Abstract
Background: Crizotinib is a tyrosine kinase inhibitor (TKI) effective in ALK/ROS-1/c-MET positive non-small cell lung cancer (NSCLC) patients. Bevacizumab is an antiangiogenic monoclonal antibody, and improves clinical benefit of NSCLC in combination with EGFR-TKIs or chemotherapy. However, the efficacy and safety of crizotinib plus bevacizumab in treating naive ALK/ROS-1/c-MET positive NSCLC patients have not been studied. Methods: In this open-label, single-arm, prospective observational study, locally advanced or metastatic ALK rearrangement/ROS-1 fusion/c-MET amplification NSCLC patients were treated with crizotinib (250 mg orally twice daily) and bevacizumab (7.5 mg/kg intravenous every three weeks) until disease progression or intolerant toxicity or death. Primary end point was progressive free survival (PFS), secondary end points were duration of response (DOR), overall response rate (ORR), disease control rate (DCR) and safety. Patients receiving ≥1 cycle of treatment were evaluated. Findings: Fourteen patients were eligible for analyzing between June 2016 and October 2017. There were 12 patients with ALK rearrangement, 1 patient with ROS-1 fusion, and 1 patient with c-MET amplification. The median follow-up time was 42.8 months. The median PFS and DOR of the patients with ALK rearrangement were 13.9 and 14.8 months respectively. Of the 12 patients, 7 gained partial response, 5 gained stable disease. The ORR and DCR were 58.3% and 100%. The PFS were 12.9 months and 1.9 months for patient with ROS-1 fusion or c-MET amplification. The most two common treatment-related adverse events were fatigue (28.6%) and rash (21.4%). 3 patients discontinued therapy because of liver damage or hemoptysis. Interpretation: This study demonstrated that crizotinib plus bevacizumab showed benefit in treating naive ALK rearrangement NSCLC patients, and the toxicity was relatively tolerant. Our results suggested that crizotinib plus bevacizumab might be a promising treatment strategy in ALK/ROS-1/c-MET positive NSCLC patients.
Keywords: Crizotinib, bevacizumab, ALK, ROS-1, c-MET, brain metastasis
Introduction
Lung cancer, of which approximately 85% are non-small cell lung cancer (NSCLC), is the most frequently diagnosed tumor and the leading cause of cancer-related mortality [1]. 50%-60% of NSCLC patients were estimated to have at least one identifiable driver mutations, including KRAS, EGFR, ALK, ROS-1, BRAF, c-MET, and several additional mutations [2]. Rearrangement of ALK is present at 3%-7% of NSCLC patients [3,4], while prevalence of ROS-1 fusion varies from 0.9% to 3.7% [5], and amplification of c-MET accounts for approximately 2.8% of NSCLC [6]. Crizotinib is a multitargeted tyrosine kinase inhibitor (TKI) against ALK rearrangement, ROS-1 fusion, and c-MET amplification, and substantially improves outcome of NSCLC patients with those mutations.
In study PROFILE 1014, median progressive free survival (PFS) and duration of response (DOR) were 10.9 months and 11.3 months in advanced ALK rearrangement non-squamous NSCLC patients receiving crizotinib as first-line treatment [7]. The study PROFILE 1001 reported remarkable antitumor activity of crizotinib in NSCLC patients harboring ROS-1 fusion with 19.2 months PFS and 17.6 months DOR [8]. Moreover, D. Ross Camidge et al. reported that untreated NSCLC patients with c-MET amplification could also benefit from crizotinib on ASCO 2014; Median DOR was 8.6 months in 12 patients evaluable for response [9]. Collectively, these findings supported that crizotinib had antitumor activity in NSCLC patients with ALK rearrangement/ROS-1 fusion/c-MET amplification. However, most responders treated with crizotinib acquired resistance within 1 year, and developed metastases in brain or liver [10,11]. Thus, novel strategies are encouraged to improve treatment status.
