Abstract
Small-molecule drugs are organic compounds affecting molecular pathways by targeting important proteins, which have a low molecular weight, making them penetrate cells easily. Small-molecule drugs can be developed from leads derived from rational drug design or isolated from natural resources. As commonly used medications, small-molecule drugs can be taken orally, which enter cells to act on intracellular targets. These characteristics make small-molecule drugs promising candidates for drug development, and they are increasingly favored in the pharmaceutical market. Despite the advancements in molecular genetics and effective new processes in drug development, the drugs currently used in clinical practice are inadequate due to their poor efficacy or severe side effects. Therefore, developing new safe and efficient drugs is a top priority for disease control and curing.
Keywords: Small-molecule drugs, compound, natural resource, molecular genetics, cancer
Small-molecule drugs are organic compounds affecting molecular pathways by targeting important proteins, which have a low molecular weight, making them penetrate cells easily. Small-molecule drugs can be developed from leads derived from rational drug design or isolated from natural resources [1-3]. As commonly used medications, small-molecule drugs can be taken orally, which enter cells to act on intracellular targets [4]. These characteristics make small-molecule drugs promising candidates for drug development, and they are increasingly favored in the pharmaceutical market [5-7]. Despite the advancements in molecular genetics and effective new processes in drug development, the drugs currently used in clinical practice are inadequate due to their poor efficacy or severe side effects. Therefore, developing new safe and efficient drugs is a top priority for disease control and curing. Since the beginning of the 21st century, all basic scientific disciplines have developed rapidly, and the resulting knowledge and techniques have been applied to the medical field [8-11].
This review summarizes the basic approaches of research to discover small-molecule drugs. Research strategies for discovering breakthrough drugs consist of discovery of lead compounds and optimization of lead compounds, each of which play important roles in how these drugs ultimately reach the pharmaceutical market (Figure 1) [12-15].
Figure 1.

Workflow of in silico approaches for small molecule drug discovery. The path leading to the development of a new drug is long and complex, representing the convergence of in silico and in vitro screenings and in vitro and in vivo testing and validation, which highlight the need for a faster track in the procedures for drug development to be met by increasing the in silico part of the process, performing via digital computing a series of time-saving evaluations that can greatly simplify the in vitro procedures.
Discovery of lead compounds
Lead compounds can be obtained from natural sources (e.g., animals, plants, and microorganisms), and their physiological processes as well as drug metabolites produced from exogenous drug applications. Prospective lead compounds can be further screened and optimized via observations of clinical side effects of tested drugs [16-19].
Obtaining lead compounds from natural products
The search for natural products and the comprehensive analysis of their novel structural, chemical, and pharmacological characteristics represent an important pathway for lead compound discovery [20]. According to statistics, approximately half of the clinical drugs currently on the market are derived from natural products and their derivatives [21-23]. For example, morphine was isolated from opium in 1808. In the 1960s, the antibiotic cephalosporin C was obtained from metabolites of cephalosporins, and a series of semi-synthetic antibiotics with considerable antibacterial activities and wide antibacterial spectra were obtained by modifying the side chains of seven amino groups [24].
In 1972, Chinese scientists isolated artemisinin from Artemisia annua L, and, through structural modification, developed artemether, artesunate, and dihydroartemisin, all of which have been shown to exhibit more effective anti-malarial activities than the originally isolated artemisinin [25-27]. In recent years, the United States isolated the anticancer active ingredient, paclitaxel, from the taxol plant, which has been approved for commercial use and is the drug of choice for clinical treatment of ovarian cancer [28].
Obtaining a lead compound from an intermediate
During the synthesis of a drug, many intermediates appear, and the structures of these intermediates and final products are often similar to one another and to the original drug [29]. Screening these compounds to obtain pharmacologically active structures is one way to obtain lead compounds (Figure 2) [30-32]. For example, from the synthesis of the antitumor drug, cytarabine, the intermediate, cyclocytidine, is obtained and also exhibits antitumor activity [33]. Compared with cytarabine, cyclocytidine has additional advantages in terms of its slow metabolism in the body, long duration of action, and minimal effects, all of which have led it to become a clinical drug for leukemia treatment [34-37].
Figure 2.

Overview of various discovery platforms for antibacterial drugs. There is a very low probability for a biologically active compounds to succeed from the pre-clinical to clinical phase of drug discovery. For this reason, reliable discovery platforms are needed to continuously produce compounds with antibacterial activity that may be lead compounds for further studies. The currently defined antibiotic discovery platforms are summarized.
Obtaining lead compounds from basic research
The rapid development of biochemistry, molecular biology, pharmacology, and other related disciplines has provided the basis and means for the improved research and development of small-molecule drugs. This period of development also provided novel targets and lead compounds for the design of small-molecule drugs, such as enzymes, receptors, ion channels, and nucleic acids [38-41]. On this basis, researchers have conducted in-depth studies on the mechanisms of action of enzymes and receptors in the body [42].
According to the structures and performances of drug targets, molecular engineering methods have been used to design various molecules (Figure 3) [43-45]. Enzymatic inhibitors, receptor agonists/antagonists, and channel blockers have all been successfully designed [46-49]. For example, the renin-angiotensin-aldosterone system is active in hypertensive patients, in which angiotensin-converting enzyme (ACE) catalyzes the conversion of angiotensin I to angiotensin II, while angiotensin II can contract vascular smooth muscle and promote aldosterone synthesis to raise blood pressure [50]. By inhibiting ACE, researchers have been able to cut off the production of angiotensin II and have developed a series of drugs, including captopril, enalapril, and fosinopril, to treat hypertension [51].
Figure 3.

Workflow for machine learning in drug discovery. Over the past decade, Machine learning methods have been recognized as the most important tools for extracting chemical compounds with important biological activities from large chemical databases. To understand the physiological and pathological phenomena, it is important to identify ligands that modulate a particular target activity. The main steps of machine learning comprise data collation, chemical descriptor calculation, classifier/model selection, and model validation.
Lead compounds discovered from metabolites
Drug metabolism often produces oxidation, reduction, alkylation, demethylation, and binding reactions [52-55]. If a drug’s metabolites have pharmacological activities, these metabolites can also be used as drugs or after structural modifications of specific functional groups as lead compounds themselves. The ad hoc protection of some functional groups has often led to highly efficacious drugs [56-58]. For example, sulfonamides, highly efficacious lead compounds, have been found in the urine of patients with staphylococcal septicaemia who were treated with prontosil after drug metabolism [59].
Lead compounds found from side effects
When drugs are applied to the human body, the biological activities produced are often diverse. Since drugs are difficult to distribute specifically to target tissues for binding to specific receptors, they have side effects in addition to therapeutic effects [60]. To reduce the side effects of a drug, researchers often carefully observe and systematically study its side effects and metabolism, and then develop new dosage protocols or propose new methods for the drug’s use. For example, vinblastine and vincristine compounds were first used as hypoglycemic drugs, but they were subsequently found to also significantly reduce white blood cells [61]. Further studies have found that vinblastine and vincristine are effective in lymphoblastic leukemia transplantation in mice, and thus, these compounds have since become clinical drugs for leukemia treatment [62].
Lead compounds found by computer-aided design
Computer-aided drug design uses computers as a tool to leverage the existing molecular structures and target information of drugs to guide the directional design of small-molecule drugs through theoretical simulations, calculations, and predictions [63-65]. Using a mathematical-combination method, related structural units are sequentially connected in the form of covalent bonds, and a compound molecular library is then established [66]. The molecular library of compounds obtained by combinatorial chemistry is directly used for large-scale screening. This method far exceeds conventional methods in terms of quantity of leads and has incomparable advantages in synthesis speed [67].
Using this highly efficient, minimal, and highly automated combinatorial chemistry and mass-screening technology, it is possible to screen two million to two billion compounds for a particular molecular target in one to two years [68-71]. The combination of computer-assisted drug design and combinatorial chemistry makes screening more accurate, efficient, and convenient than many other methods. This computer-assisted technology has contributed to a major change in the methodology of small-molecule drug discovery, marking the beginning of a new era in the development of small-molecule drugs [72].
ATG4B has been proposed as a drug target. There is increasing evidence that modulation of ATG4B by either si/shRNA-mediated knockdown or the expression of a dominant negative construct yields beneficial results in multiple cancer models, including breast, pancreatic, and lung cancer. Several ATG4B agonists and inhibitors have been described in the literature, identified either by structure-guided molecular docking of compounds in silico, or by screening chemical libraries of compounds with known activity (Table 1).
Table 1.
In vivo models and potential biomarkers for ATG4B inhibition in cancer
| Cancer Type | Therapeutic Modality | In Vivo Model | Biomarker |
|---|---|---|---|
| Breast cancer | siRNA ATG4B/Trastuzumab | MCF7 xenograft | HER2, ATG4B |
| Colorectal cancer | Tioconazole | HCT-116 Xenograft | none |
| Colorectal cancer | S130/Caloric restriction | HCT-116 Xenograft | none |
| Colorectal cancer | UAMC2526/oxaliplatin | HT-29 Xenograft | LC3 conversion |
| Glioblastoma | NSC185058/Chloroquine | M83 glioma xenograft | none |
| Lung adenocarcinoma | Doxicylcin-inducible ATG4B C74A | GEMM | K-Ras mutation |
| Osteosarcoma | NSC185058/starvation | SAOS Xenograft | none |
| Pancreatic ductal adenocarcinoma | Doxicyclin-inducible ATG4B C74A | GEMM | K-Ras mutation |
| Prostate cancer | ATG4B C74A/doxorubicin | PC-3 Xenograft | none |
Optimization of lead compounds
After the structure of a lead compound is determined, it is often found to have poor pharmacokinetic properties and/or substantial side effects [73]. These adverse reactions often make lead compounds clinically unusable. Therefore, it is often necessary to optimize the structure of the lead compound to improve biological activity, reduce toxicity, increase specificity, and/or improve pharmacokinetic behavior, in order to improve the drug-forming properties of the lead compound and apply it in the clinical setting [74-77].
