEF measured by conventional echocardiography at three measurement time points of chemotherapy. B, D, F: Left ventricular longitudinal layer-specific strain (LS) measured by 2D-STI technology at three measurement time points of chemotherapy.
Compared to layer specific strains at baseline, GLS and layer specific LSs (endo-PM, endo-AP, mid-PM, mid-AP and epi-AP) decreased significantly in the third cycle of chemotherapy (P<0.05); layer specific LSs (epi-MV and epi-PM) decreased significantly in the sixth cycle of chemotherapy (P<0.05; Table 2; Figure 5).
Table 2.
Comparison of layer specific LS among different segments at three time points of chemotherapy (x̅ ± sd)
| Variables (%) | Baseline | Cycle 3 (AD≤360 mg/m2) | Cycle 6 (AD≤480 mg/m2) | P values |
|---|---|---|---|---|
| LS endo-MV | -19.4±2.7 | -18.4±3.1 | -18.4±3.1 | 0.1235 |
| endo-PM | -22.3±3.5 | -20.6±3.8 | -19.8±4.3 | 0.0023 |
| endo-AP | -30.5±7.7 | -27.7±7.6 | -26.9±8.5 | 0.0140 |
| mid-MV | -18.4±2.6 | -17.6±2.8 | -17.3±2.9 | 0.0878 |
| mid-PM | -20.1±3.0 | -18.6±3.5 | -18.1±3.8 | 0.0063 |
| mid-AP | -23.7±5.9 | -21.4±6.0 | -20.9±6.8 | 0.0230 |
| epi-MV | -17.9±2.3 | -16.9±2.6 | -16.5±2.5 | 0.0212 |
| epi-PM | -18.3±3.1 | -17.2±2.9 | -16.7±3.4 | 0.0282 |
| epi-AP | -18.9±4.5 | -17.0±4.9 | -16.7±5.6 | 0.0294 |
| GLS | -21.1±3.4 | -19.5±3.6 | -19.0±4.1 | 0.0066 |
Note: P values were based on multilevel models with the repeated time points as the level 1 and the individual subject as the level 2. GLS: Global longitudinal strain.
Figure 5.
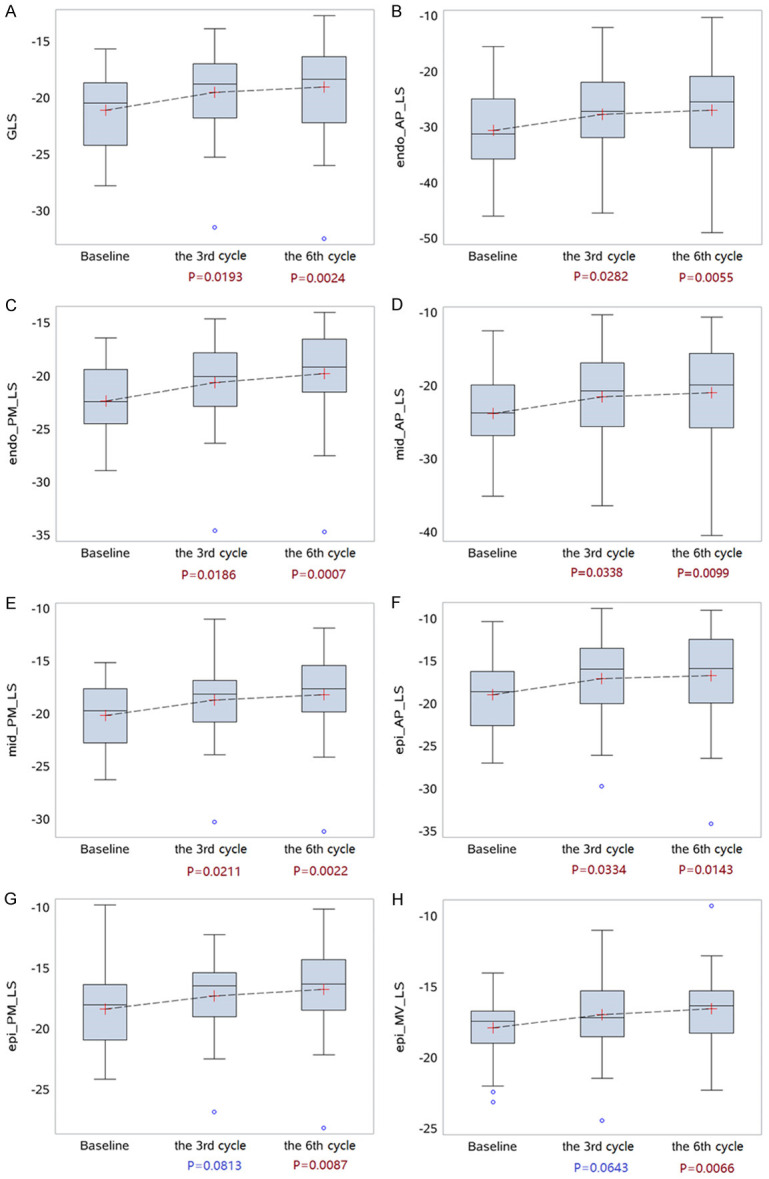
Box plots for the trends of GLS, LS in different layers and segments among three time points of chemotherapy. A: Box plots for the trends of GLS; B-H: LS in different layers and segments among three time points of chemotherapy. GLS: Global longitudinal strain.