Angiogenesis is reported to participate in the growth and development of tumor. Therefore, anti-angiogenesis therapy plays a particularly important role in controlling tumor growth. Vascular endothelial growth factor (VEGF) is the principal regulator of angiogenesis and stimulates proangiogenic signaling pathways by binding to its receptor VEGFR2 [12,13]. Bevacizumab is a recombinant humanized monoclonal antibody targeting VEGF, and improves PFS, overall response rate (ORR) of NSCLC patients in combination with chemotherapy or targeted therapy. The pivotal study E4599 demonstrated a two-month improvement in overall survival (OS) in recurrent or advanced non-squamous NSCLC patients without brain metastases or clinically significant hemoptysis treated with bevacizumab [14]. The study NEJ026 reported the median PFS of patients in the erlotinib plus bevacizumab arm was 16.9 months comparing with 13.3 months in the erlotinib alone arm [15]. On ASCO 2019, Helena A Yu et al. reported that the ORR was 80% and median PFS was 19 months in 49 NSCLC patients receiving osimertinib plus bevacizumab [16]. A retrospective study including 208 NSCLC patients with EGFR-mutant and brain metastases identified that EGFR-TKIs plus bevacizumab prolonged PFS and OS compared with EGFR-TKIs alone [17]. However, the efficacy of crizotinib plus bevacizumab has not been studied to date. Thus, we conducted this study to explore the efficacy and safety of crizotinib plus bevacizumab in treatment naive ALK/ROS-1/c-MET positive NSCLC patients.
Methods
Study design and participants
This open-label, single arm, prospective observational study enrolled NSCLC patients with ALK rearrangement or ROS-1 fusion or c-MET amplification with the aim to explore the efficacy and the safety of crizotinib plus bevacizumab. The study was approved by Medical Ethics Committee of PLA General Hospital (No. S2016-046-01). All procedures involving human participants were in accordance with the ethical standards of the national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Inclusion criteria were as follows: 1) eligible patients treated in General Hospital of Chinese PLA were histologically or cytologically confirmed locally advanced or metastatic non-squamous NSCLC with ALK rearrangement or ROS-1 fusion or c-MET amplification. The gene status was determined by IHC, FISH, or NGS; 2) patients should be ≥18 years old; 3) patients received no previous systemic treatment; 4) Eastern Cooperative Oncology Group performance status (ECOG PS) was 0-2 (on a scale of 0-5); 5) patients were expected to survive for 12 weeks or longer; 6) patients were required to have at least one measurable lesion at baseline according to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1); 7) patients had adequate organ function and laboratory results in one week before enrollment; 8) patients with untreated or locally treated brain metastases (such as radiotherapy) were eligible on the condition that the metastases were neurologically stable for at least 2 weeks before enrollment; 9) patients should provide written informed consent before enrollment.
Exclusion criteria were as follows: 1) patients had received previous chemotherapy, targeted therapy, immunotherapy, biologic therapy or other investigational agent prior to this study; 2) patients had history of other tumors; 3) patients had unresolved nausea, vomiting or diarrhea, interstitial lung disease, liver disease, active bleeding, neurologically unstable central nervous systemic (CNS) disease, and other diseases unproper for this study assessed by researchers.
Treatment
Enrolled patients were treated with crizotinib (250 mg orally twice daily) and bevacizumab (7.5 mg/kg intravenous every three weeks) in continuous 21-day treatment cycles. Treatment continued until disease progression or intolerant toxicity or death or withdraw of consent. The dosage of crizotinib was identical with that of the single drug therapy approved by American Food and Drug Administration. The dosage of bevacizumab was set as 7.5 mg/kg according to previous study, in which bevacizumab was in combination with chemotherapy [18].
Outcomes and assessments
Patients receiving ≥1 cycle of treatment were evaluated. Tumor assessments were performed at baseline (within 28 days before enrollment) and every 6 weeks during treatment. Computed tomography or magnetic resonance imaging was independently assessed by two physicians. Safety evaluations were performed at baseline and every 3 weeks. Clinical responses were assessed by two doctors independently and categorized as either complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to RECIST v1.1. PFS was defined as the time from the start of treatment to the date of disease progression or death of any cause. DOR was defined as the time from first documented PR or CR to the date of first disease progression or death from any cause. ORR was defined as the proportion of patients with CR or PR. DCR was defined as the proportion of patients with CR or PR or SD. Adverse events were classified and graded according to Common Terminology Criteria for Adverse Events version 4.03. Primary end point was PFS, secondary end points were DOR, ORR, DCR and safety.