The primary strategies for lead compound optimization are as follows: (1) changing the metabolic pathway to improve metabolic stability, (2) structural optimization to reduce toxicity risks in drug design, (3) structural modifications to improve water solubility, (4) promoting the passage of compounds through the blood-brain barrier, (5) reducing cardiac toxicity, and (6) improving plasma stability [78-81].
Prodrug modification strategies
Prodrugs, also known as drug precursors or precursor drugs, are compounds with no pharmacological activity that can be metabolized in the body and converted into substances with specific pharmacological activity [82]. Prodrug design involves improving poor metabolic stability, potential toxicity, low water-solubility, and poor blood-brain barrier permeability in target compounds [83-86]. To solve these prodrug problems, the originating drugs are often connected with non-toxic compounds to form new compounds; a new compound may then improve the shortcomings of the original drug. After a new drug is metabolized in the body, it can be decomposed into the active drug and non-toxic compounds through metabolic processes, such as the action of enzymatic or hydrolysis reactions, to exert its efficacy in the body [87-89].
Prodrug modification strategies usually include esterification, acylation, amidation, and other methods [90]. For example, the oral bioavailability of the antibiotic ampicillin is 40%. Polar carboxyl esterification has been used to obtain the prodrug, pivampicillin, which has increased lipophilicity and an oral bioavailability reaching 95% [91]. Additionally, clinical application of clopidogrel has shown that its cardiotoxicity risk is associated with clopidogrel resistance [92]. A prodrug strategy was used to modify clopidogrel thiolactone, the metabolic intermediate of clopidogrel, to obtain vicagrel. Specifically, vicagrel does not need CYP2C19 to metabolize into the thiolactone form, which can effectively reduce clopidogrel resistance [93-96].
At the same time, the toxicity risk has been shown to be greatly reduced due to the lower effective dose of vicagrel. As another example, a dual Src/Abl inhibitor, pyrazolo[3,4-d]pyrimidine derivative, has been shown to have nanomolar-level activity at both Src and Abl enzymatic levels, but its cellular activity is not high, which is probably due to poor water solubility (only 0.05 mg ml-1) [97]. The solubility of an acylated prodrug was 600 times higher, and the corresponding level of cellular activity also significantly improved [98].
A γ-secretase inhibitor and N-methyl dihydropyridine fragment were combined to form a chemical delivery system prodrug. After administration, the 2 h brain concentration reached 345 ng·g-1, which was about 1.5 times that of the original compound (240 ng·g-1) [99]. Therefore, prodrug modification by chemical-delivery systems can effectively improve the cerebral permeability of compounds, and increase their intracerebral concentration [100-102].
Strategies for changing a compound’s lipid solubility
Most metabolic enzymes in the body have active pockets that bind to lipophilic groups. By reducing a compound’s lipophilic properties, the binding activity between compounds and metabolic enzymes is weakened, thus delaying the metabolism of compounds in vivo and improving metabolic stability [103-105]. The anti-thrombin factor Xa inhibitor, developed by the Takeda company of Japan, has a strong inhibitory activity of FXa (IC50 = 28 nmol·L-1) [106]. Although this compound has strong biological activity, it has a very high elimination rate in human liver microsomes, reaching 91.2% [107].
By replacing the seven-membered lactam ring with a six-membered and five-membered lactam ring, the anti-thrombin factor Xa inhibitor has reduced fat solubility, a reduced elimination rate, and an increased activity; when the R group was replaced by a six-member cyclourea group, the obtained compound, TAK-442, had the strongest inhibitory activity and the best metabolic stability of any known anti-thrombin factor Xa derivatives, and is currently being tested in a phase-II clinical study [108].
As another example, a powerful phosphodiesterase inhibitor, PDE4D, developed by Novartis, has a solubility of only 2.3 μg·mL-1, and bioavailability of only 8% in rats due to its large aromatic conjugate region [109,110]. Researchers succeeded in replacing one of its benzene rings with a cyclohexane or piperidine ring, while removing the other oxadiazole ring and introducing various substituents [111]. The solubility of the resulting compounds improved greatly. PDE4D is an antibacterial drug with good bacteriostatic activity and a strong inhibitory effect on topoisomerase IV [112]. The inhibitory activity (IC50) of hERG is 3 μmol-1. Other compounds were obtained by replacing quinolines with quinolones with greater polarity. The lipid-soluble logD7.4 decreased by 0.6-1.6 units, and hERG inhibition decreased significantly (IC50 > 30 μmol-1) [113]. Through quantitative structure-activity relationship analysis, Levoin has been shown to have a lipid solubility (clogP, clogD, or polar surface area PSA) and aromatic properties that are closely related to hERG inhibitory activity [114]. The fat-soluble aromatic ring in the drug molecule generates a π-π hydrophobic effect with the hERG potassium channel. Reducing liposolubility of molecules, through methods such as introducing electron-withdrawing groups or polar groups on aromatic rings of drug molecules, or replacing benzene rings with heterocyclic rings through bioisosterism, can effectively hinder the hydrophobic effect and reduce hERG-inhibiting activity [115].
Adenosine receptor A2A antagonists can be used to treat Parkinson’s disease [116]. The A2A antagonist lead compound reported by Merck (IC50 = 5.5 nmol·L-1) has a good selectivity to adenosine receptor A1 but has strong hERG inhibitory activity (IC50 = 1.5 μmol·L-1) [117]. In terms of useful strategies for liposolubility reduction, compounds a and b were obtained by replacing the end benzene ring with pyrazole, and the lipid-soluble clogP of the resulting compounds decreased by 1.9 and 0.7 units, respectively, whereas hERG inhibitory activity decreased significantly (IC50 > 60 μmol·L-1) while maintaining affinity with A2A receptors and selectivity to A1 receptors [118].
Broad-spectrum antimicrobial agents have good bacteriostatic activity and have a strong inhibitory effect on topoisomerase IV. Their hERG inhibitory activity (IC50) is 3 μmol·L-1 [119]. When new compounds were obtained by replacing quinolines with quinolones with greater polarity, lipid-soluble logD7.4 decreased, and hERG inhibitory activity decreased significantly [120].
Bioisosterism strategies
Many compounds contain metabolizable groups. One important strategy to improve metabolic stability, reduce potential toxicity, and improve plasma stability of a compound is the principle of bioisosterism, in which metabolizable groups are replaced with stable bioisosteres [121-123]. The compound 5-(2,8-bis(trifluoromethyl)quinolin-4-yloxymethyl)isoxazole-3-carboxylic acid ethyl ester has activity against Mycobacterium tuberculosis (minimum inhibitory concentration [MIC] = 0.9 μmol·L-1), and the compound is prone to being deactivated by CYP catalytic oxidation and ester hydrolysis [124].
To effectively reduce the compound’s oxidative metabolic rate, the linking group between quinoline and isoxazole is structurally optimized, containing a trans-double bonded compound [125]. A bioisosterism strategy not only enhances antibacterial activity, but also improves pharmacokinetics. The liver metabolites of nimesulide, especially nitroreductive products, are closely related to nimesulide’s toxicity [126]. Aromatic nitrogroup products can be oxidized into quinone imines and electrophilic products by the P450 enzyme and monoamine oxidase in human liver microbodies [127]. Electrophilic products can further covalently bind to some strong nucleophilic substances (such as proteins and DNA) in the body, which may lead to the production of hepatotoxicity.
In subsequent research on the modification of nimesulide, it was found that arranging the nitrobenzene structure into a pyridine ring structure improved enzymatic activity and selectivity, and also maintained anti-inflammatory activity in rats [128]. The successful application of this strategy avoids aromatic nitro structures and significantly reduces the expected toxicity risk of lead compounds. Novel P2X7 receptor antagonists have been developed by Roche Pharmaceuticals. This lead compound is unstable in human plasma, causing 50% of the prototype drug to be degraded after 4 h of plasma incubation [129]. Therefore, the researchers replaced the urea group in the compound structure with an amide group to obtain a new compound that greatly improved plasma stability while maintaining its inhibitory activity on P2X7 receptors (IC50 = 23 nmol·L-1) [130].
In the process of drug discovery, we often encounter problems with lead compounds, such as poor medicinal properties, poor pharmacokinetic characteristics, toxic effects, and side effects. To improve the drug potency of lead compounds and to accelerate the process of new drug development, structural optimization of lead compounds has become a key link in current drug development [131-134]. Effective approaches to optimize lead compound structure include the rational use of closed metabolic sites, skeleton modifications, reduction of vigilant structural reactivity, chiral changes and methyl strategies to change conformation and increase molecular rigidity to achieve conformational restriction, as well as changing the water-solubility and fat-solubility of lead [135,136]. The flexible application of the above methods can improve the pharmacokinetic characteristics of lead compounds, prolong the time of drug action in vivo, enhance metabolic stability, improve bioavailability, reduce hERG inhibitory activity, reduce adverse effects on the heart, and reduce adverse effects on the liver, kidneys, and other organs [137-139].
Research and development of mimetic small-molecule drugs
Imitative small-molecule drugs are also known as “me-too” drugs. This kind of drug is used to conduct full research on the pharmacological, toxicological, metabolic, and clinical effects and mechanisms of known drugs. Imitative small molecule drugs are then used as the lead compound for structural modifications to obtain new drugs [140-142]. In addition, new chemical entities with the same mechanism of action, or with similar or enhanced effects or certain characteristics, are developed to avoid patent protection of the original drug [143]. This innovative approach to research and develop small-molecule drugs is known as the “me-too” strategy. In small-molecule drug research and development, the “me-too” strategy is carried out from three aspects: bioelectronic-equivalent row replacement, prodrug design, and chiral drug research [144-146].
Replacement with bioelectronic isosteres
The number of electrons in the outermost layer of elements within the same family in the periodic table is equal. Additionally, the physical and chemical properties of elements within the same family are similar to one another. This relationship is extended to atoms, ions, or molecules of equal outer electrons, which are called electron isosteres [147]. When the physical and chemical properties of the atoms, groups, or molecules under consideration are associated with biological activities, groups with similar physical and chemical properties and the same valence bonds capable of producing similar biological activities are called bioisosteres [148-150]. For example, replacing the imidazole ring with a furan ring and thiazole ring yields ranitidine and famotidine, respectively, and the H2 receptor antagonism of these drugs is stronger than that of cimetidine [151]. The use of bioisosteres to replace a group of lead compounds one by one to obtain a series of new compounds is a classic method for pharmaceutical chemists to study drugs [152].
