Abstract
Progestin administration serves as the optimal conservative treatment method for women with endometrial cancer or precancer lesions who want to preserve fertility. However, there are still at least 30% of patients in which progestin resistance occurs. LASS2 (Ceramide Synthase 2) has been reported to be involved in chemotherapy resistance, whether it also plays a role in progestin resistance is not clear. Here, we explored the detailed mechanism by which Nrf2/LASS2 contributes to progestin resistance and disease progression. Methods: IHC assays were performed to estimate the expression pattern of Nrf2 and LASS2. Moreover, it bears three antioxidant response elements (ARE) in the promoter region of LASS2 gene, therefore, Luciferase assays were performed to determine if Nrf2 regulates LASS2 by binding with these ARE sequence. Western Blot assays were used to determine the expression of Nrf2 and LASS2 protein among various endometrial cell lines. Relative mRNA expression levels were detected by RT-PCR. Cellular growth was monitored with CCK-8 tests. Apoptosis was determined with Annexin V-PI staining and flow cytometry analysis. siRNA knockdown was performed to investigate the effects of Nrf2 on cell proliferation. Result: Nrf2/LASS2 is highly expressed in endometrial cancer tissue, as compared to expression levels in normal endometrial tissue. Proliferation assays demonstrated that overexpression of Nrf2/LASS2 resulted in progestin resistance. Conversely, knockdown of LASS2 increased apoptosis and decreased cell viability. In addition, metformin overcame progestin resistance by down-regulating Nrf2/LASS2 expression. Conclusion: Our findings provide new insight into the mechanism of progestin resistance in type I endometrial cancer. Nrf2/LASS2 may not only be a possible marker for predicting the prognosis of endometrial cancer but also serve as a potential therapeutic target.
Keywords: Nrf2, LASS2, Type I endometrial cancer, proliferation, progestin resistance
Introduction
Endometrial cancer is the most common gynecological tumor in developed countries [1]. Statistics suggest that the rise in occurrence of endometrial cancers is due to rising incidences of obesity and type II diabetes, delays in child-bearing age, and decreases in the rate of hysterectomy for benign disease [2]. For the majority of woman diagnosed with early stage endometrial cancer, surgery is the first choice for therapy, especially in China. However, there is a growing number of young women with endometrial cancer who want to preserve their fertility [3,4]. In women who desire to preserve fertility, progestin therapy is regarded as a temporizing therapeutic strategy for endometrial lesions. Though 70% of patients respond well to progestin therapy, at least 30% of patients ultimately experience disease progression due to progestin resistance [5]. Thus, recent studies have been focused on elucidating mechanisms of progestin resistance and extensive work has been done to investigate and characterize the role of the estrogen receptor (ER) and the progestin receptor (PR) in progestin resistance. Unfortunately, these studies have not been able to fully uncover the mechanisms of progestin resistance [2]. Therefore, there is an urgent need to find new markers that play a role in progestin therapy.
Nuclear factor erythroid-derived factor 2-related factor (Nrf2) belongs to the Cap’-n’-Collar (CNC) family of transcription factors [6]. Nrf2 is regarded as a master regulator of cellular response to oxidative stress and binds to the antioxidant response element (ARE) to subsequently activate genes which play important roles in protecting cells from intrinsic or exogenous oxidative stress [7]. Recent studies have demonstrated that the anti-oxidative function of Nrf2 may have deleterious effects and may serve a tumorigenic function. For example, Nrf2 activation is associated with biological processes such as activating anti-apoptotic genes, promoting the growth of tumor cells, and activating a series of drug metabolism genes that allow cancer cells to become resistant to chemotherapy and radiotherapy [8,9]. Furthermore, studies have suggested that Nrf2 may be a novel therapeutic target to reverse drug resistance [10,11]. Zhou et al. demonstrated that inhibition of Nrf2 signaling could be used to re-sensitize gemcitabine-resistant pancreatic cancers [12]. In line with studies showing that Nrf2 plays a role in cancer drug resistance, our previous endometrial cancer studies also showed that Nrf2 expression was clearly responsible for chemoresistance and progestin resistance [13,14].
LASS2, a synthesizer of ceramides with broad tissue distribution, is involved in multiple intracellular signaling processes such as apoptosis, senescence, proliferation, growth and differentiation [15,16]. Schiffmann et al. showed that the mRNA levels of LASS2, LASS4, LASS5 and LASS6 were elevated in tumor samples with high ceramide levels, indicating that an increase in specific ceramides correlates with the development of breast cancer [16-18]. LASS2 also has been found to be induced by estrogen, which was accompanied by an increase in the relative ceramide species in MCF-7 cells. Furthermore, upregulation of LASS2 increased cancer cell proliferation [18,19]. Recent studies demonstrate that LASS2 plays an important role in cancer progression and chemoresistance due to its interaction with proton pumps, suggesting that LASS2 may regulate the tumor microenvironment. However, the mechanism by which LASS2 is regulated and its potential oncogenic role are still unclear. In this study, we hypothesize that LASS2, which possesses three ARE sequences, is a downstream mediator of Nrf2 and participates in the progression of endometrial cancer and progestin resistance.