Statistical analysis
Continuous variables were described as median and 95% confidential interval (CI) or range. Categorical data variables of adverse events were reported as frequencies and percentages. Fisher’s exact test and Wilcoxon rank-sum test were used to compare continuous and categorical variables between two groups, respectively. The Kaplan-Meier method was used to estimate the probability of PFS, DOR and OS. PRISM version 7.0 (GraphPad Software, La Jolla, CA, USA) and SPSS version 20.0 (IBM Corp., Armonk, NY, USA) were used for all statistical analysis. P<0.05 (two-sided) was defined as statistical significance.
Results
Clinical characteristics
A total of 16 patients were enrolled between June 2016 and October 2017, while two patients strongly requested to quit the study and withdrew the consent in 2018. Detailed clinical characteristics of the 14 patients were showed in Table 1. 12 patients harbored ALK rearrangement, 1 patient harbored ROS-1 fusion, and 1 patient harbored c-MET amplification (Figure 1). Of the 14 patients, 8 (57.1%) were female and 6 (42.9%) were male. The median age was 47.5 (Range: 27-65) years old. 4 (28.6%) patients had a history of smoking and 10 (71.4%) patients were non-smokers. 3 (21.4%) patients were staged as locally advanced and 11 (78.6%) were metastatic disease. As for ECOG PS, 12 (85.7%) patients scored 1, 2 (14.3%) scored 2. 1 patient had symptomatic brain metastases and received radiotherapy, 2 patients had asymptomatic brain metastases. 6 (42.9%) patients had pleural effusion at baseline.
Table 1.
Clinical characteristics of the whole patients and mPFS of ALK positive patients
| Characteristics | N (n=14) | mPFS (n=12) |
|---|---|---|
| Age-years | ||
| Median | 47.5 | |
| Range | 27-65 | |
| <60 | 11* | 14.7 |
| ≥60 | 3# | 13.2 |
| Sex | ||
| Female | 8# | 15.2 |
| Male | 6* | 12.6 |
| Smoking | ||
| Yes | 4* | 12.9 |
| No | 10# | 15.0 |
| ECOG | ||
| 1 | 12*,# | 14.7 |
| 2 | 2 | 7.0 |
| Stage of disease | ||
| Locally advanced | 3 | 15.3 |
| Metastatic | 11*,# | 13.2 |
| Pleural effusion | ||
| Yes | 6 | 15.2 |
| No | 8*,# | 12.6 |
| Brain metastases | ||
| Yes | 3 | 13.2 |
| No | 11*,# | 15.2 |
| Gene status | ||
| ALK (+) | 12 | 13.9 |
| ROS-1 (+) | 1# | 12.9 |
| C-MET (+) | 1* | 1.9 |
mPFS: median PFS;
indicated the c-MET (+) patient;
indicated the ROS-1 (+) patient.
Figure 1.
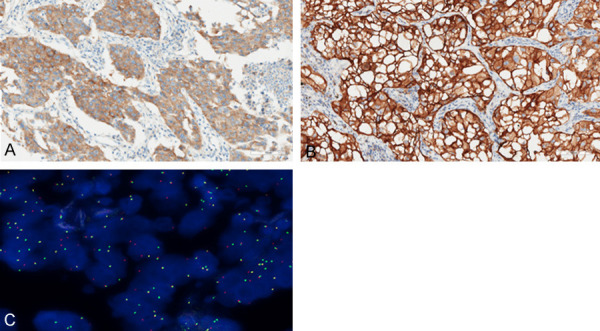
A. The typical IHC image of the ALK (Ventana) (+) patient; B. The IHC image of the c-MET (+++); C. The FISH image of the ROS-1 (+).
Efficacy of crizotinib plus bevacizumab treatment
The median follow-up time was 42.8 months (range: 8.0-44.8 months). Of the 12 patients with ALK rearrangement, the median PFS were 13.9 months (Figure 2) and median DOR was 14.8 months (Figure 3). Median OS was not reached, the 3-year-survival rate was 79.5% (Figure 4). The PFS and OS of the patient with c-MET amplification were 1.9 months and 23.9 months. The PFS, DOR and OS of patient with ROS-1 fusion were 12.9 months, 10.6 months and 27.1 months, respectively.