Prodrug design
A prodrug refers to a compound that has little or no activity in vitro and releases an active substance after the action of an enzyme or non-enzyme in the body to ultimately produce a pharmacological effect [153]. For example, lactam antibiotics have a carboxyl group in position 2, and due to strong polarity and acidity, oral absorption is poor [154]. Ampicillin, in the form of ions in the gastrointestinal tract, has a bioavailability of only 20%-30% [155]. Application of a prodrug design to yield pivampicillin, which is obtained by esterification of carboxylic acid, yields increased fat solubility and an improved bioavailability of 95%. Pivampicillin has an in vivo antibacterial effect that is two to four times greater than that of ammonia benzyl ester, and yields a high blood drug concentration and short half-life [156]. When carbenicillin is taken orally, it is easy broken down by gastric acid in the stomach, leading to an unstable drug effect. The carboxyl esterification of the side chain to carindacillin yields a drug that is not easily decomposed by gastric acid, and thus can be taken orally, with significant improvement in bioavailability [157].
Chiral drug design
Stereochemistry has occupied a great role in the manufacture and development of pharmaceuticals. Chiral properties play an important role in the determination of pharmacological actions of drugs [158]. In recent years, there has been considerable interest in chiral separation to isolate and examine both enantiomers. For example, the specific drug, omeprazole, developed by Astmzenaca for the treatment of gastric ulcers, is the world’s first proton-pump inhibitor that has undergone clinical application [159]. The S atom in the omeprazole molecule is an asymmetric atom. The l-isomer (esomeprazole) obtained through chiral design of the drug has a slow metabolism in the body and is repeatedly generated through internal circulation, leading to a higher blood concentration and longer maintenance time [160-163].
Known small-molecule drug-extension research and development
To shorten development cycles, reduce risks, and improve the success rates of small-molecule drugs, the “new use of old medicine” has attracted increasing attention from researchers [164]. “New use of old medicine” refers to the development of new indications, or new uses, of drugs that have been marketed for other purposes previously [165]. Owing to the detailed pharmacokinetics and safety data of drugs already on the market, the development of new applications can be quickly evaluated in phase-II clinical trials, which can save approximately 40% on the costs of research and development and can shorten the development cycle [166-168]. For example, aspirin has expanded from a conventional anti-inflammatory analgesic to a small-molecule drug that can dilute blood, prevent thrombosis, and reduce the incidence of stroke [169]. The original methoxypyrimidine, for the treatment of pneumonia, was approved for the treatment of AIDS [170].
The pregnancy drug mifepristone has since been approved for severe psychiatric depression. Thalidomide is a synthetic glutamic acid derivative that was once called a reaction stop, and has a calming and antiemetic effect [171]. In 1998, thalidomide was approved by the FDA for the treatment of ENL. In 2006, the FDA approved its combination with dexamethasone for the treatment of multiple myeloma (MM) [172]. Metformin, a biguanide compound from Galega officinalis, has been used to treat hyperglycemia since the 1950s and is one of the most widely prescribed diabetic drugs. Numerous studies have shown that metformin, in addition to being used in the treatment of diabetes, can also be used to treat off-label diseases such as polycystic ovary syndrome, nonalcoholic steatohepatitis, and HIV-related metabolic abnormalities [173].
In tumor therapy, the mechanism of metformin is becoming increasingly clear, and the application of metformin in the field of non-diabetic diseases is increasing. A series of promising early clinical trials are underway, making it highly likely that this classic drug will be turned into a novel anticancer drug [174].
New technology for small-molecule drug discovery
Drug discovery in the 1960s and 1970s relied primarily on cell and animal models that mostly used phenotypic screenings [175-177]. After entering the 21st century, with the rapid development of computational biology, bioinformatics, molecular biology, and chemical biology, the molecular targets of small-molecule drugs have since been elucidated [175-177]. The Nikolovska-Coleska lab conducted a high throughput screening (HTS) campaign with a library of 53,000 synthetic small molecules to identify Mcl-1 inhibitors, which led to the identification of UMI-59 (Ki = 1.55 μM). This molecule inhibited Mcl-1/Bid interaction and displayed greater selectivity toward Mcl-1 compared to other Bcl-2 proteins in the FP assay. Additionally, the genome project and proteome project are new approaches for drug design. The application of computational biology, bioinformatics, molecular biology, and chemical biology makes it more efficient to discover novel targets for drug action [178].
Methods such as selecting targets for important diseases, designing lead compounds, and optimizing lead structures using combinatorial chemistry and high-throughput screening methods have recently been developed [179-181]. In 2009, the discovery and subsequent clinical trials of an NAE inhibitor, MLN4924 (pevonedistat), set a milestone that validated the neddylation pathway as an effective anticancer target. Thereafter, there has been continuous effort to seek more neddylation inhibitors.
Now, a decade later, high-throughput screening, virtual screening, as well as structural-based design have yielded a diverse collection of small-molecule inhibitors of neddylation, and some have shown promising anticancer activities (Figure 4). Therefore, high-throughput screening, virtual screening, structure-based drug design, and optimization of lead compounds have become common techniques for small-molecule drug discovery.
Figure 4.
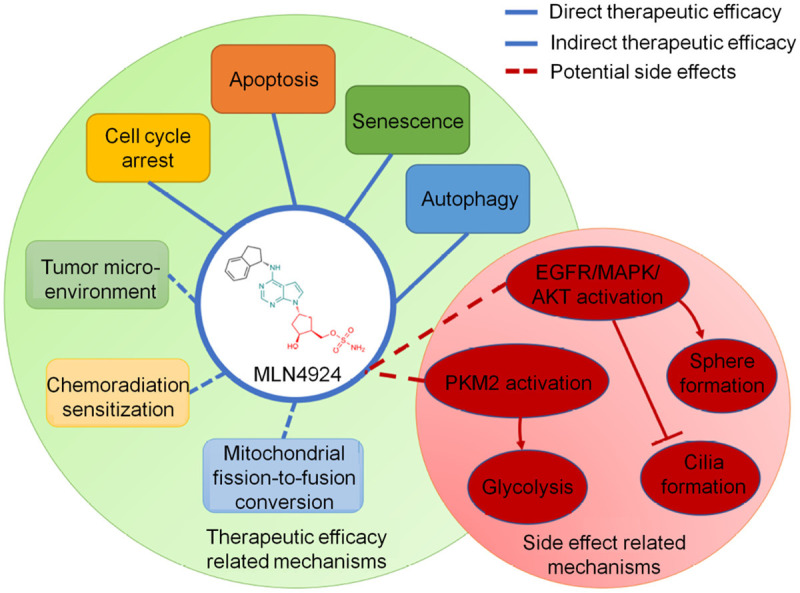
The first-in-class NAE inhibitor, MLN4924. A scheme of the mechanisms of MLN4924 regarding to its therapeutic efficacy and side effect.
Outlook
In conclusion, the creation of novel structures and biologically active drugs via the basic pathways of small-molecule drug discovery and the instant identification of new chemical entities (NCEs) are highly respected and lucrative approaches in the medical community. However, this kind of small-molecule drug research is difficult to develop, and the risks are often great. Further research and development of known drugs can extend to small-molecule drug research and development. In the establishment of a compound library, high-throughput screening based on a certain molecular target can obtain the complex crystal structure of small molecules and target proteins, and many classic drugs can be exploited to have novel uses for the treatment of other diseases and disorders. Once life science enters the post-genomic era, scientists will be able to find and discover new genes from a large number of gene-sequencing results and deeply study their functions and regulatory networks. Such an approach will further improve the quality and efficiency of innovative drug research through a large number of bioinformational databases, compound-information databases, biochips, and other high-tech technologies. However, human biology is so complex that drug discovery has not been as efficient as might be expected. The cost and efficiency of clinical trials are rate-limiting steps in drug discovery. Due to these factors, the significance of new technologies in drug discovery can be leveraged in terms of the following three strategies: (1) if a drug target is correct, find the most effective regulation mode and molecule; (2) demonstrate the effectiveness of the target as early as possible; and (3) take advantage of the fact that effective drugs can be found even when a clear target is not identified.
Acknowledgements
We would like to thank Prof. Wei Lv for data analysis and critical discussion of the manuscript. This study was supported partly by grants from the National Natural Science Foundation of China (81972214, 81772932, 81472202, 81201535, 81302065, 81671716, 81372175 and 81472209), Natural Science Foundation of Hunan Province of China (2020WK2020), Shanghai Natural Science Foundation (20ZR1472400), Wu Jieping Medical Foundation (320.6750.14326) and Construction of Clinical Medical Center for Tumor Biological Samples in Nantong (HS2016004).
Disclosure of conflict of interest
None.