Materials and methods
Cell lines and culture of endometrial primary cells
Endometrial cancer cell lines Ishikawa, Hec-1A and KLE were maintained in our lab. Ishikawa and Hec-1A cell lines are estrogen-responsive cell lines derived from a well-differentiated endometrioid carcinoma. Endometrial primary cells were collected from patients with benign endometrial lesions, followed by digestion with type I collagenase (Sigma, American), then cultured in MEM-F12 medium with 10% FBS.
Plasmids, siRNA, and transfections
LASS2 plasmids were purchased from Harmonious One Biotech Co., Ltd (Shanghai, China). Nrf2 small interfering RNA was purchased from RiboBio Co., Ltd (Guangzhou, China) and Qiagen (Germany). The pEGFP-LASS2, PLKO-shLASS2, pcDNA-Nrf2 plasmid and siNrf2 (target sequence are listed in Table S2) were transfected into endometrial cells using the Lipofectamine 3000 transfection reagent (Invitrogen, USA, L3000015), respectively. The transfection efficiency of target gene was examined by western blot.
Clinical endometrial cancer specimens and IHC assay
IHC assay of 120 clinical endometrial tissue samples were conducted to determine the variability of Nrf2 expression between normal endometrial tissues and carcinomas. Another cohort of endometrial samples was used to investigate the expression of LASS2 proteins to correlate the relationship of Nrf2/LASS2 expression level with cancer stage. Different endometrial tissue samples were constructed in a tissue microarray including benign endometrial tissues: proliferation phase (PP) and Secretory phase (SP), simple hyperplasia, endometrial atypical hyperplasia (EAH) cases, endometrial cancer (CA). Samples were stained with Nrf2 (Abcam, ab62352, 1:200) and CerS2 (Sigma, HPA027262, 1:200). IHC staining and scoring were performed as described (19). The expression of Nrf2/LASS2 was determined by obtaining the mean of integrated optical density (Mean of IOD), this value was obtained by Image J plus 6.0.
Quantitative real time PCR
Total RNA was extracted and reverse transcribed as previously described [4]. Primers for Nrf2, CerS2 and GAPDH genes were synthesized by Sangon Biotech Corporation (Shanghai, China) (Table S1). Real-time PCR reactions were set up with 5 μl SYBR Green PCR Master Mix (Takara, Japan, RR036A, RR420A), 1.0 μl of a 10 μM primer mixture, and 4 μl of cDNA template. The PCR conditions were 95°C for 5 min, followed by 35 cycles of 94°C for 1 min, 61°C for 30 s and 72°C for 45 s. GAPDH served as a loading control for the expression of target genes. Each real-time PCR experiment was repeated three times. Replacing template with water served as a negative control.
Cell viability and apoptosis assay
Cell viability was measured using the Cell Counting Kit-8 (Dojin Laboratory, Kumamoto, Japan, CK04). Ishikawa cells were seeded onto 96-well plates at a concentration of 30,000 cells/mL. After overnight starvation in Opti-MEM medium, various treatments were performed, such as E2 (β-Estradiol from Sigma Aldrich, E-8875), MPA (Medroxyprogesterone 17-acetate from Sigma Aldrich, M-1629), and the cells were incubated for another 48 hrs. The plates were then processed with CCK-8 staining as previously described [18,19]. Apoptotic cells were detected using an Annexin V-FITC staining kit (BD Pharmingen, CA, 559763) and analyzed using flow cytometry.
Western blot analysis
Western blotting was performed as previously reported [4,19]. Briefly, Ishikawa cells with indicated treatments were harvested and lysed with RIPA buffer, after determining protein concentration, proteins (50 μg) were loaded and separated by SDS-PAGE. After transferring to PVDF membranes, proteins were detected using specific antibodies. The proteins were detected by enhanced chemiluminescence (thermo Fisher, USA). α-Tubulin was employed as an internal control.
Dual luciferase assay
The dual luciferase assay was performed according to the manufacturer’s instructions (Promega, e1910). Ishikawa cells were co-transfected with PGL 4.27-LASS2-ARE plasmids, pRL-SV40-Renalla plasmid and Nrf2 plasmid. Firefly and Renilla luciferase values were measured using the dual luciferase assay kit and Thermo Scientific Varioskan LUX. For relative luciferase activity calculations, the Firefly value was normalized to the Renilla value.