There were no significant differences in parameters such as left ventricular CSs, RSs and LVtw among three time points of chemotherapy (Table 3).
Table 3.
Comparison of layer specific CS and RS among different segments at three time points of chemotherapy (x̅ ± sd)
| Variables (%) | Baseline | Cycle 3 (AD≤360 mg/m2) | Cycle 6 (AD≤480 mg/m2) | P value |
|---|---|---|---|---|
| CS endo-MV | -19.8±6.6 | -18.7±6.0 | -19.3±5.2 | 0.7021 |
| Mid-MV | -14.4±3.5 | -13.6±3.7 | -14.0±3.7 | 0.6304 |
| Epi-MV | -10.6±2.9 | -10.0±2.8 | -9.4±3.4 | 0.2426 |
| Endo-PM | -22.7±5.8 | -21.4±11.7 | -20.3±6.8 | 0.4549 |
| Mid-PM | -15.7±4.0 | -14.0±3.4 | -14.4±3.8 | 0.1278 |
| Epi-PM | -10.7±3.7 | -9.6±3.5 | -9.7±3.4 | 0.3118 |
| Endo-AP | -22.7±7.4 | -26.6±9.0 | -27.2±10.8 | 0.0612 |
| Mid-AP | -17.0±5.2 | -18.4±5.3 | -19.0±7.5 | 0.3711 |
| Epi-AP | -13.4±5.0 | -12.9±4.5 | -13.2±6.7 | 0.9371 |
| GCS | -16.3±2.8 | -16.1±3.6 | -16.5±3.9 | 0.8792 |
| LVtw | 13.6±7.7 | 13.9±6.0 | 14.6±8.1 | 0.8077 |
| RS | ||||
| MV | 32.9±20.0 | 32.4±21.3 | 35.4±18.7 | 0.7862 |
| PM | 40.9±23.5 | 35.5±22.4 | 33.6±28.6 | 0.3260 |
| AP | 23.2±17.9 | 25.4±16.8 | 27.8±18.7 | 0.5095 |
| GRS | 32.3±11.9 | 31.1±11.9 | 32.3±12.4 | 0.8745 |
Note: P-based on the comparison results in different periods among 2-level models (cumulative dose groups). GCS: global circumferential strain; GRS: global radial strain; LVtw: left ventricular twist.
After anthracycline treatment was finished, GLS decreased by more than 15% in 5 patients, and the LVEF decreased by more than 10% in 3 patients but did not exceed the lower limit of normal value.