References
- 1.Ibarrola-Villava M, Cervantes A, Bardelli A. Preclinical models for precision oncology. Biochim Biophys Acta Rev Cancer. 2018;1870:239–246. doi: 10.1016/j.bbcan.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Raju G, Pavitra E, Merchant N, Lee H, Prasad G, Nagaraju G, Huh Y, Han Y. Targeting autophagy in gastrointestinal malignancy by using nanomaterials as drug delivery systems. Cancer Lett. 2018;419:222–232. doi: 10.1016/j.canlet.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Long Q, Fu D, Zhu D, Ji Y, Han L, Zhang B, Xu Q, Liu B, Li Y, Wu S, Yang C, Qian M, Xu J, Liu S, Cao L, Chin Y, Lan E, Coppé J, Sun Y. Targeting SPINK1 in the damaged tumour microenvironment alleviates therapeutic resistance. Nat Commun. 2018;9:4315. doi: 10.1038/s41467-018-06860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abou-El-Enein M, Grainger D, Kili S. Registry contributions to strengthen cell and gene therapeutic evidence. Mol Ther. 2018;26:1172–1176. doi: 10.1016/j.ymthe.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17:147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacAskill M, Saif J, Condie A, Jansen M, MacGillivray T, Tavares A, Fleisinger L, Spencer H, Besnier M, Martin E, Biglino G, Newby D, Hadoke P, Mountford J, Emanueli C, Baker A. Robust revascularization in models of limb ischemia using a clinically translatable human stem cell-derived endothelial cell product. Mol Ther. 2018;26:1669–1684. doi: 10.1016/j.ymthe.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vi L, Baht G, Soderblom E, Whetstone H, Wei Q, Furman B, Puviindran V, Nadesan P, Foster M, Poon R, White J, Yahara Y, Ng A, Barrientos T, Grynpas M, Mosely M, Alman B. Macrophage cells secrete factors including LRP1 that orchestrate the rejuvenation of bone repair in mice. Nat Commun. 2018;9:5191. doi: 10.1038/s41467-018-07666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, Huang K, Jie Z, Wu Y, Chen J, Chen Z, Fang X, Shen S. CircFAT1 sponges miR-375 to promote the expression of Yes-associated protein 1 in osteosarcoma cells. Mol Cancer. 2018;17:170. doi: 10.1186/s12943-018-0917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Deng X, Yu C, Zhao G, Zhou J, Zhang G, Li M, Jiang D, Quan Z, Zhang Y. Synergistic inhibitory effects of capsaicin combined with cisplatin on human osteosarcoma in culture and in xenografts. J Exp Clin Cancer Res. 2018;37:251. doi: 10.1186/s13046-018-0922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Tsai H, Cheng Y, Lin C, Huang Y, Tsai C, Xu G, Wang S, Fong Y, Tang C. CTGF promotes osteosarcoma angiogenesis by regulating miR-543/angiopoietin 2 signaling. Cancer Lett. 2017;391:28–37. doi: 10.1016/j.canlet.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Herrera M, Llorens C, Rodríguez M, Herrera A, Ramos R, Gil B, Candia A, Larriba MJ, Garre P, Earl J, Rodríguez-Garrote M, Caldés T, Bonilla F, Carrato A, García-Barberán V, Peña C. Differential distribution and enrichment of non-coding RNAs in exosomes from normal and Cancer-associated fibroblasts in colorectal cancer. Mol Cancer. 2018;17:114. doi: 10.1186/s12943-018-0863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Song P, Jiang T, Dai D, Wang H, Sun J, Zhu L, Xu W, Feng L, Shin VY, Morrison H, Wang X, Jin H. Heat shock factor 1 epigenetically stimulates glutaminase-1-dependent mTOR activation to promote colorectal carcinogenesis. Mol Ther. 2018;26:1828–1839. doi: 10.1016/j.ymthe.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan G, Shao B, Liu Q, Zeng Y, Fu C, Chen A, Chen Q. circFMN2 sponges miR-1238 to promote the expression of LIM-homeobox Gene 2 in prostate cancer cells. Mol Ther Nucleic Acids. 2020;21:133–146. doi: 10.1016/j.omtn.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Parry A, Hoare M, Bihary D, Hänsel-Hertsch R, Smith S, Tomimatsu K, Mannion E, Smith A, D’Santos P, Russell I, Balasubramanian S, Kimura H, Samarajiwa S, Narita M. NOTCH-mediated non-cell autonomous regulation of chromatin structure during senescence. Nat Commun. 2018;9:1840. doi: 10.1038/s41467-018-04283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Huang T, Zhong X, Zhang H, Cong X, Xu H, Lu G, Yu F, Xue S, Lv Z, Fu D. Proteogenomic characterization and comprehensive integrative genomic analysis of human colorectal cancer liver metastasis. Mol Cancer. 2018;17:139. doi: 10.1186/s12943-018-0890-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng D, Ye Z, Wu J, Zhou R, Fan X, Wang G, Huang Y, Wu J, Sun H, Wang M, Bin J, Liao Y, Li N, Shi M, Liao W. Macrophage correlates with immunophenotype and predicts anti-PD-L1 response of urothelial cancer. Theranostics. 2020;10:7002–7014. doi: 10.7150/thno.46176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw B, Cheng D, Acharya C, Ettenger R, Lyerly H, Cheng Q, Kirk A, Chambers E. An age-independent gene signature for monitoring acute rejection in kidney transplantation. Theranostics. 2020;10:6977–6986. doi: 10.7150/thno.42110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao X, Wang W, Li Y, Yang D, Li X, Shen C, Liu Y, Ke X, Guo S, Guo Z. HSP90AA1-mediated autophagy promotes drug resistance in osteosarcoma. J Exp Clin Cancer Res. 2018;37:201. doi: 10.1186/s13046-018-0880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laissue P. The forkhead-box family of transcription factors: key molecular players in colorectal cancer pathogenesis. Mol Cancer. 2019;18:5. doi: 10.1186/s12943-019-0938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadremomtaz A, Ali A, Jouyandeh F, Balalaie S, Navari R, Broussy S, Mansouri K, Groves M, Asghari S. Molecular docking, synthesis and biological evaluation of Vascular Endothelial Growth Factor (VEGF) B based peptide as antiangiogenic agent targeting the second domain of the Vascular Endothelial Growth Factor Receptor 1 (VEGFR1D2) for anticancer application. Signal Transduct Target Ther. 2020;5:76. doi: 10.1038/s41392-020-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su R, Cao S, Ma J, Liu Y, Liu X, Zheng J, Chen J, Liu L, Cai H, Li Z, Zhao L, He Q, Xue Y. Knockdown of SOX2OT inhibits the malignant biological behaviors of glioblastoma stem cells via up-regulating the expression of miR-194-5p and miR-12. Mol Cancer. 2018;16:171. doi: 10.1186/s12943-017-0737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Niu M, Liu Y, Tang J, Chen W, Qian G, Zhang M, Shi Y, Lin J, Li X, Li R, Xiao X, Li G, Wang J. Screening for susceptibility-related factors and biomarkers of Xianling Gubao capsule-induced liver injury. Front Pharmacol. 2020;11:810. doi: 10.3389/fphar.2020.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Y, Wang C, Becker S, Hurst K, Nogueira L, Findlay V, Camp E. miR-145 antagonizes SNAI1-mediated stemness and radiation resistance in colorectal cancer. Mol Ther. 2018;26:744–754. doi: 10.1016/j.ymthe.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Yang S. Multispecific drugs: the fourth wave of biopharmaceutical innovation. Signal Transduct Target Ther. 2020;5:86. doi: 10.1038/s41392-020-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckert E, Nace R, Tonne J, Evgin L, Vile R, Russell S. Generation of a tumor-specific chemokine gradient using oncolytic vesicular stomatitis virus encoding CXCL9. Mol Ther Oncolytics. 2019;16:63–74. doi: 10.1016/j.omto.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abba M, Patil N, Leupold J, Saeed M, Efferth T, Allgayer H. Prevention of carcinogenesis and metastasis by Artemisinin-type drugs. Cancer Lett. 2018;429:11–18. doi: 10.1016/j.canlet.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Wang Z, Ye C, Zhao B, Li Z, Yang Y. RNA sequencing reveals the expression profiles of circRNA and indicates that circDDX17 acts as a tumor suppressor in colorectal cancer. J Exp Clin Cancer Res. 2018;37:325. doi: 10.1186/s13046-018-1006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng L, Meng T, Chen L, Wei W, Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. 2020;5:11. doi: 10.1038/s41392-020-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besnier M, Gasparino S, Vono R, Sangalli E, Facoetti A, Bollati V, Cantone L, Zaccagnini G, Maimone B, Fuschi P, Silva D, Schiavulli M, Aday S, Caputo M, Madeddu P, Emanueli C, Martelli F, Spinetti G. miR-210 enhances the therapeutic potential of bone-marrow-derived circulating proangiogenic cells in the setting of limb ischemia. Mol Ther. 2018;26:1694–1705. doi: 10.1016/j.ymthe.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Fernández Y, Brown H, Patiño-García A, Heymann D, Blanco-Prietoac M. Oral administration of edelfosine encapsulated lipid nanoparticles causes regression of lung metastases in pre-clinical models of osteosarcoma. Cancer Lett. 2018;430:193–200. doi: 10.1016/j.canlet.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Gushchina L, Bhattacharya S, McElhanon K, Choi J, Manring H, Beck E, Alloush J, Weisleder N. Treatment with recombinant human MG53 protein increases membrane integrity in a mouse model of limb girdle muscular dystrophy 2B. Mol Ther. 2017;25:2360–2371. doi: 10.1016/j.ymthe.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia W, Zhen M, Li L, Zhou C, Sun Z, Liu S, Zhao Z, Li J, Wang C, Bai C. Gadofullerene nanoparticles for robust treatment of aplastic anemia induced by chemotherapy drugs. Theranostics. 2020;10:6886–6897. doi: 10.7150/thno.46794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki S, Kobunai T, Kitayama J, Nagawa H. DNA methylation and sensitivity to antimetabolites in cancer cell lines. Oncol Rep. 2008;19:407–412. [PubMed] [Google Scholar]