Colony formation assays
Cells were resuspended at a final concentration at 50 cells/ml and seeded at a density of 100 cells in 6-well plate in complete medium. After 24 hours, the cells were treated with different drugs at the indicated concentrations, media (with drug) was changed every three days and cells were incubated for a total of 14 days. The clonies were fixed with methanol and stained with crystal violet. The number of colonies with > 50 cells were counted under a dissecting microscope. The survival fraction (SFs) values were calculated using the following equation: SF = colonies counted/cells seeded × (plating efficiency/100), which takes the individual plating inconsistencies into consideration.
Statistical analysis
Data are represented as mean ± standard deviation (SD) and analyzed by using SPSS V19.0. Comparisons among multiple groups were made with one-way analysis of variance (ANOVA) followed by Dunnet t-test. Statistical significance between the treated and untreated groups was analyzed by Student’s t test, and the statistical significance was set at P < 0.05.
Results
Nrf2 regulates LASS2 and its functional effects on endometrial cancer cells
Previous studies demonstrated that Nrf2 regulation its downstream tartget genes mainly depends on its binding with the AREs sequence in the target gene promoter regions. In this study, we identified three putative AREs in the LASS2 promoter in which Nrf2 could potentially regulate LASS2 expression (Figure 1A). As shown in Figure 1B, co-transfection of Nrf2 and plasmid of each single ARE site has no significant effect on LASS2 transcription, but co-transfection of Nrf2 and plasmid of the LASS2 full length sequence result in remarkable increase in luciferase activity, indicating that the full length sequence of a protein including three AREs is necessary for Nrf2 regulation of LASS2 expression. Moreover, as a Nrf2 inducer, t-BHQ could upregulate both Nrf2 and LASS2 proteins (Figure 1C), following the similar effect of t-BHQ on proliferation was observed (Figure 1D). To further investigate the role of LASS2 in endometrial cancer development, LASS2 silence has been performed. Elevated early apoptosis rate has been founded after knocking down LASS2 (Figure 1E and 1F).
Figure 1.
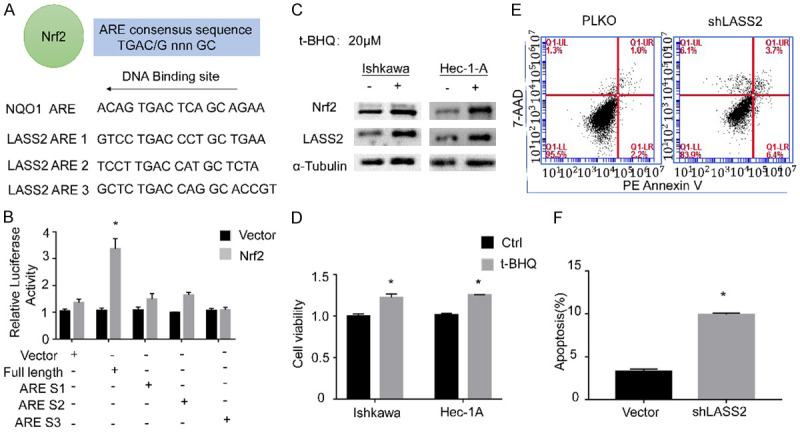
Nrf2 regulates LASS2 and its functional effects on endometrial cancer cells. Dual luciferase assays to examine if Nrf2 drives LASS2 expression by binding with its AREs (A, B). Western blot was performed to determine the effect of t-BHQ on Nrf2 and LASS2 expression in protein levels and proliferation activity was evaluated by CCK8 assay (C, D). The apoptosis effect after knocking down LASS2 was monitored by FCM (E, F).
Nrf2 and LASS2 are positively correlated with endometrial cancer development
Higher expressions of Nrf2 and LASS2 were observed in endometrial carcinoma tissues when compared with the benign endometrial tissue (Figure 2A). The gradually increasing staining intensity of LASS2 correlates with the pathological progress of the endometrial lesion, the peak of LASS2 expression appears in endometrial cancer, while the Nrf2 peak intensities occur in EAH (Figure 2B and 2C). To confirm the relationship between Nrf2 and LASS2, western blot analyses were performed. We found that Nrf2 and LASS2 were highly expressed in endometrial cancer cell lines and barely expressed in endometrial primary cells and stromal cells (Figure 2D and 2E). These data imply that high expression of Nrf2 and LASS2 may be an indicative biomarker in the development of endometrial cancer and may play important roles in endometrial cancer development. The positive correlation between Nrf2 and LASS2 was supported by the alanlysis of the TCGA data base (Figure 2F).
Figure 2.