Consistency test
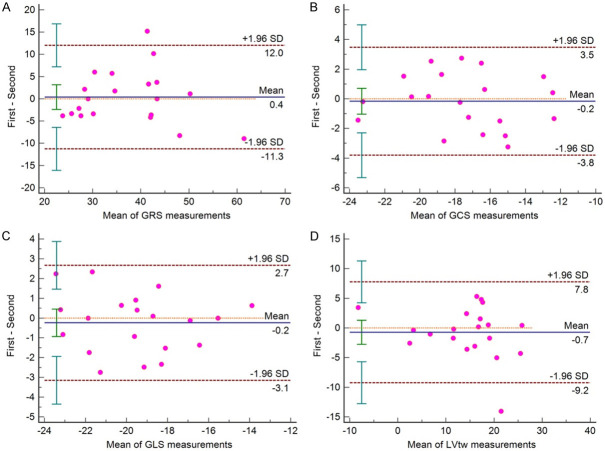
GLS, GCS, GRS and LVtw showed good intra-observer consistency and inter-observer consistency. Taking Mean ± 1.96 SD as the consistency interval, the measured values beyond that were less than 10% (only one value outside the interval for GCS, GLS and LVtw in the intra-operator assessment, and only one value outside the interval for GRS and LVtw in the inter-operator assessment). It suggested that strain parameters such as GLS, GCS, GRS and LVtw had good consistency (Figures 6, 7).
Figure 6.
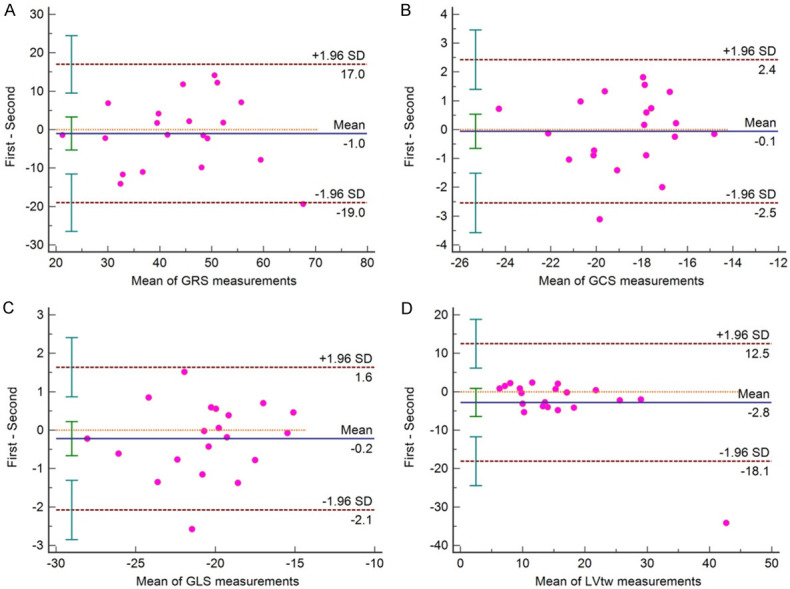
Intraoperator (2 measurements) Bland-Altman plots. A: GRS is global circumferential strain, 5% (1/20) measurement is out of limit of agreement; B: GCS is global radial strain, 5% (1/20) measurement is out of limit of agreement; C: GLS is global longitudinal strain, 5% (1/20) measurement is out of limit of agreement; D: LVtw is left ventricular twist, 5% (1/20) measurement is out of limit of agreement.
Figure 7.
Interoperator (2 operators) Bland-Altman plots. A: GRS is global circumferential strain, 5% (1/20) measurement is out of limit of agreement; B: GCS is global radial strain, no measurement is out of limit of agreement; C: GLS is global longitudinal strain, no measurement is out of limit of agreement; D: LVtw is left ventricular twist, 5% (1/20) measurement is out of limit of agreement.