Figure 2.
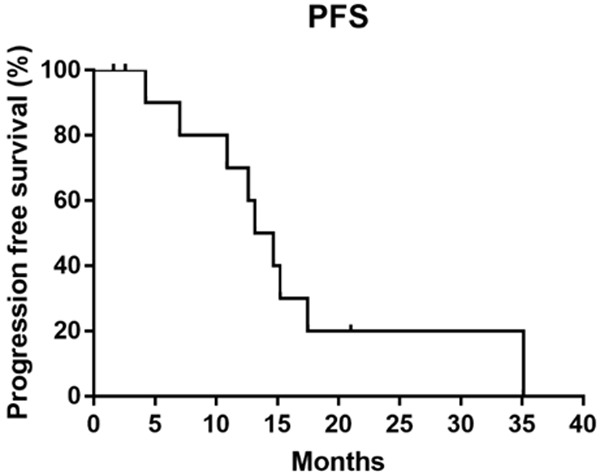
The median PFS of 12 ALK positive patients was 13.9 months.
Figure 3.
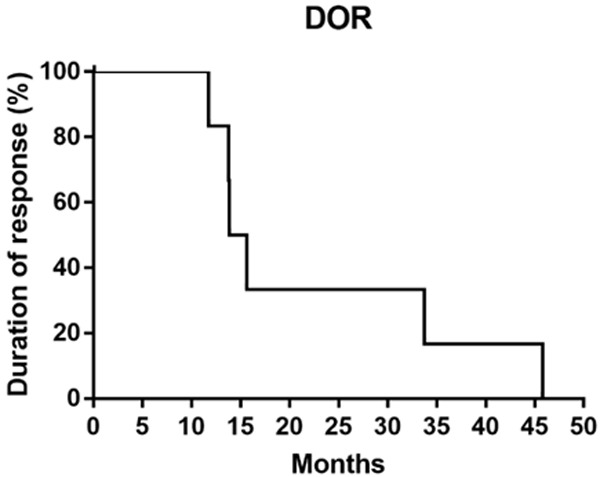
The median DOR of 12 ALK positive patients was 14.8 months.
Figure 4.
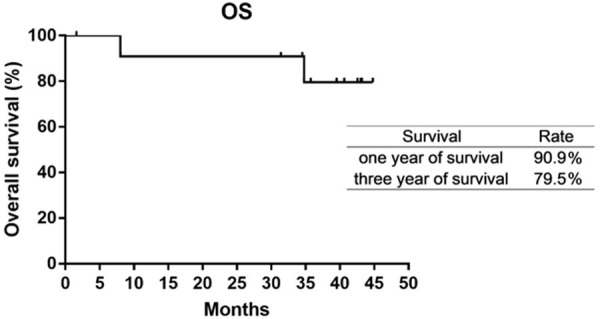
The OS was not reached. The 1-year-survival rate and 3-year-survial rate were 90.9% and 79.5%.
The best percentage changes of target lesions were showed in Figure 5. Tumors of all 14 patients retracted to some extent. 6 patients were evaluated as SD with the tumor retraction <30%, 6 patients obtained a retraction ratio ≥50%. Of the 12 patients with ALK rearrangement, 7 gained PR, and 5 gained SD. The ORR and DCR were 58.3% and 100% respectively (Table 2). The patient with c-MET amplification gained SD, while the patient with ROS-1 fusion gained PR.
Figure 5.
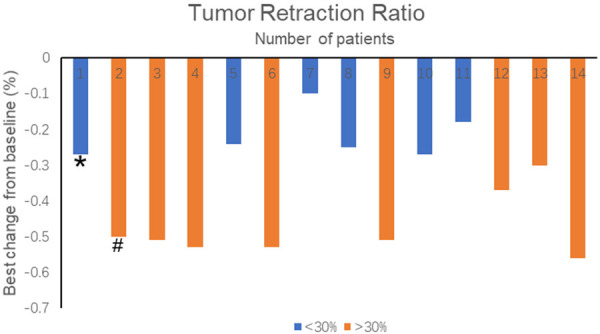
Best percentage change from baseline in tumor volume after treatment. *indicated the c-MET (+) patient and #indicated the ROS-1 (+) patient.