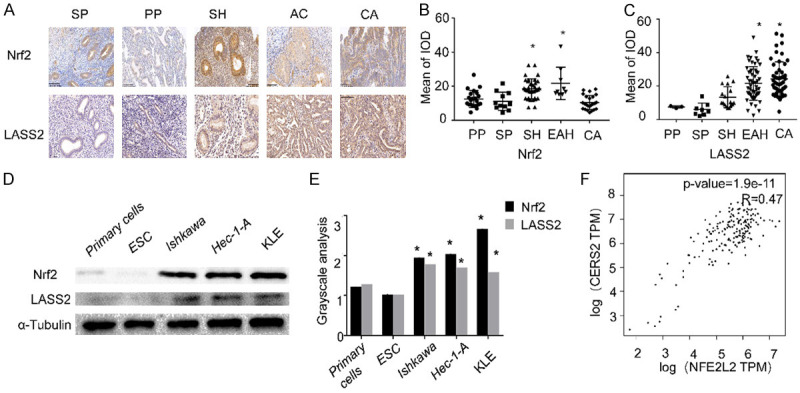
Nrf2 and LASS2 are positively correlated in endometrial cancers development. The expression of Nrf2 and LASS2 in benign and endometrial cancer tissues was assessed using IHC staining. Representative images were captured at 200 × magnification (A). Nrf2/LASS2 IHC mean of IOD in endometrial lesion tissues are presented (B, C). The expression of Nrf2 and LASS2 in benign and endometrial cancer cells was assessed using western blot (D) along with protein grayscale analysis (E). Analysis of the TCGA database displaying the relationship between Nrf2 and LASS2 (F).
Exogenous estrogen increase the expression of Nrf2/LASS2 which results in a decrease in long-term survival
To explore the effect of estrogen on Nrf2/LASS2 expression, RT-qPCR, western blot analysis and proliferation assays were performed. We found that the expression of Nrf2/LASS2 mRNA and protein were elevated with the addition of estrogen, meanwhile, the enhanced proliferation activity has also been observed. Furthermore, knockdown of LASS2 could alleviate the proliferative effects induced by estrogen (Figure 3A-C). Moreover, analysis of the TCGA database shows high expression of Nrf2 in endometrial cancers is associated with a decrease in long-term survival (Figure 3D). These data suggests that Nrf2/LASS2 is associated with hormone response and may participate in long-term, estrogen-stimulated development of endometrial cancer.
Figure 3.
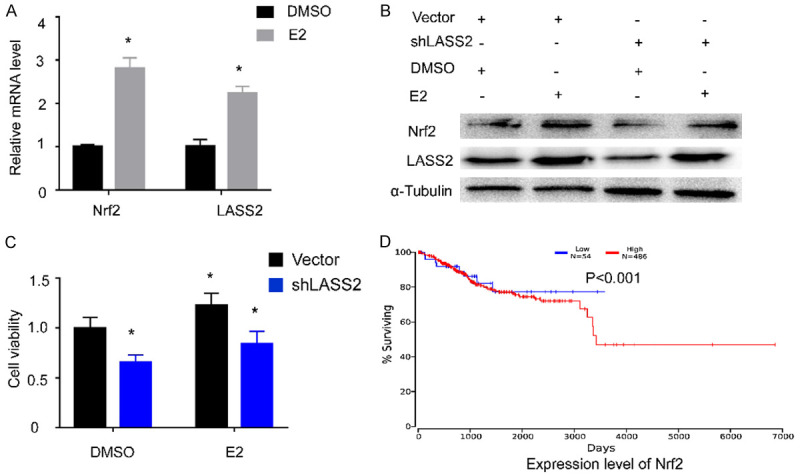
Exogenous estrogen increase the expression of Nrf2/LASS2 which results in a decrease in long-term survival. RT-qPCR was used to detect mRNA levels of Nrf2 and LASS2 after estrogen stimulation (A), Western blot and proliferation analysis showing the effect of exogenous estrogen on Nrf2 and LASS2 expression (B, C). Analysis of the TCGA database showing high expression of Nrf2 correlates with a decrease in overall survival of endometrial cancer patients (D).
Nrf2 and LASS2 expression regulates progestin sensitivity in endometrial cancer cells
To determine the role of Nrf2 and LASS2 expression in progestin resistance, we stably overexpressed or knocked down Nrf2 or LASS2 in human endometrial cancer cells. The protein levels of Nrf2 and LASS2 were measured using Western blot to confirm the effects of transfection and knockdown (Figure 4A-C). As shown in Figure 4A and 4B, the cells with lower levels of Nrf2 or LASS2 is more sensitive to progestin treatment compared with cells that have high levels of both proteins. On the contrary, if we knockdown each of the proteins, a decreasing proliferative activity was observed (Figure 4C and 4D). These data implies that Nrf2 or LASS2 plays an essential role in progestin resistance.
Figure 4.