Discussion
In this study, we found that 2D-STI technology could identify subclinical myocardial injury, which was more sensitive than conventional echocardiography. Using the 2D-STI technology, the absolute values of strains in three-layers of each left ventricular segment (LS and CS) expressed gradient characteristics. LS and CS decreased layer by layer from the endo-myocardium to the epi-myocardium, and increased gradually from the basal segment to the apical segment, while RS had no such gradient characteristics. We concluded that the myocardium in the apical segment was more susceptible to the influence of chemotherapy drugs, wherein the whole thickness of myocardium was affected in the early stage, and the epi-myocardium was affected later than the endo-myocardium and mid-myocardium.
2D-STI technology can objectively evaluate the global and segmental motion of left ventricular myocardium in longitudinal, circumferential and radial directions by tracking the relative motion of speckles in the myocardium, so it can realize precise accurate quantitative analysis on the regional and global systolic and diastolic functions of the myocardium [6]. In previous studies, 2D-STI technology have been widely used in diagnosis of various of heart diseases, such as heart failure, coronary artery disease, valvular heart disease, and cardio myopathies [7,8]. Several publications have showed that GLS is useful in detecting early dysfunction of left ventricle by chemotherapy [9-11]. Even in patients with breast cancer showing normal LVEF, standard anti-cancer treatment could reduce GLS values in addition to other conventional echocardiographic parameters [12,13]. The layer-specific strain is a more accurate strain analysis technology based on the 2D-STI. By tracking the stable acoustic speckles in the endo-myocardium, mid-myocardium and epi-myocardium respectively, the strains in different layers of myocardium can be calculated, which can reflect the change of cardiac contraction function more sensitively [14]. Several recent studies have shown that assessment of myocardial layer-specific strain may allow for detection of early subclinical cardiac dysfunction in children after anthracycline therapy despite normal LVEF [15,16]. Layer specific strain technology was utilized in this study to analyze the functional changes of different myocardial layers of breast cancer patients in the early cycle of anthracycline chemotherapy. Furthermore, we explored the value of layer specific strain technology in identifying and monitoring early cardiac toxicity of anthracycline drugs.
In this study, 2D-STI technology was used to analyze the patients’ left ventricular function. It was found that GLS and LSs in some segmental myocardia decreased obviously in the third cycle of chemotherapy (P<0.05). However, neither cardiac structural parameters nor functional parameter (LVEF) in conventional echocardiography appeared a remarkable decline in the whole chemotherapy cycle. Our results showed that subclinical myocardial function damage had occurred when the cumulative dose of anthracycline reached 360 mg/m2, which was consistent with the results in previous studies [17,18]. The LVEF is not as sensitive as STI in monitoring the cardiotoxicity of anthracycline drugs due to the fact that LVEF is susceptible to cardiac preload, afterload and myocardial contractility. In addition, the circumferential strain compensation maintains LVEF normal when GLS decreases, which affects the sensitivity of LVEF monitoring LV systolic function.
This study showed that the absolute values of myocardial LS and CS had gradient characteristics in different cycles of chemotherapy. They decreased layer by layer from endo-myocardium to epi-myocardium, which might be correlated with different myocardial tensions of different layers of myocardium caused by different curvature radiuses of various layers of myocardium. Another reason might be that the alignment directions of various layers of myocardium were different, so the thickening rate of left ventricular wall during systole was mainly contributed from the endocardial contraction, only 1/3 of which was contributed from the epicardial contraction [19,20]. The segmental distributions of LS and CS also had gradient characteristics. They increased gradually from the basal segment to the apical segment, which is consistent with the results of the study about layer-specific myocardial strain in normal people by Shi Jing et al [19]. The reason might be that the geometric center of the left ventricle was located at the apex of the heart, and the oblique spiral muscle bundles in the inner and outer layers of each segment moved towards the apex of the heart during the systole, which would reduce the ventricular cavity along the longitudinal direction, then the apex of the heart produced huge deformation to ensure the most efficient blood pumping, and thus the LS and CS of the apex were higher than that of other segments.