Table 2.
Responses and events in treatment
| Investigator-assessed whole-body responses for all patients (n=14) | |
|---|---|
| Efficacy parameter | N (%) |
| Complete response | 0 (0%) |
| Partial response | 7 (58.3%) |
| Stable disease | 5 (41.7%) |
| Progressive disease | 0 (0%) |
| Reason for trial ending | N (%) |
| Lung PD | 2 (14.3%) |
| Brain PD | 7 (50.0%) |
| Other PD | 1 (7.1%) |
| Adverse effect | 3 (21.4%) |
| not reached | 1 (7.1%) |
PD: progression disease.
To explore the efficacy of crizotinib combined with bevacizumab on patients with brain metastases, causes of disease progression were evaluated. Of the 12 ALK positive patients, 6 (50.0%) developed PD in brain, 2 (16.7%) in lung and 1 (8.3%) in axillary lymph nodes. The median time of PD in brain metastases were 12.9 months (range: 7.0-15.2 months). 3 patients with brain metastases at baseline developed new lesions in brain during treatment. Of the 9 patients without brain metastases at baseline, 3 (33.3%) developed brain metastases. The patient with ROS-1 fusion developed brain metastases and demonstrated severe symptoms during treatment.
In addition, subgroup analysis was performed in ALK positive patients. The median PFS of patients aged <60 years was 14.7 months, while that of those aged ≥60 years was 13.2 months. The median PFS was 15.2 months in female and 12.6 months in male. The median PFS of never smokers was 15.0 months and that of smokers was 12.9 months. The median PFS of locally advanced and metastatic patients were 15.3 months and 13.2 months. The median PFS was 15.2 months in patients with pleural effusion at baseline, and 12.6 months in patients without pleural effusion at baseline. The median PFS of patients without brain metastases at baseline were 15.2 months comparing to 13.2 months in patients with brain metastases at baseline. Due to small sample size in this study, comparisons of subgroups were not conducted.
Safety of crizotinib plus bevacizumab treatment
The most two common treatment-related adverse events were fatigue (28.6%) and rash (21.4%). Other reported adverse events were nausea (14.3%), vomiting (7.1%), edema (7.1%), and pain (7.1%). Most (86.7%) adverse events were grade 1 or 2. 2 patients reported grade 3 or 4 aminotransferase elevation and were not managed well by dosage reduction. Therefore, treatment discontinuation was required for both patients. 1 patient reported grade 1 hemoptysis and dropped out the study. No treatment related death was observed in this study. Interstitial lung disease, active bleeding and hypertension were not reported in this study. All treatment-related adverse events were described in Table 3.
Table 3.
Treatment-related adverse events and grades in patients (n=14)
| Adverse Events | grade 1-2 | grade 3-4 |
|---|---|---|
| Nausea | 2 | 0 |
| Vomiting | 1 | 0 |
| Fatigue | 4 | 0 |
| Edema | 1 | 0 |
| Pain | 1 | 0 |
| Hemoptysis | 1 | 0 |
| Rash | 3 | 0 |
| Aminotransferase elevation | 0 | 2 |
Discussion
Crizotinib or bevacizumab alone has showed clinical benefit in NSCLC patients. However, efficacy of crizotinib in combination with bevacizumab is still elusive. In present study, the median PFS (13.9 vs 10.9 months) and DOR (14.8 vs 11.3 months) of naive ALK positive NSCLC patients receiving crizotinib combined with bevacizumab were longer than those of patients receiving crizotinib alone in PROFILE 1014 study. These results suggested that the combination therapeutic strategy might have better efficacy. However, the ORR in our study was lower than that in PROFILE 1014 study. When compared with a retrospective multicenter cohort study enrolling 273 ALK positive patients treated with crizotinib alone as first-line treatment in China, the median PFS was 13.9 vs 15.5 months, ORR was 58.3% vs 74.1% and DCR was 100% vs 94.8% [19]. Furthermore, our subgroup analysis showed that patients of age <60, female, never smoking, locally advanced stage, and those with pleural effusion or without brain metastasis at baseline tended to have longer PFS. Thomas E. Stinchcombe et al. also revealed that female was associated with better PFS and OS, while patients with baseline ECOG PS of 1 had worse OS [20]. Similar with PROFILE 1014 study, most adverse events were grade 1 or 2, and no treatment related death was reported in our study. Notably, 2 patients discontinued treatment due to grade 3/4 liver damage, a common adverse event in patients treated with crizotinib, suggesting attention to monitoring liver function should be paid. Taken together, this study showed that crizotinib plus bevacizumab was a promising therapeutic strategy in ALK positive NSCLC patients and the toxicity was well tolerable.