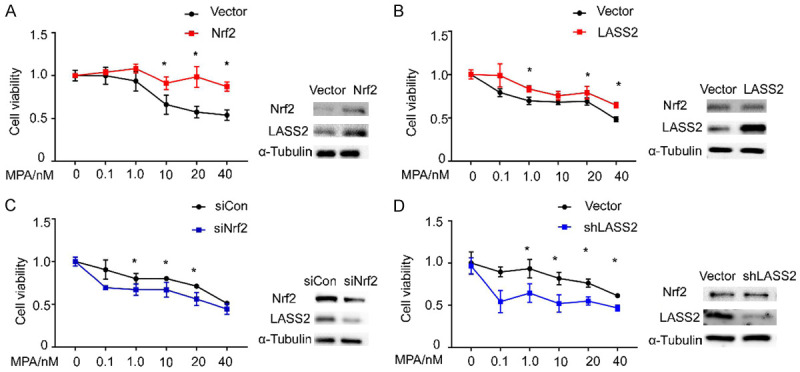
Nrf2 and LASS2 regulates progestin sensitivity in endometrial cancer cells. Relative cell viability was measured using CCK-8. Results demonstrate that Nrf2 and LASS2 expression have an impact on progestin resistance. Overexpression of Nrf2 or LASS2 results in increased viability (A, B) while knockdown of Nrf2 or LASS2 results in decreased cell viability (C, D). Western blots was performed to determine the efficiency of gene overexpression and knockdown.
Progestin withdrawal resulted in on-going Nrf2/LASS2 decline in protein level and attenuated proliferative activity
As hormone withdrawal usually causes “breakdown” of the endometrium, we assessed whether removal of MPA may change the expression of Nrf2 and LASS2. The change pattern of Nrf2 expression was confirmed in our previous study. As expected, LASS2 shows similar expression trend after removal of MPA, the on-going decline of LASS2 was observed (Figure 5A). Meanwhile, the withdrawal also resulted in continued attenuated cell proliferative activity (Figure 5B).
Figure 5.
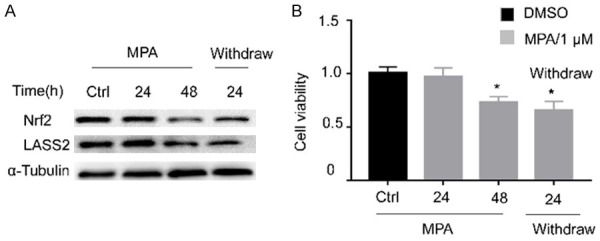
Progestin withdrawal resulted in on-going Nrf2/Lass2 decline in protein level and attenuated proliferative activity. After 1 uM MPA treatment for the indicated 24 h, 48 h, and withdraw for 24 h after a 48 h treatment, followed by MPA withdrawl, the Nrf2 and LASS2 protein expression was determined by Western blot (A), the cellular growth was detected by CCK8 assay (B).
Nrf2/LASS2 mediates metformin-enhanced progestin sensitivity
As shown above, Nrf2 and LASS2 play important roles in progestin resistance. Metformin is able to reverse progestin resistance in patients with endometrial cancers [20]. Based on these findings, we examined the role of metformin in Nrf2/LASS2 mediated progestin resistance to see whether metformin conferred sensitivity to endometrial cancers via Nrf2/LASS2. It was found that metformin suppresses Nrf2/LASS2 protein expression in a dose-dependent manner (Figure 6A). In addition, metformin significantly enhances progestin response in LASS2 knockdown cells (Figure 6B) and rescues from the proliferative effect of LASS2 overexpression (Figure 6C). The colony formation assay shows a similar result that metformin enhances the inhibitory effect of progestin on endometrial cancer (Figure 6D and 6E). This demonstrates the important role of metformin in progestin resistance in endometrial cells.
Figure 6.
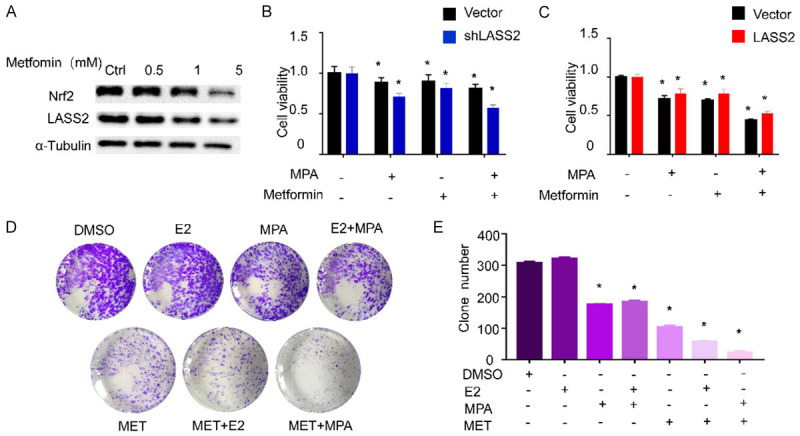
Nrf2/LASS2 mediate metformin-enhanced progestin sensitivity. Western blot analysis was used to detect the changes of Nrf2 and LASS2 protein levels after metformin treatment (A). CCK-8 was used to measure the relative cell viability after LASS2 knockdown (B) or LASS2 overexpression (C) cells. Colony formation assays shows the effects of exogenous drug on colony formation (D). Quantification of colony formation assays (E).