Numerous studies have demonstrated that the cardiotoxicity of anthracycline drugs is dose-dependent, and different doses of chemotherapy drugs have different degrees of myocardial damage [5,21]. This study showed that with the increase of drug dosage, the LS of left ventricular decreased gradually layer by layer, the LS in three layers of the apical segments (epi-, mid- and endo-myocardium) and two layers of intermedial segments (mid- and endo-myocardium) decreased significantly in the third cycle of chemotherapy, and the LS in the outer layer of basal segments and intermedial segments also decreased significantly in the sixth cycle of chemotherapy. Comprehensive analysis showed that when the drug dose reached 360 mg/m2, the longitudinal contraction of most segments had been damaged, especially for the whole layer of apical segments, which resulted in the decrease of GLS. When the drug dose reached 480 mg/m2, the range of involvement extended to the outer layer of the basal and middle segments. The results suggested that the apical segments were more susceptible to chemotherapy drugs, and the epi-myocardium was injured later than the mid- and endo-myocardium. A previous study showed that when the cumulative dose of anthracycline drug reached 240 mg/m2, the longitudinal contractile function of the endo-myocardium was impaired, therefore the endo-myocardium damage appeared firstly in different myocardium layers [22]. Our study failed to identify which layer of myocardium was firstly damaged, which was likely related to the different selection of chemotherapy cycle. In our study, there was no significant difference in the parameters CS and RS during chemotherapy which could be explained by that CS and RS were mainly produced by the circumferential fibers in the mid-myocardium. The curvature radius of the circumferential fibers was smaller than that of the longitudinal fibers, so the pressure they withstood was also lower than that withstood by the longitudinal fibers. Therefore, the decrease of CS and RS was later than that of LS [23].
In this observational study, firstly, we could only assess correlations, not cause-and-effect relationships between parameters due to limited follow-up time; secondly, we just focused on the cardiotoxicity of anthracycline drugs, but these drugs could be used in combination with other drugs. For example, the combined application of trastuzumab might also cause myocardial depression. Theoretically, trastuzumab has little effects on cardiac function, which needs to be confirmed by further study. It is also a future research direction in our center.
Acknowledgements
This work was supported by the grants from Nantong Municipal Health Commission (WKZL2018013) and Nantong Science and Technology Bureau (MSZ18229). We thank Tonglin Xu, B.M., for providing the clinical data of patients for this study, Jiabao Zhu (M.M.) and Tong Si (M.M.) for collecting data for this study, we also thank all the participants from the Third Affiliated Hospital of Nantong University.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coe-bergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Juan CP, Michael SE, Javier G, Luigi PB, Joseph C, Scott DF, Jennifer EL, Liza YS, Patrizio L. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Yu AF, Raikhelkar J, Zabor EC, Tonorezos ES, Moskowitz CS, Adsuar R, Mara E, Huie K, Oeffinger KC, Steingart RM, Liu JE. Two-dimensional speckle tracking echocardiography detects subclinical left ventricular systolic dysfunction among adult survivors of childhood, adolescent, and young adult cancer. Biomed Res Int. 2016;2016:936–951. doi: 10.1155/2016/9363951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamorano JL, Lancellotti P, Muñoz DR, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Fernandez TL, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 5.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7):viil55–viil66. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 6.Chan J, Hanekom L, Wong C, Leano R, Cho GY, Marwick TH. Differentiation of subendocardial and transmural infarction using two-dimensional strain rate imaging to assess short-axis and long-axis myocardial function. J Am Coll Cardiol. 