Despite crizotinib improved prognosis of NSCLC patients with ALK rearrangement, progression disease in brain was frequent during treatment. A recent retrospective study demonstrated that brain metastases were considerably common at the time of diagnosis and disease progression in NSCLC patients with ALK rearrangement treated with TKIs [21]. Retrospective analysis of the PROFILE 1005 and 1007 studies also reported similar results [22]. Previous study suggested that these might attribute to the insufficient therapeutic exposure dose of crizotinib [23]. The P-glycoprotein overexpression could reduce drug levels in tumor cells [24]. The blood-brain barrier contains P-glycoprotein and other drug efflux transporters preventing crizotinib from entering into brain, which accelerates the CNS progression [25,26]. Kienast Y et al. reported bevacizumab could prevent an early angiogenic switch that was mandatory for brain outgrowth of non-squamous NSCLC cells [27]. Thus, the application of bevacizumab might correct the formation of pathologic micro-vessels and increase the drug concentration in brain. Retrospective analysis of the AVAiL study showed that relapse or metastasis in brain was less frequent in the bevacizumab arm than in the control arm [28]. These findings inferred that bevacizumab might have the potential to prevent brain metastasis. However, in our study, crizotinib plus bevacizumab didn’t show satisfying efficacy in preventing brain metastases, which was probably due to the small sample size in our study.
Abnormal blood vessels at the tumor site were reported to be related with primary resistance through inhibiting drug penetration and result in insufficient drug dose at the target lesion [29,30]. Therefore, addition of anti-angiogenesis drugs pretreatment, such as bevacizumab, might overcome the resistance. Moreover, previous studies suggested that tumors might escape anti-angiogenesis treatment by upregulation of EGFR and FGFR pathways, uncoupled tumor dependency on blood supply through p53 mutation and growth pattern variation [31-33]. VEGF and EGFR signaling pathways were independent but interrelated. It was reported that EGFR pathways modulated angiogenesis by up-regulating VEGF [34]. Thus, the inhibition of angiogenesis via down-regulating VEGF is postulated to contribute to the mechanism of action of EGFR TKIs. Preclinical model identified that EFGR blockades plus VEGF antibody reduced micro-vessel density and metastases [35]. Thus, further molecular mechanism researches on the 7 patients developing brain metastases would be of great help to unveil the reason for crizotinib plus bevacizumab failing in the prevention of brain metastases.
There were some limitations in this study. Firstly, the sample size was small, and only one patient with ROS-1 fusion or c-MET amplification was included. We initially planned to enroll 30 or more patients. However, during the enrollment, second-generation ALK inhibitors, such as alectinib and ceritinib, showed remarkably better efficacy than crizotinib and were approved as first-line treatment. The majority of NSCLC patients harboring ALK rearrangement chose alectinib or ceritinib as first line treatment, which made the enrollment very difficult for our study. In addition, there was no control group in this study. We could only compare the efficacy of crizotinib plus bevacizumab with that of crizotinib alone in previous studies. Thus, cautions on interpretation of our results should be taken because of the heterogeneity and bias between our study and others. However, to our knowledge, this was the first study focusing on the efficacy and safety of crizotinib plus bevacizumab as first-line therapy for ALK/ROS-1/c-MET positive NSCLC.
In conclusion, crizotinib plus bevacizumab is effective and tolerant in treating naive ALK positive NSCLC patients. Our results demonstrated that crizotinib plus bevacizumab might be a promising treatment strategy for ALK/ROS-1/c-MET positive NSCLC patients. Further prospective studies and researches on molecular mechanism are needed to investigate whether and how crizotinib plus bevacizumab prevent brain metastases.