Proposed signaling pathway showing Nrf2/LASS2 regulation of the progression of endometrial cancer and progestin response
As shown in Figure 7, We proposed there is a feedback loop between Nrf2 signaling pathways and high levels of estrogen. Decreased Nrf2 contributes to enhanced progestin sensitivity, type I endometrial cancer patients with higher Nrf2 expression are at greater risk for disease progression.
Figure 7.
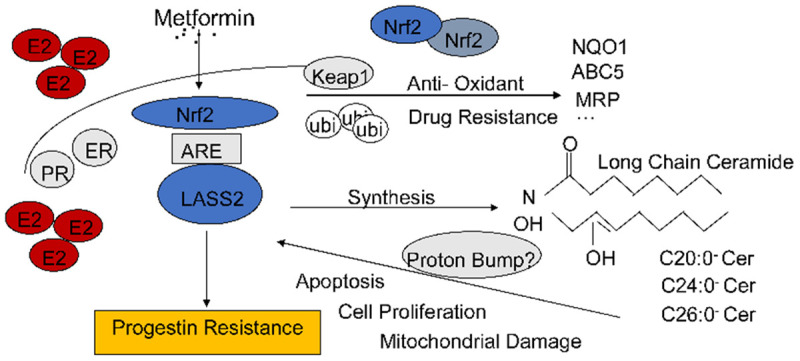
Proposed signaling pathway showing Nrf2/LASS2 regulation of the progression of endometrial cancer and progestin response. There is a feedback loop between Nrf2 signaling pathways and high levels of estrogen. Decreased Nrf2 contributes to enhanced progestin sensitivity, type I endometrial cancer patients with higher Nrf2 expression are at greater risk for disease progression.
Discussion
Incidences of endometrial cancer are on the rise, and though the typical age for most cases of endometrial cancer diagnosis are after menopause, there is a growing concern for patients of child-bearing age who wish to preserve fertility [21]. Though surgery is the preferred and standard treatment for endometrial cancer, progestin treatment is a more conservative method used as a temporary measure for young woman who with plans for pregnancy [22]. However, there is no consensus on the duration of this treatment and whether it is safe to prolong progestin-based therapy, considering the overwhelming rate of disease progression and relapse. Especially due to the uncertain effectiveness of progestin treatment, women who have successfully carried out a full-term pregnancy or who have failed in their attempts to conceive, are encouraged to undergo definitive surgery [4]. Researchers have demonstrated that comprehensive hysteroscopic evaluation and lesion resection plus progestin therapy seem to be an effective and safe fertility sparing therapy for patients with EAH or EEC, and 45% of complete response patient had been pregnant [23]. This data shed light on patients who desire the preservation of fertility. It has become increasingly important and necessary to find a safe and effective fertility-sparing treatment with better tolerability and fewer side effects. Unfortunately, there is still a lack of understanding of progestin resistance. As a continuation of our preliminary work on mechanisms of Nrf2 and progestin resistance, we found that LASS2 might be a downstream target gene of Nrf2, promoting the endometrial cancer progression and progestin sensitivity.
In our previous work, we found that Nrf2 was correlated with progestin resistance in endometrial cancers. Nrf2 is the primary transcription factor that binds to the ARE promoter of phase II genes and induces their expression. Researchers have found that ischemia and reperfusion (I/R)-induced Nrf2 activation directly upregulates estrogen sulfotransferase and in turn increases estrogen breakdown, limiting Nrf2 activity and gender-dependently affecting I/R injury. This study reveals a feedback mechanism between Nrf2 and estrogen in liver ischemia and reperfusion, and demonstrates that exogenous estrogen can further increase hepatic Nrf2 activity, which ultimately results in the formation of cancer in some estrogen-responsive tissues [24,25]. Son HJ et al. found that up-regulation of Nrf2 protein expression by estrogen alleviated colitis in early-induced mice, while abnormal high expression of Nrf2 increased inflammation and promoted tumor formation in late-induced mice [26]. This evidence implicates that Nrf2 signaling plays an important role in type I endometrial cancers, which are usually caused by long-term estrogen stimulation without progestin antagonism. Furthermore, high levels of estrogen provide feedback for the expression of Nrf2, which negatively correlates with the progression of endometrial cancer and progestin resistance.