- 34.Tzelepis K, De Braekeleer E, Aspris D, Barbieri I, Vijayabaskar M, Liu W, Gozdecka M, Metzakopian E, Toop H, Dudek M, Robson S, Hermida-Prado F, Yang Y, Babaei-Jadidi R, Garyfallos D, Ponstingl H, Dias J, Gallipoli P, Seiler M, Buonamici S, Vick B, Bannister A, Rad R, Prinjha R, Marioni J, Huntly B, Batson J, Morris J, Pina C, Bradley A, Jeremias I, Bates D, Yusa K, Kouzarides T, Vassiliou G. SRPK1 maintains acute myeloid leukemia through effects on isoform usage of epigenetic regulators including BRD4. Nat Commun. 2018;9:5378. doi: 10.1038/s41467-018-07620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escobar G, Barbarossa L, Barbiera G, Norelli M, Genua M, Ranghetti A, Plati T, Camisa B, Brombin C, Cittaro D, Annoni A, Bondanza A, Ostuni R, Gentner B, Naldini L. Interferon gene therapy reprograms the leukemia microenvironment inducing protective immunity to multiple tumor antigens. Nat Commun. 2018;9:2896. doi: 10.1038/s41467-018-05315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Qin W, Huo Y, Li X, Shi Q, Rasko J, Janin A, Zhao W. Advances in targeted therapy for malignant lymphoma. Signal Transduct Target Ther. 2020;5:15. doi: 10.1038/s41392-020-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stankovicová M, Rauko P, Bachratá M, Blesová M, Sveda P. In vitro antileukemic activity and chemical transformation of the 5’-chloro-5’-deoxy derivative of cyclocytidine. Neoplasma. 1995;42:255–258. [PubMed] [Google Scholar]
- 38.Shastri A, Choudhary G, Teixeira M, Gordon-Mitchell S, Ramachandra N, Bernard L, Bhattacharyya S, Lopez R, Pradhan K, Giricz O, Ravipati G, Wong L, Cole S, Bhagat T, Feld J, Dhar Y, Bartenstein M, Thiruthuvanathan V, Wickrema A, Ye B, Frank D, Pellagatti A, Boultwood J, Zhou T, Kim Y, MacLeod A, Epling-Burnette P, Ye M, McCoon P, Woessner R, Steidl U, Will B, Verma A. Antisense STAT3 inhibitor decreases viability of myelodysplastic and leukemic stem cells. J Clin Invest. 2018;128:5479–5488. doi: 10.1172/JCI120156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kryeziu K, Bruun J, Guren T, Sveen A, Lothe R. Combination therapies with HSP90 inhibitors against colorectal cancer. Biochim Biophys Acta Rev Cancer. 2019;1871:240–247. doi: 10.1016/j.bbcan.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Chen E, Yang F, He H, Li Q, Zhang W, Xing J, Zhu Z, Jiang J, Wang H, Zhao X, Liu R, Lei L, Dong J, Pei Y, Yang Y, Pan J, Zhang P, Liu S, Du L, Zeng Y, Yang J. Alteration of tumor suppressor BMP5 in sporadic colorectal cancer: a genomic and transcriptomic profiling based study. Mol Cancer. 2018;17:176. doi: 10.1186/s12943-018-0925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Wang C, Becker S, Hurst K, Nogueira L, Findlay V, Camp E. miR-145 Antagonizes SNAI1-mediated stemness and radiation resistance in colorectal cancer. Mol Ther. 2018;26:744–754. doi: 10.1016/j.ymthe.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalerba P, Dylla S, Park I, Liu R, Wang X, Cho R, Hoey T, Gurney A, Huang E, Simeone D, Shelton A, Parmiani G, Castelli C, Clarke M. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25:1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullis P, Hope M. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chuang Y, Chu C, Cheng S, Liao L, Chu T, Chen N, Paldino A, Hsia Y, Chen C, Lo W. Annealing-modulated nanoscintillators for nonconventional X-ray activation of comprehensive photodynamic effects in deep cancer theranostics. Theranostics. 2020;10:6758–6773. doi: 10.7150/thno.41752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Y, Lv Z, Yu F, Chang Z, Cong X, Zhong X, Lu G, Zhu J, Fu D. MiRNA-302a/d inhibits the self-renewal capability and cell cycle entry of liver cancer stem cells by targeting the E2F7/AKT axis. J Exp Clin Cancer Res. 2018;37:252. doi: 10.1186/s13046-018-0927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma J, Kala S, Yung S, Chan T, Wong A. Blocking stemness and metastatic properties of ovarian cancer cells by targeting p70S6K with dendrimer nanovector-based siRNA delivery. Mol Ther. 2018;26:70–83. doi: 10.1016/j.ymthe.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng S, Deng Q, Zhang Y, Ye H, Yu X, Zhang Y, Han G, Luo P, Wu M, Yu Y, Han W. Non-platelet-derived CXCL4 differentially regulates cytotoxic and regulatory T cells through CXCR3 to suppress the immune response to colon cancer. Cancer Lett. 2019;443:1–12. doi: 10.1016/j.canlet.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Yu F, Liu J, Wu Z, Xie W, Zhong X, Hou L, Wu W, Lu H, Jiang X, Jiang J, Cao Z, Cong G, Shi M, Jia C, Lu G, Song Y, Chai L, Lv Z, Wu C, Ma Y, Fu D. Tumor suppressive microRNA-124a inhibits stemness and enhances gefitinib sensitivity of non-small cell lung cancer cells by targeting ubiquitin-specific protease 14. Cancer Lett. 2018;427:74–84. doi: 10.1016/j.canlet.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Zhang F, Yu G, Wang Z, Chen X. Zwitterionic-to-cationic charge conversion polyprodrug nanomedicine for enhanced drug delivery. Theranostics. 2020;10:6629–6637. doi: 10.7150/thno.47849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fares J, Fares M, Khachfe H, Salhab H, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin F, Yang R, Wei Y, Wang D, Zhu Y, Wang X, Lu Y, Wang Y, Zen K, Li L. HIF-1α-induced miR-23a~27a~24 cluster promotes colorectal cancer progression via reprogramming metabolism. Cancer Lett. 2019;440-441:211–222. doi: 10.1016/j.canlet.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 53.Feng H, Lin J, Hsu S, Lan W, Kuo C, Tian Y, Sun D, Huang R. Low folate metabolic stress reprograms DNA methylation-activated sonic hedgehog signaling to mediate cancer stem cell-like signatures and invasive tumour stage-specific malignancy of human colorectal cancers. Int J Cancer. 2017;141:2537–2550. doi: 10.1002/ijc.31008. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Li X, Mo Z, Fan C, Tang L, Xiong F, Guo C, Xiang B, Zhou M, Ma J, Huang X, Wu X, Li Y, Li Gui, Zeng Z, Xiong W. Effects of tumor metabolic microenvironment on regulatory T cells. Mol Cancer. 2018;17:168. doi: 10.1186/s12943-018-0913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuo D, Shogren K, Zang J, Jewison D, Waletzki B, Miller A, Okuno S, Cai Z, Yaszemski M, Maran A. Inhibition of STAT3 blocks protein synthesis and tumor metastasis in osteosarcoma cells. J Exp Clin Cancer Res. 2018;37:244. doi: 10.1186/s13046-018-0914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diao X, Huestis M. New synthetic cannabinoids metabolism and strategies to best identify optimal marker metabolites. Front Chem. 2019;7:109. doi: 10.3389/fchem.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yasuyuki A, Uimook C, Cristina C, Koontz S, Tajima M, Sweeney C, Black M, Feldman S, Dinauer M, Malech H. Myeloid conditioning with c-kit-targeted CAR-T cells enables donor stem cell engraftment. Mol Ther. 2018;26:1181–1197. doi: 10.1016/j.ymthe.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maryam M, Negin P, Afsane B, Bahrami A, Atkin S, Sahebkar A. Role of the NLRP3 inflammasome in cancer. Mol Cancer. 2018;17:158. doi: 10.1186/s12943-018-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren L, Feng W, Shao J, Ma J, Xu M, Zhu B, Zheng N, Liu S. Diethyldithiocarbamate-copper nanocomplex reinforces disulfiram chemotherapeutic efficacy through light-triggered nuclear targeting. Theranostics. 2020;10:6384–6398. doi: 10.7150/thno.45558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bai X, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152–163. doi: 10.1016/j.ctrv.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Fahrer J. Switching off DNA repair-how colorectal cancer evades targeted therapies through adaptive mutability. Signal Transduct Target Ther. 2020;5:19. doi: 10.1038/s41392-020-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shastri A, Choudhary G, Teixeira M, Gordon-Mitchell S, Verma A. Antisense STAT3 inhibitor decreases viability of myelodysplastic and leukemic stem cells. J Clin Invest. 2018;128:5479–5488. doi: 10.1172/JCI120156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu H, Li H, Liang Y, Du Xin, Song H. Phage-delivered sensitisation with subsequent antibiotic treatment reveals sustained effect against antimicrobial resistant bacteria. Theranostics. 2020;10:6310–6321. doi: 10.7150/thno.42573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boohaker R, Sambandam V, Segura I, Miller J, Suto M, Xu B. Rational design and development of a peptide inhibitor for the PD-1/PD-L1 interaction. Cancer Lett. 2018;434:11–21. doi: 10.1016/j.canlet.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 65.Boohaker R, Sambandam V, Segura I, Miller J, Suto M, Xu B. Rational design and development of a peptide inhibitor for the PD-1/PD-L1 interaction. Cancer Lett. 2018;434:11–21. doi: 10.1016/j.canlet.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 66.Burton C, Bartee E. Syncytia formation in oncolytic virotherapy. Mol Ther Oncolytics. 2019;15:131–139. doi: 10.1016/j.omto.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di L, Liu L, Yan Y, Fu R, Li Y, Xu Y, Cheng Y, Wu Z. Discovery of a natural small-molecule compound that suppresses tumor EMT, stemness and metastasis by inhibiting TGFβ/BMP signaling in triple-negative breast cancer. J Exp Clin Cancer Res. 2019;38:134. doi: 10.1186/s13046-019-1130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yokoyama Y, Watanabe T, Tamura Y, Hashizume Y, Miyazono K, Ehata S. Autocrine BMP-4 signaling is a therapeutic target in colorectal cancer. Cancer Res. 2017;77:4026–4038. doi: 10.1158/0008-5472.CAN-17-0112. [DOI] [PubMed] [Google Scholar]