Disclosure of conflict of interest
None.
References
- 1.Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Ganti AK, Govindan R, Grannis FW Jr, Jahan T, Jahanzeb M, Johnson DH, Kessinger A, Komaki R, Kong FM, Kris MG, Krug LM, Le QT, Lennes IT, Martins R, O’Malley J, Osarogiagbon RU, Otterson GA, Patel JD, Pisters KM, Reckamp K, Riely GJ, Rohren E, Simon GR, Swanson SJ, Wood DE, Yang SC Members NN-SCLCP. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 2.Sequist LV, Heist RS, Shaw AT, Fidias P, Rosovsky R, Temel JS, Lennes IT, Digumarthy S, Waltman BA, Bast E, Tammireddy S, Morrissey L, Muzikansky A, Goldberg SB, Gainor J, Channick CL, Wain JC, Gaissert H, Donahue DM, Muniappan A, Wright C, Willers H, Mathisen DJ, Choi NC, Baselga J, Lynch TJ, Ellisen LW, Mino-Kenudson M, Lanuti M, Borger DR, Iafrate AJ, Engelman JA, Dias-Santagata D. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol. 2011;22:2616–2624. doi: 10.1093/annonc/mdr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi YL, Takeuchi K, Soda M, Inamura K, Togashi Y, Hatano S, Enomoto M, Hamada T, Haruta H, Watanabe H, Kurashina K, Hatanaka H, Ueno T, Takada S, Yamashita Y, Sugiyama Y, Ishikawa Y, Mano H. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68:4971–4976. doi: 10.1158/0008-5472.CAN-07-6158. [DOI] [PubMed] [Google Scholar]
- 4.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, Lim Choi Y, Satoh Y, Okumura S, Nakagawa K, Mano H, Ishikawa Y. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 6.Bubendorf L, Lantuejoul S, de Langen AJ, Thunnissen E. Nonsmall cell lung carcinoma: diagnostic difficulties in small biopsies and cytological specimens: number 2 in the series “pathology for the clinician” edited by peter dorfmuller and alberto cavazza. Eur Respir Rev. 2017;26:170007. doi: 10.1183/16000617.0007-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 8.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, Doebele RC, Le LP, Zheng Z, Tan W, Stephenson P, Shreeve SM, Tye LM, Christensen JG, Wilner KD, Clark JW, Iafrate AJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camidge DR, Ou SHI, Shapiro G, Otterson GA, Villaruz LC, Villalona-Calero MA, Iafrate AJ, Varella-Garcia M, Dacic S, Cardarella S, Zhao W, Tye L, Stephenson P, Wilner KD, James LP, Socinski MA. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2014;32:8001–8001. [Google Scholar]
- 10.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, Jessop NA, Wain JC, Yeo AT, Benes C, Drew L, Saeh JC, Crosby K, Sequist LV, Iafrate AJ, Engelman JA. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012;4:120ra117. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou SH, Janne PA, Bartlett CH, Tang Y, Kim DW, Otterson GA, Crino L, Selaru P, Cohen DP, Clark JW, Riely GJ. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25:415–422. doi: 10.1093/annonc/mdt572. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw. 2009;20:158–163. doi: 10.1684/ecn.2009.0170. [DOI] [PubMed] [Google Scholar]
- 13.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 14.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 15.Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, Nakachi I, Gemma A, Azuma K, Kurimoto F, Tsubata Y, Fujita Y, Nagashima H, Asai G, Watanabe S, Miyazaki M, Hagiwara K, Nukiwa T, Morita S, Kobayashi K, Maemondo M. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 16.Yu HA, Schoenfeld AJ, Makhnin A, Kim R, Rizvi H, Tsui D, Falcon C, Houck-Loomis B, Meng F, Yang JL, Tobi Y, Heller G, Ahn L, Hayes SA, Young RJ, Arcila ME, Berger M, Chaft JE, Ladanyi M, Riely GJ, Kris MG. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: a phase 1/2 single-group open-label trial. JAMA Oncol. 2020;6:1048–1054. doi: 10.1001/jamaoncol.2020.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang T, Zhang Y, Li X, Zhao C, Chen X, Su C, Ren S, Yang N, Zhou C. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur J Cancer. 