Our result is consistent with the above hypothesis as we found that Nrf2 could also bind to ARE promoters and positively regulate the expression of LASS2. Exogenous estrogen stimulation elevated both mRNA and protein expression of Nrf2 and LASS2, which promotes proliferation of endometrial cancer cells. Immunohistological analysis of endometrial cancer cells and tissue show that the expression of Nrf2 and LASS2 is higher in malignant cells/tissues as compared with benign cells/tissues (Figure 2). These data imply that Nrf2/LASS2 play important roles in the progression of endometrial cancer. Upon further testing of progestin sensitivity, we found that lower expression of Nrf2/LASS2 would sensitize endometrial cancer cells, resulting in a more effective progestin treatment (Figure 4), and progestin have impact on Ishkawa cells viability even after 24 hrs withdrawal which may attribute to progestin could decrease the expression of Nrf2/LASS2 protein (Figure 5). These may provide a new sight on progestin therapy strategies. We further demonstrate that metformin may be a good adjuvant to sensitizing progestin response (Figure 6). The above results are consistent with the data from TCGA showing that the expression of Nrf2 positively correlated with LASS2 and is associated with a poor prognosis for endometrial cancer patients (Figures 2 and 3).
Progestin resistance is an growing field that researchers have been intensively studied. Although there are several signaling pathways may involve in progestin resistance, the exact mechanism is still unclear. There are still no effective treatments for endometrial cancer and no agents to enhance sensitivity to progestin therapy. Here, we identified LASS2 as a novel Nrf2 target gene. In type I endometrial cancers, there is a feedback loop involving estrogen and the high expression of Nrf2, which results in low response to progestin and ultimately progestin resistance. Our study provides in vitro, proof-of-concept evidence that targeting Nrf2/LASS2 may be a promising approach to inhibiting endometrial cancer development and improving the efficacy of progestin therapy. In addition, overexpression of Nrf2/LASS2 may be useful biomarkers to estimate cancer stage and potential resistance to progestin. As such, future studies should focus on identifying the molecular mechanisms by which LASS2 affects the progestin response and reasons for differences in LASS2 function in different tissue contexts.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grants 81872111, 81672562, 81370074), National Key Technology R&D Program of China (2019YFC1005200 and 2019YFC1005201), Shanghai Municipal Science and Technology Committee of Shanghai outstanding academic leaders plan (19XD1423100), the project of Outstanding Medical Doctor for ZZ, the cross project of Medical and Engineering (YG2016MS27), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20181714), the Shanghai Municipal Public Health Bureau (grant XYQ2013119), and the “Chenxing Project” from Shanghai Jiao Tong University to Z.Z.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 2.Jerzak KJ, Duska L, Mackay HJ. Endocrine therapy in endometrial cancer: an old dog with new tricks. Gynecol Oncol. 2019;153:175–183. doi: 10.1016/j.ygyno.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Tomao F, Peccatori F, Del Pup L, Franchi D, Zanagnolo V, Panici PB, Colombo N. Special issues in fertility preservation for gynecologic malignancies. Crit Rev Oncol Hematol. 2016;97:206–219. doi: 10.1016/j.critrevonc.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Falcone F, Laurelli G, Losito S, Di Napoli M, Granata V, Greggi S. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J Gynecol Oncol. 2017;28:e2. doi: 10.3802/jgo.2017.28.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Medina T, Bajo J, Folgueira G, Haya J, Ortega P. Atypical endometrial hyperplasia treatment with progestogens and gonadotropin-releasing hormone analogues: long-term follow-up. Gynecol Oncol. 1999;73:299–304. doi: 10.1006/gyno.1998.5322. [DOI] [PubMed] [Google Scholar]
- 6.Chartoumpekis DV, Wakabayashi N, Kensler TW. Keap1/nrf2 pathway in the frontiers of cancer and non-cancer cell metabolism. Biochem Soc Trans. 2015;43:639–644. doi: 10.1042/BST20150049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, Jedrychowski MP, Costa ASH, Higgins M, Hams E, Szpyt J, Runtsch MC, King MS, McGouran JF, Fischer R, Kessler BM, McGettrick AF, Hughes MM, Carroll RG, Booty LM, Knatko EV, Meakin PJ, Ashford MLJ, Modis LK, Brunori G, Sévin DC, Fallon PG, Caldwell ST, Kunji ERS, Chouchani ET, Frezza C, Dinkova-Kostova AT, Hartley RC, Murphy MP, O’Neill LA. Itaconate is an anti-inflammatory metabolite that activates nrf2 via alkylation of keap1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menegon S, Columbano A, Giordano S. The dual roles of nrf2 in cancer. Trends Mol Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Chu XY, Liu YM, Zhang HY. Activating or inhibiting nrf2? Trends Pharmacol Sci. 2017;38:953–955. doi: 10.1016/j.tips.