- 69.Dong R, Gong Y, Meng W, Yuan M, Zhu H, Ying M, He Q, Cao J, Yang B. The involvement of M2 macrophage polarization inhibition in fenretinide-mediated chemopreventive effects on colon cancer. Cancer Lett. 2017;388:43–53. doi: 10.1016/j.canlet.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Wang Q, Qian B, Schäfer M, Wolfgang G, Arianeb M, Eduard R. Fluorescence-guided fiber-optic micronavigation using microscopic identification of vascular boundary of liver segment and tumors. Theranostics. 2020;10:6136–6148. doi: 10.7150/thno.45973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y, He J, Li Y, Lv S, Cui H. NUSAP1 potentiates chemoresistance in glioblastoma through its SAP domain to stabilize ATR. Signal Transduct Target Ther. 2020;5:44. doi: 10.1038/s41392-020-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li C, Huang J, Cheng Y, Zhang Y. Traditional Chinese medicine in depression treatment: from molecules to systems. Front Pharmacol. 2020;11:586. doi: 10.3389/fphar.2020.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ylä-Herttuala S. The pharmacology of gene therapy. Mol Ther. 2017;25:1731–1732. doi: 10.1016/j.ymthe.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu G, Ma Z, Cheng Y, Hu W, Deng C, Jiang S, Li T, Chen F, Yang Y. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol Cancer. 2018;17:20. doi: 10.1186/s12943-018-0769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang S, Ding J. Ubiquitination and SUMOylation in the chronic inflammatory tumor microenvironment. Biochim Biophys Acta Rev Cancer. 2018;1870:165–175. doi: 10.1016/j.bbcan.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Wang D, Hu G, Du Y, Zhang C, Lu Q, Lv N, Luo S. Aberrant activation of hedgehog signaling promotes cell proliferation via the transcriptional activation of forkhead Box M1 in colorectal cancer cells. J Exp Clin Cancer Res. 2017;36:23. doi: 10.1186/s13046-017-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng M, Huang J, Tong A, Yang H. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol Ther Oncolytics. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma YS, Yu F, Zhong X, Lu G, Cong X, Xue S, Xie W, Hou L, Pang L, Wu W, Zhang W, Cong L, Liu T, Long H, Sun R, Sun H, Lv Z, Wu C, Fu D. miR-30 family reduction maintains self-renewal and promotes tumorigenesis in NSCLC-initiating cells by targeting oncogene TM4SF. Mol Ther. 2018;26:2751–2765. doi: 10.1016/j.ymthe.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nejadnik H, Jung K, Theruvath A, Kiru L, Liu A, Wu W, Sulchek T, Pratx G, Daldrup-Link H. Instant labeling of therapeutic cells for multimodality imaging. Theranostics. 2020;10:6024–6034. doi: 10.7150/thno.39554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Igase M, Shibutani S, Kurogouchi Y, Noriyuki Fujiki, Hwang C, Coffey M, Noguchi S, Nemoto Y, Takuya M. Combination therapy with reovirus and atm inhibitor enhances cell death and virus replication in canine melanoma. Mol Ther Oncolytics. 2019;15:49–59. doi: 10.1016/j.omto.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ratnayake G, Bain A, Fletcher N, Howard C, Khanna K, Thurecht K. RNA interference to enhance radiation therapy: targeting the DNA damage response. Cancer Lett. 2018;439:14–23. doi: 10.1016/j.canlet.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 82.Keck M, Hmazzou R, Llorens-Cortes C. Orally active aminopeptidase a inhibitor prodrugs: current state and future directions. Curr Hypertens Rep. 2019;21:50. doi: 10.1007/s11906-019-0957-4. [DOI] [PubMed] [Google Scholar]
- 83.Wu B, Pan X, Chen X, Chen M, Shi K, Xu J, Zheng J, Niu T, Chen C, Shuai X, Liu Y. Epigenetic drug library screening identified an LSD1 inhibitor to target UTX-deficient cells for differentiation therapy. Signal Transduct Target Ther. 2019;4:11. doi: 10.1038/s41392-019-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoon J, Kim G, Jarhad D, Kim H, Shin Y, Qu S, Sahu P, Kim H, Lee H, Wang S, Kong Y, Chang T, Ogando N, Kovacikova K, Snijder E, Posthuma C, Hemert M, Jeong L. Design, synthesis, and anti-RNA virus activity of 6’-fluorinated-aristeromycin analogues. J Med Chem. 2019;62:6346–6362. doi: 10.1021/acs.jmedchem.9b00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giordano G, Parcesepe P, D’Andrea MR, Coppola L, Di Raimo T, Remo A, Manfrin E, Fiorini C, Scarpa A, Amoreo CA, Conciatori F, Milella M, Caruso FP, Cerulo L, Porras A, Pancione M. JAK/Stat5-mediated subtype-specific lymphocyte antigen 6 complex, locus G6D (LY6G6D) expression drives mismatch repair proficient colorectal cancer. J Exp Clin Cancer Res. 2019;38:28. doi: 10.1186/s13046-018-1019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, Chen L, Sun X, Yang Q, Guo C. Matrine: a promising natural product with various pharmacological activities. Front Pharmacol. 2020;11:588. doi: 10.3389/fphar.2020.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu X, Jiang J, Meng H. Transcytosis - an effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery. Theranostics. 2019;9:8018–8025. doi: 10.7150/thno.38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Robertis M, Mazza T, Fusilli C, Loiacono L, Poeta M, Sanchez M, Massi E, Lamorte G, Diodoro M, Pescarmona E, Signori E, Pesole G, Vescovi A, Garcia-Foncillas J, Fazio V. EphB2 stem-related and EphA2 progression-related miRNA-based networks in progressive stages of CRC evolution: clinical significance and potential miRNA drivers. Mol Cancer. 2018;17:169. doi: 10.1186/s12943-018-0912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salomone S. Editorial: new paradigms in neuroscience and related targets for drug discovery. Front Pharmacol. 2020;11:750. doi: 10.3389/fphar.2020.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hutzen B, Ghonime M, Lee J, Mardis E, Wang R, Lee D, Cairo M, Roberts R, Cripe T, Cassady K. Immunotherapeutic challenges for pediatric cancers. Mol Ther Oncolytics. 2019;15:38–48. doi: 10.1016/j.omto.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fox K. Acute coronary syndromes in 2010: progress from trials to practice. Nat Rev Cardiol. 2011;8:68–70. doi: 10.1038/nrcardio.2010.207. [DOI] [PubMed] [Google Scholar]
- 92.Guan N, Zhao Y, Wang C, Li J, Chen X, Piao X. Anticancer drug response prediction in cell lines using weighted graph regularized matrix factorization. Mol Ther Nucleic Acids. 2019;17:164–174. doi: 10.1016/j.omtn.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu H, Wang S, Zhou S, Meng Q, Ma X, Song X, Wang L, Jiang W. Drug resistance-related competing interactions of lncRNA and mRNA across 19 cancer types. Mol Ther Nucleic Acids. 2019;16:442–451. doi: 10.1016/j.omtn.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Small D, Farid N, Payne C, Konkoy C, Jakubowski J, Winters K, Salazar D. Effect of intrinsic and extrinsic factors on the clinical pharmacokinetics and pharmacodynamics of prasugrel. Clin Pharmacokinet. 2010;49:777–798. doi: 10.2165/11537820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 95.Huang R, Zhou P. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5:60. doi: 10.1038/s41392-020-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsai Y, Zeng T, Abou-Kheir W, Yeh H, Yin J, Lee Y, Chen W, Liu Y. Disruption of ETV6 leads to TWIST1-dependent progression and resistance to epidermal growth factor receptor tyrosine kinase inhibitors in prostate cancer. Mol Cancer. 2018;17:42. doi: 10.1186/s12943-018-0785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu X, Li T, Shen S, Wang J, Abdou P, Gu Z, Mo R. Advances in engineering cells for cancer immunotherapy. Theranostics. 2019;9:7889–7905. doi: 10.7150/thno.38583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He X, Luan F, Yang Y, Wang Z, Zhao Z, Fang J, Wang M, Zuo M, Li Y. Passiflora edulis: an insight into current researches on phytochemistry and pharmacology. Front Pharmacol. 2020;11:617. doi: 10.3389/fphar.2020.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krokhotin A, Du H, Hirabayashi K, Popov K, Kurokawa T, Wan X, Ferrone S, Dotti G, Dokholyan N. Computationally guided design of single-chain variable fragment improves specificity of chimeric antigen receptors. Mol Ther Oncolytics. 2019;15:30–37. doi: 10.1016/j.omto.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fassunke J, Müller F, Keul M, Michels S, Dammert M, Schmitt A, Plenker D, Lategahn J, Heydt C, Brägelmann J, Tumbrink H, Alber Y, Klein S, Heimsoeth A, Dahmen I, Fischer R, Scheffler M, Ihle M, Priesner V, Scheel A, Wagener S, Kron A, Frank K, Garbert K, Persigehl T, Püsken M, Haneder S, Schaaf B, Rodermann E, Engel-Riedel W, Felip E, Smit E, Merkelbach-Bruse S, Reinhardt H, Kast S, Wolf J, Rauh D, Büttner R, Sos M. Overcoming EGFRG724S-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun. 2018;9:4655. doi: 10.1038/s41467-018-07078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tzchori I, Falah M, Shteynberg D, Levin Ashkenazi D, Loberman Z, Perry L, Flugelman M. Improved patency of ePTFE grafts as a hemodialysis access site by seeding autologous endothelial cells expressing Fibulin-5 and VEGF. Mol Ther. 2018;26:1660–1668. doi: 10.1016/j.ymthe.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhong X, Chen B, Liu M, Yang Z. The role of adaptor protein CARD9 in colitis-associated cancer. Mol Ther Oncolytics. 2019;15:1–6. doi: 10.1016/j.omto.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhullar K, Lagarón N, McGowan E, Parmar I, Jha A, Hubbard B, Rupasinghe H. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17:48. doi: 10.1186/s12943-018-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin L, Liu Y, Li H, Li P, Fuchs J, Shibata H, Iwabuchi Y, Lin J. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer. 2011;105:212–220. doi: 10.1038/bjc.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valenzuela R, Flores I, Urrutia B, Fuentes F, Sabat P, Llanos C, Cuitino L, Urzua C. New pharmacological strategies for the treatment of non-infectious uveitis. A minireview. Front Pharmacol. 2020;11:655. doi: 10.3389/fphar.2020.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y, Jin M, Wang Y, Zhu J, Tan R, Zhao J, Ji X, Jin C, Jia Y, Ren T, Xing J. MCU-induced mitochondrial calcium uptake promotes mitochondrial biogenesis and colorectal cancer growth. Signal Transduct Target Ther. 2020;5:59. doi: 10.1038/s41392-020-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song Y, Ye M, Zhou J, Wang Z, Zhu X. Restoring E-cadherin expression by natural compounds for anticancer therapies in genital and urinary cancers. Mol Ther Oncolytics. 