2019;121:98–108. doi: 10.1016/j.ejca.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing P, Ma D, Wang Q, Hao X, Wang M, Wang Y, Shan L, Xin T, Liang L, Liang H, Du Y, Zhang Z, Li J. Impact of crizotinib on long-term survival of -positive advanced non-small-cell lung cancer: a Chinese multicenter cohort study. Chin J Cancer Res. 2019;31:481–488. doi: 10.21147/j.issn.1000-9604.2019.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stinchcombe TE, Jänne PA, Wang X, Bertino EM, Weiss J, Bazhenova L, Gu L, Lau C, Paweletz C, Jaslowski A, Gerstner GJ, Baggstrom MQ, Graziano S, Bearden J, Vokes EE. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1448–1455. doi: 10.1001/jamaoncol.2019.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons CP, Koinis F, Fallon MT, Fearon KC, Bowden J, Solheim TS, Gronberg BH, McMillan DC, Gioulbasanis I, Laird BJ. Corrigendum to “Prognosis in advanced lung cancer - A prospective study examining key clinicopathological factors”[Lung Cancer 88 (2015) 304-309] . Lung Cancer. 2017;108:256. doi: 10.1016/j.lungcan.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, Schnell P, Wilner KD, Wiltshire R, Camidge DR, Crino L. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J. Clin. Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang I, Zaorsky NG, Palmer JD, Mehra R, Lu B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015;16:e510–e521. doi: 10.1016/S1470-2045(15)00013-3. [DOI] [PubMed] [Google Scholar]
- 24.Katayama R, Sakashita T, Yanagitani N, Ninomiya H, Horiike A, Friboulet L, Gainor JF, Motoi N, Dobashi A, Sakata S, Tambo Y, Kitazono S, Sato S, Koike S, John Iafrate A, Mino-Kenudson M, Ishikawa Y, Shaw AT, Engelman JA, Takeuchi K, Nishio M, Fujita N. P-glycoprotein mediates ceritinib resistance in anaplastic lymphoma kinase-rearranged non-small cell lung cancer. EBioMedicine. 2016;3:54–66. doi: 10.1016/j.ebiom.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27(Suppl 3):iii42–iii50. doi: 10.1093/annonc/mdw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusthoven CG, Doebele RC. Management of brain metastases in ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 2016;34:2814–2819. doi: 10.1200/JCO.2016.67.2410. [DOI] [PubMed] [Google Scholar]
- 27.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 28.Ilhan-Mutlu A, Osswald M, Liao Y, Gommel M, Reck M, Miles D, Mariani P, Gianni L, Lutiger B, Nendel V, Srock S, Perez-Moreno P, Thorsen F, von Baumgarten L, Preusser M, Wick W, Winkler F. Bevacizumab prevents brain metastases formation in lung adenocarcinoma. Mol Cancer Ther. 2016;15:702–710. doi: 10.1158/1535-7163.MCT-15-0582. [DOI] [PubMed] [Google Scholar]
- 29.Lovly CM, Shaw AT. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clin Cancer Res. 2014;20:2249–2256. doi: 10.1158/1078-0432.CCR-13-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakasuka T, Ichihara E, Makimoto G, Maeda Y, Kiura K. Primary resistance to alectinib was lost after bevacizumab combined chemotherapy in ALK-rearranged lung adenocarcinoma. J Thorac Oncol. 2019;14:e168–e169. doi: 10.1016/j.jtho.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Cascone T, Herynk MH, Xu L, Du Z, Kadara H, Nilsson MB, Oborn CJ, Park YY, Erez B, Jacoby JJ. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest. 2011;121:1313–1328. doi: 10.1172/JCI42405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joanne LY, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 33.Leenders WP, Küsters B, de Waal RM. Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium. 2002;9:83–87. doi: 10.1080/10623320212006. [DOI] [PubMed] [Google Scholar]
- 34.Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, Radinsky R, Dinney CP. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- 35.Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:1007–21. viii. doi: 10.1016/j.hoc.2004.06.002. [DOI] [PubMed] [Google Scholar]