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 10.No JH, Kim YB, Song YS. Targeting nrf2 signaling to combat chemoresistance. J Cancer Prev. 2014;19:111–117. doi: 10.15430/JCP.2014.19.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao AM, Ke ZP, Wang JN, Yang JY, Chen SY, Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma bel-7402/adm cells to doxorubicin via inhibiting pi3k/akt/nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Zhou Y, Yang M, Wang K, Liu Y, Zhang M, Yang Y, Jin C, Wang R, Hu R. Digoxin sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine via inhibiting nrf2 signaling pathway. Redox Biol. 2019;22:101131. doi: 10.1016/j.redox.2019.101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Zhou Y, Yang M, Wang K, Liu Y, Zhang M, Yang Y, Jin C, Wang R, Hu R. Digoxin sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine via inhibiting nrf2 signaling pathway. Redox Biol. 2019;22:101131. doi: 10.1016/j.redox.2019.101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai M, Yang L, Liao H, Liang X, Xie B, Xiong J, Tao X, Chen X, Cheng Y, Chen X, Feng Y, Zhang Z, Zheng W. Metformin sensitizes endometrial cancer cells to chemotherapy through idh1-induced nrf2 expression via an epigenetic mechanism. Oncogene. 2018;37:5666–5681. doi: 10.1038/s41388-018-0360-7. [DOI] [PubMed] [Google Scholar]
- 15.Laviad El, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH Jr, Futerman AH. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem. 2008;283:5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- 16.Schiffmann S, Sandner J, Birod K, Wobst I, Angioni C, Ruckhaberle E, Kaufmann M, Ackermann H, Lotsch J, Schmidt H, Geisslinger G, Grosch S. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis. 2009;30:745–752. doi: 10.1093/carcin/bgp061. [DOI] [PubMed] [Google Scholar]
- 17.Ruckhaberle E, Rody A, Engels K, Gaetje R, Von Minckwitz G, Schiffmann S, Grosch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann D, Lucks J, Fuchs S, Schiffmann S, Schreiber Y, Ferreiros N, Merkens J, Marschalek R, Geisslinger G, Grosch S. Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int J Biochem Cell Biol. 2012;44:620–628. doi: 10.1016/j.biocel.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Wegner MS, Wanger RA, Oertel S, Brachtendorf S, Hartmann D, Schiffmann S, Marschalek R, Schreiber Y, Ferreirós N, Geisslinger G, Grösch S. Ceramide synthases cers4 and cers5 are upregulated by 17beta-estradiol and gper1 via ap-1 in human breast cancer cells. Biochem Pharmacol. 2014;92:577–589. doi: 10.1016/j.bcp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Sivalingam V, Mcvey R, Gilmour K, Ali S, Roberts C, Renehan A, Kitchener H, Crosbie E. A presurgical window-of-opportunity study of metformin in obesity-driven endometrial cancer. Lancet. 2015;385(Suppl 1):S90. doi: 10.1016/S0140-6736(15)60405-6. [DOI] [PubMed] [Google Scholar]
- 21.Luo L, Luo B, Zheng Y, Zhang H, Li J, Sidell N. Oral and intrauterine progestogens for atypical endometrial hyperplasia. Cochrane Database Syst Rev. 2018;12:cd009458. doi: 10.1002/14651858.CD009458.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone receptor status predicts response to progestin therapy in endometriosis. J Clin Endocrinol Metab. 2018;103:4561–4568. doi: 10.1210/jc.2018-01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang B, Xu Y, Zhu Q, Xie L, Shan W, Ning C, Xie B, Shi Y, Luo X, Zhang H, Chen X. treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol Oncol. 2019;153:55–62. doi: 10.1016/j.ygyno.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Ansell PJ, Espinosa-Nicholas C, Curran EM, Judy BM, Philips BJ, Hannink M, Lubahn DB. In vitro and in vivo regulation of antioxidant response element-dependent gene expression by estrogens. Endocrinology. 2004;145:311–317. doi: 10.1210/en.2003-0817. [DOI] [PubMed] [Google Scholar]
- 25.Rui W, Zou Y, Lee J, Nambiar SM, Lin J, Zhang L, Yang Y, Dai G. Nuclear factor erythroid 2-related factor 2 deficiency results in amplification of the liver fat-lowering effect of estrogen. J Pharmacol Exp Ther. 2016;358:14–21. doi: 10.1124/jpet.115.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son HJ, Sohn SH, Kim N, Lee HN, Lee SM, Nam RH, Park JH, Song CH, Shin E, Na HY, Kim JS, Lee DH, Surh YJ. Effect of estradiol in an azoxymethane/dextran sulfate sodium-treated mouse model of colorectal cancer: implication for sex difference in colorectal cancer development. Cancer Res Treat. 2019;51:632–648. doi: 10.4143/crt.2018.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