2019;14:130–138. doi: 10.1016/j.omto.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin S, Hu F, Miao Y, Hu H, Lei Q, Zhang Q, Li Q, Wang H, Chen Z, Guo A. Identification of STAB1 in multiple datasets as a prognostic factor for cytogenetically normal AML: mechanism and drug indications. Mol Ther Nucleic Acids. 2019;18:476–484. doi: 10.1016/j.omtn.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen E, Yang F, He H, Li Q, Zhang W, Xing J, Zhu Z, Jiang J, Wang H, Zhao X, Liu R, Lei L, Dong J, Pei Y, Yang Y, Pan J, Zhang P, Liu S, Du L, Zeng Y, Yang J. Alteration of tumor suppressor BMP5 in sporadic colorectal cancer: a genomic and transcriptomic profiling based study. Mol Cancer. 2018;17:176. doi: 10.1186/s12943-018-0925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu H, Li J, Deng K, Zhou W, Wang C, Wang Q, Li K, Zhao H, Huang S. Tumor acidity activated triphenylphosphonium-based mitochondrial targeting nanocarriers for overcoming drug resistance of cancer therapy. Theranostics. 2019;9:7033–7050. doi: 10.7150/thno.35748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xie J, Bi Y, Zhang H, Dong S, Teng L, Lee R, Yang Z. Cell-penetrating peptides in diagnosis and treatment of human diseases: from preclinical research to clinical application. Front Pharmacol. 2020;11:697. doi: 10.3389/fphar.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo L, Zhang Y, Wei R, Wang C, Feng M. Lipopolysaccharide-anchored macrophages hijack tumor microtube networks for selective drug transport and augmentation of antitumor effects in orthotopic lung cancer. Theranostics. 2019;9:6936–6948. doi: 10.7150/thno.37380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwartz J. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163:713–721. doi: 10.1111/j.1476-5381.2011.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sivanandam V, LaRocca C, Chen N, Fong Y, Warner S. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Mol Ther Oncolytics. 2019;13:93–106. doi: 10.1016/j.omto.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cui W, Aouidate A, Wang S, Yu Q, Li Y, Yuan S. Discovering anti-cancer drugs via computational methods. Front Pharmacol. 2020;11:733. doi: 10.3389/fphar.2020.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Deng Q, Lim Y, Anand R, Yu Y, Kim J, Zhou W, Zheng J, Tempest P, Levorse D, Zhang X, Greene S, Mullins D, Culberson C, Sherborne B, Parker E, Stamford A, Ali A. Use of molecular modeling aided design to dial out hERG liability in adenosine A(2A) receptor antagonists. Bioorg Med Chem Lett. 2015;25:2958–2962. doi: 10.1016/j.bmcl.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 117.van Rensburg H, Legoabe L, Terre’Blanche G, Van der Walt M. 2-Benzylidene-1-indanone analogues as dual adenosine A1/A2a receptor antagonists for the potential treatment of neurological conditions. Drug Res (Stuttg) 2019;69:382–391. doi: 10.1055/a-0808-3993. [DOI] [PubMed] [Google Scholar]
- 118.Fu L, Zhang L. Serotonylation: a novel histone H3 marker. Signal Transduct Target Ther. 2019;4:15. doi: 10.1038/s41392-019-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chakraborty S, Dlie Z, Chakraborty S, Roy S, Mukherjee B, Besra S, Dewanjee S, Mukherjee A, Ojha P, Kumar V, Sen R. Aptamer-functionalized drug nanocarrier improves hepatocellular carcinoma toward normal by targeting neoplastic hepatocytes. Mol Ther Nucleic Acids. 2020;20:34–49. doi: 10.1016/j.omtn.2020.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun D, Chen J, Wang Y, Ji H, Peng R, Jin L, Wu W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics. 2019;9:6885–6900. doi: 10.7150/thno.36510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.da Silva R, Lima E, Novaes M, Osorio-de-Castro C. The high “cost” of experimental drugs obtained through health litigation in Brazil. Front Pharmacol. 2020;11:752. doi: 10.3389/fphar.2020.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laoharawee K, DeKelver R, Podetz-Pedersen K, Rohde M, Sproul S, Nguyen H, Nguyen T, St Martin S, Ou L, Tom S, Radeke R, Meyer K, Holmes M, Whitley C, Wechsler T, McIvor R. Dose-dependent prevention of metabolic and neurologic disease in murine MPS II by ZFN-mediated in vivo genome editing. Mol Ther. 2018;26:1127–1136. doi: 10.1016/j.ymthe.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marshall M, Issa Y, Jakubauskas B, Stoskute M, Elackattu V, Marshall J, Bogue W, Nguyen D, Hauck Z, Rue E, Karumuthil-Melethil S, Zaric V, Bosland M, van Breemen R, Givogri M, Gray S, Crocker S, Bongarzone E. Long-term improvement of neurological signs and metabolic dysfunction in a mouse model of Krabbe’s disease after global gene therapy. Mol Ther. 2018;26:874–889. doi: 10.1016/j.ymthe.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahamadi-Fesharaki R, Fateh A, Vaziri F, Solgi G, Siadat S, Mahboudi F, Rahimi-Jamnani F. Single-chain variable fragment-based bispecific antibodies: hitting two targets with one sophisticated arrow. Mol Ther Oncolytics. 2019;14:38–56. doi: 10.1016/j.omto.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boelsterli U, Ho H, Zhou S, Leow K. Bioactivation and hepatotoxicity of nitroaromatic drugs. Curr Drug Metab. 2006;7:715–727. doi: 10.2174/138920006778520606. [DOI] [PubMed] [Google Scholar]
- 126.Huang P, Wang G, Su Y, Zhou Y, Huang W, Zhang R, Yan D. Stimuli-responsive nanodrug self-assembled from amphiphilic drug-inhibitor conjugate for overcoming multidrug resistance in cancer treatment. Theranostics. 2019;9:5755–5768. doi: 10.7150/thno.36163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fu C. Gasdermin: a novel therapeutic target for tumour treatment by activating anti-tumour immunity. Signal Transduct Target Ther. 2020;5:69. doi: 10.1038/s41392-020-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cui H, Kong H, Peng F, Wang C, Zhang D, Tian J, Zhang L. Inferences of individual drug response-related long non-coding RNAs based on integrating multi-omics data in breast cancer. Mol Ther Nucleic Acids. 2020;20:128–139. doi: 10.1016/j.omtn.2020.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meng G, Fei Z, Fang M, Li B, Chen A, Xu C, Xia M, Yu D, Wei J. Fludarabine as an Adjuvant improves newcastle disease virus-mediated antitumor immunity in hepatocellular carcinoma. Mol Ther Oncolytics. 2019;13:22–34. doi: 10.1016/j.omto.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cherian D, Bhuvan T, Meagher L, Heng T. Biological considerations in scaling up therapeutic cell manufacturing. Front Pharmacol. 2020;11:654. doi: 10.3389/fphar.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daquinag A, Dadbin A, Snyder B, Wang X, Sahin A, Ueno N, Kolonin M. Non-glycanated decorin is a drug target on human adipose stromal cells. Mol Ther Oncolytics. 2017;6:1–9. doi: 10.1016/j.omto.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang T, Song C, Zheng L, Xia L, Li Y, Zhou Y. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol Cancer. 2019;18:62. doi: 10.1186/s12943-019-0967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sobot D, Mura S, Rouquette M, Vukosavljevic B, Cayre F, Buchy E, Pieters G, Garcia-Argote S, Windbergs M, Desmaële D, Couvreur P. Circulating Lipoproteins: a trojan horse guiding squalenoylated drugs to LDL-accumulating cancer cells. Mol Ther. 2017;25:1596–1605. doi: 10.1016/j.ymthe.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xiao B, Xu Z, Viennois E, Zhang Y, Zhang Z, Zhang M, Han M, Kang Y, Merlin D. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol Ther. 2017;25:1628–1640. doi: 10.1016/j.ymthe.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Siegler E, Kim Y, Chen X, Siriwon N, Mac J, Rohrs J, Bryson P, Wang P. Combination cancer therapy using chimeric antigen receptor-engineered natural killer cells as drug carriers. Mol Ther. 2017;25:2607–2619. doi: 10.1016/j.ymthe.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee A, Jones R, Huang P. Pazopanib in advanced soft tissue sarcomas. Signal Transduct Target Ther. 2019;4:16. doi: 10.1038/s41392-019-0049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Santiago C, Keuthan C, Boye S, Boye S, Imam A, Ash J. A drug-tunable gene therapy for broad-spectrum protection against retinal degeneration. Mol Ther. 2018;26:2407–2417. doi: 10.1016/j.ymthe.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bianchini F, Portioli E, Ferlenghi F, Vacondio F, Andreucci E, Biagioni A, Ruzzolini J, Peppicelli S, Lulli M, Calorini L, Battistini L, Zanardi F, Sartori A. Cell-targeted c(AmpRGD)-sunitinib molecular conjugates impair tumor growth of melanoma. Cancer Lett. 2019;446:25–37. doi: 10.1016/j.canlet.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 139.Caine M, Chung T, Kilpatrick H, Bascal Z, Willis S, Tang Y, de Baere T, Dreher M, Lewis A. Evaluation of novel formulations for transarterial chemoembolization: combining elements of Lipiodol emulsions with Drug-eluting Beads. Theranostics. 2019;9:5626–5641. doi: 10.7150/thno.34778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou Y, Zhang R, Rahman K, Cao Z, Zhang H, Peng C. Diverse pharmacological activities and potential medicinal benefits of geniposide. Evid Based Complement Alternat Med. 2019;2019:4925682. doi: 10.1155/2019/4925682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Naidoo J, Stanek L, Ohno K, Trewman S, Samaranch L, Hadaczek P, O’Riordan C, Sullivan J, San Sebastian W, Bringas J, Snieckus C, Mahmoodi A, Mahmoodi A, Forsayeth J, Bankiewicz K, Shihabuddin L. Extensive transduction and enhanced spread of a modified AAV2 capsid in the non-human primate CNS. Mol Ther. 2018;26:2418–2430. doi: 10.1016/j.ymthe.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wada A, Terashima T, Kageyama S, Yoshida T, Narita M, Kawauchi A, Kojima H. Efficient prostate cancer therapy with tissue-specific homing peptides identified by advanced phage display technology. Mol Ther Oncolytics. 