2006;48:2026–2033. doi: 10.1016/j.jacc.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 8.Tarascio M, Leo LA, Klersy C, Murzilli R, Moccetti T, Faletra FF. Speckle-tracking layer-specific analysis of myocardial deformation and evaluation of scar transmurality in chronic ischemic heart disease. J Am Soc Echocardiogr. 2017;30:667–675. doi: 10.1016/j.echo.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 10.Fang S, Zhang Z, Wang Y, Jiang F, Yang K, He F, Zhang C. Predictive value of left ventricular myocardial strain by four-dimensional speckle tracking echocardiography combined with red cell distribution width in heart failure with preserved ejection fraction. Echocardiography. 2019;36:1074–1083. doi: 10.1111/echo.14373. [DOI] [PubMed] [Google Scholar]
- 11.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d’Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 12.Colombo A, Cipolla C, Beggiato M, Cardinale D. Cardiac toxicity of cardiac toxicity of anticancer agents. Curr Cardiol Rep. 2013;15:362. doi: 10.1007/s11886-013-0362-6. [DOI] [PubMed] [Google Scholar]
- 13.Yao MY, Lv Q, Xie MX, Wang XF, Qin XJ, He L, Zhang L, Deng Y, Pei M. Evaluation of left ventricular global systolic function after epirubicin chemotherapy in patients with breast cancer by two-dimensional speckle tracking imaging. Chin J Ultrason. 2013;22:576–577. [Google Scholar]
- 14.Bachner-Hinezon N, Ertracht O, Malka A, Leitman M, Vered Z, Binah O, Adam D. Layer-specific strain analysis: investigation of regional deformations in a rat model of acute versus chronic myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;303:549–558. doi: 10.1152/ajpheart.00294.2012. [DOI] [PubMed] [Google Scholar]
- 15.Li HR, Liu C, Zhang G, Wang C, Sun P, Du GQ, Tian JW. The early alteration of left ventricular strain and dys-synchrony index in breast cancer patients undergoing anthracycline therapy using layer-specific strain analysis. Echocardiography. 2019;36:1675–1681. doi: 10.1111/echo.14460. [DOI] [PubMed] [Google Scholar]
- 16.Kana Y, Ken T, Sachie S, Mariko Y, Takeshi I, Maki K, Katsumi A, Hiroyuki T, Junya F, Masahiro S, Masaki N, Toshiaki S. In-depth insight into the mechanisms of cardiac dysfunction in patients with childhood cancSer after anthracycline treatment using layer-specific strain analysis. Circ J. 2018;82:715–723. doi: 10.1253/circj.CJ-17-0874. [DOI] [PubMed] [Google Scholar]
- 17.Tan Y, Hu B, Leng QQ, Lei JR, Zhou Z, Zhu M, Guo RQ. Early evaluation of cardiotoxicity by two-dimensional speckle tracking layer-specific imaging in patients with anthracycline chemotherapy. J Clin Ultrasound Med. 2019;21:166–172. [Google Scholar]
- 18.Luo RL, Cui HY, Huang DM, Li GS. Early assessment of the left ventricular function by epirubicin-induced cardiotoxicity in postoperative breast cancer patients. Echocardiography. 2017;34:1601–1609. doi: 10.1111/echo.13693. [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Pan CZ, Shu XH, Chen HY, Kong DH. Quantitative analysis of left ventricular layer-specific strain in normal adults using speckle tracking imaging. Chin J Ultrason. 2015;24:378–381. [Google Scholar]
- 20.Niu HY, Huang XL, Zhang JX. Assessment of myocardial strain transmural gradient by 2-dimension strain imaging in essential hypertension patients. Chin J Ultrasound Med. 2012;28:716–719. [Google Scholar]
- 21.Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cadiac injury: can we identify strategies for cardio-protection? Prog Cardiovasc Dis. 2010;53:105–113. doi: 10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X, Zhao XL, Wang SC, Xu H, Chen DF. Evaluation on the effect of anthracycline-induced cardiotoxity on left ventricular myocardial strain by speckle tracking layered strain technique in breast cancer patients. J Clin Ultrasound Med. 2018;20:397–400. [Google Scholar]
- 23.Kosmala W, Plaksej R, Strotmann JM, Weigel C, Herrmann S, Niemann M, Mende H, Störk S, Angermann CE, Wagner JA, Weidemann F. Progression of left ventricular functional abnormalities in hypertensive patients with heart failure: an ultrasonic two-dimensional speckle tracking study. J Am Soc Echocardiogr. 2008;21:1309–1317. doi: 10.1016/j.echo.2008.10.006. [DOI] [PubMed] [Google Scholar]