2019;12:138–146. doi: 10.1016/j.omto.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li C, Mu N, Gu C, Liu M, Yang Z, Yin Y, Chen M, Wang Y, Han Y, Yu L, Ma H. Metformin mediates cardioprotection against aging-induced ischemic necroptosis. Aging Cell. 2020;19:e13096. doi: 10.1111/acel.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trunečka P. Once-daily tacrolimus in liver transplantation: a ‘me-too drug’, or a therapeutic advantage. Curr Opin Organ Transplant. 2017;22:118–122. doi: 10.1097/MOT.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 145.Hendriksen H, Groenink L. Back to the future of psychopharmacology: a perspective on animal models in drug discovery. Eur J Pharmacol. 2015;759:30–41. doi: 10.1016/j.ejphar.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 146.Wang L, Plump A, Ringel M. Racing to define pharmaceutical R&D external innovation models. Drug Discov Today. 2015;20:361–370. doi: 10.1016/j.drudis.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 147.Karakatsani M, Kugelman T, Ji R, Murillo M, Wang S, Niimi Y, Small S, Duff K, Konofagou E. Unilateral focused ultrasound-induced blood-brain barrier opening reduces phosphorylated Tau from the rTg4510 mouse model. Theranostics. 2019;9:5396–5411. doi: 10.7150/thno.28717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen X, Wang Y, Ma N, Tian J, Shao Y, Zhu B, Wong Y, Liang Z, Zou C, Wang J. Target identification of natural medicine with chemical proteomics approach: probe synthesis, target fishing and protein identification. Signal Transduct Target Ther. 2020;5:72. doi: 10.1038/s41392-020-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Böker K, Lemus-Diaz N, Rinaldi Ferreira R, Schiller L, Schneider S, Gruber J. The impact of the CD9 tetraspanin on lentivirus Infectivity and exosome secretion. Mol Ther. 2018;26:634–647. doi: 10.1016/j.ymthe.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kumar A, Ahmad A, Vyawahare A, Khan R. Membrane trafficking and subcellular drug targeting pathways. Front Pharmacol. 2020;11:629. doi: 10.3389/fphar.2020.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kondru S, Potnuri A, Allakonda L, Konduri P. Histamine 2 receptor antagonism elicits protection against doxorubicin-induced cardiotoxicity in rodent model. Mol Cell Biochem. 2018;441:77–88. doi: 10.1007/s11010-017-3175-x. [DOI] [PubMed] [Google Scholar]
- 152.Scherwitzl I, Hurtado A, Pierce CM, Vogt S, Pampeno C, Meruelo D. Systemically administered sindbis virus in combination with immune checkpoint blockade induces curative anti-tumor immunity. Mol Ther Oncolytics. 2018;9:51–63. doi: 10.1016/j.omto.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ji N, Yang Y, Cai C, Lei Z, Wang J, Gupta P, Shukla S, Ambudkar S, Kong D, Chen Z. Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1-and ABCG2-overexpressing cancer cells. Cancer Lett. 2019;440-441:82–93. doi: 10.1016/j.canlet.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang W, Zhao H, Wan J, Zhou Y, Xu Q, Zhao Y, Yang X, Gan L. pH-and photothermal-driven multistage delivery nanoplatform for overcoming cancer drug resistance. Theranostics. 2019;9:3825–3839. doi: 10.7150/thno.33958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Balakirski G, Merk H. Cutaneous allergic drug reactions: update on pathophysiology, diagnostic procedures and differential diagnosic. Cutan Ocul Toxicol. 2017;36:307–316. doi: 10.1080/15569527.2017.1319379. [DOI] [PubMed] [Google Scholar]
- 156.Dimopoulos G, Siempos I, Korbila I, Manta K, Falagas M. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447–455. doi: 10.1378/chest.07-0149. [DOI] [PubMed] [Google Scholar]
- 157.Velingkaar N, Mezhnina V, Poe A, Makwana K, Tulsian R, Kondratov RV. Reduced caloric intake and periodic fasting independently contribute to metabolic effects of caloric restriction. Aging Cell. 2020;19:e13138. doi: 10.1111/acel.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun Q, Hu Y, Gu Y, Huang J, He J, Luo L, Yang Y, Yin S, Dou C, Wang T, Fu X, He L, Qi S, Zhu X, Yang S, Wei X, Cheng W. Deciphering the regulatory and catalytic mechanisms of an unusual SAM-dependent enzyme. Signal Transduct Target Ther. 2019;4:17. doi: 10.1038/s41392-019-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feng S, Wang W. Bioactivities and structure-activity relationships of natural tetrahydroanthraquinone compounds: a review. Front Pharmacol. 2020;11:799. doi: 10.3389/fphar.2020.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peura D, Le Moigne A, Wassel H, Pollack C. Analysis of the symptom response to esomeprazole 20 mg over days 1-4 of a 14-day course of treatment for frequent heartburn: results of two randomised controlled trials. BMJ Open Gastroenterol. 2019;6:e000278. doi: 10.1136/bmjgast-2019-000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Polonini H, Silva S, Loures S, Almy R, Balland A, Brandão M, Ferreira A. Compatibility of proton pump inhibitors in a preservative-free suspending vehicle. Eur J Hosp Pharm Sci Pract. 2018;25:150–156. doi: 10.1136/ejhpharm-2016-001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Song W, Wang X, Lv W, Xu X, Tian M. The effect of early Helicobacter pylori eradication on the healing of ESD-induced artificial ulcers: a retrospective study. Medicine (Baltimore) 2019;98:e15807. doi: 10.1097/MD.0000000000015807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cao X, Jin X, Liu B. The involvement of stress granules in aging and aging-associated diseases. Aging Cell. 2020;19:e13136. doi: 10.1111/acel.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Selman M, Rousso C, Bergeron A, Son H, Krishnan R, El-Sayes N, Varette O, Chen A, Le Boeuf F, Tzelepis F, Bell J, Crans D, Diallo J. Multi-modal potentiation of oncolytic virotherapy by vanadium compounds. Mol Ther. 2018;26:56–69. doi: 10.1016/j.ymthe.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li L, Li X, Xia Y, Chu Y, Zhong H, Li J, Liang P, Bu Y, Zhao R, Liao Y, Yang P, Lu X, Jiang S. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front Pharmacol. 2020;11:786. doi: 10.3389/fphar.2020.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu N, Yu M, Yin T, Jiang Y, Liao X, Tang J, Li Y, Qin D, Li D, Wang Y. Progression of malignant pleural effusion during the early stage of gefitinib treatment in advanced EGFR-mutant lung adenocarcinoma involving complex driver gene mutations. Signal Transduct Target Ther. 2020;5:63. doi: 10.1038/s41392-020-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang Z, Fu S, Zhao J, Zhao W, Shen Z, Wang D, Duan J, Bai H, Wan R, Yu J, Wang S, Chen H, Chen B, Wang L, Wang J. Transbronchoscopic patient biopsy-derived xenografts as a preclinical model to explore chemorefractory-associated pathways and biomarkers for small-cell lung cancer. Cancer Lett. 2019;440-441:180–188. doi: 10.1016/j.canlet.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 168.Zhuang H, Shi S, Yuan Z, Chang J. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 2019;18:21. doi: 10.1186/s12943-019-0950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Han Y, Guo W, Ren T, Huang Y, Wang S, Liu K, Zheng B, Yang K, Zhang H, Liang X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019;440-441:116–125. doi: 10.1016/j.canlet.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 170.Cheng C, Deng T, Lin FC, Cai Y, Zink J. Supramolecular nanomachines as stimuli-responsive gatekeepers on mesoporous silica nanoparticles for antibiotic and cancer drug delivery. Theranostics. 2019;9:3341–3364. doi: 10.7150/thno.34576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Condello M, Mancini G, Meschini S. The Exploitation of liposomes in the inhibition of autophagy to defeat drug resistance. Front Pharmacol. 2020;11:787. doi: 10.3389/fphar.2020.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guerrero A, Guiho R, Herranz N, Uren A, Withers D, Martínez-Barbera J, Tietze L, Gil J. Galactose-modified duocarmycin prodrugs as senolytics. Aging Cell. 2020;19:e13133. doi: 10.1111/acel.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Z, Yang X, Duan C, Li J, Tong R, Fan Y, Feng J, Cao R, Zhong W, Feng X, Zhang H, Cai L. Identification and characterization of mammaglobin-A epitope in heterogenous breast cancers for enhancing tumor-targeting therapy. Signal Transduct Target Ther. 2020;5:82. doi: 10.1038/s41392-020-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hong S, Lee H, Kwon O, Song N, Lee H, Kang S, Kim J, Kim M, Kim W, Cha H. Large-scale pharmacogenomics based drug discovery for ITGB3 dependent chemoresistance in mesenchymal lung cancer. Mol Cancer. 2018;17:175. doi: 10.1186/s12943-018-0924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim S, Rait A, Garrido-Sanabria E, Pirollo K, Harford J, Chang E. Nanotherapeutics for gene modulation that prevents apoptosis in the brain and fatal neuroinflammation. Mol Ther. 2018;26:84–94. doi: 10.1016/j.ymthe.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jaime-Ramirez A, Yu J, Caserta E, Yoo J, Zhang J, Lee T, Hofmeister C, Lee J, Kumar B, Pan Q, Kumar P, Baiocchi R, Teknos T, Pichiorri F, Kaur B, Old M. Reolysin and histone deacetylase inhibition in the treatment of head and neck squamous cell carcinoma. Mol Ther Oncolytics. 2017;5:87–96. doi: 10.1016/j.omto.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hugle M, Czaplinski S, Habermann K, Vogler M, Fulda S. Identification of Smac mimetics as novel substrates for p-glycoprotein. Cancer Lett. 2019;440-441:126–134. doi: 10.1016/j.canlet.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 178.Donati B, Lorenzini E, Ciarrocchi A. BRD4 and cancer: going beyond transcriptional regulation. Mol Cancer. 2018;17:164. doi: 10.1186/s12943-018-0915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moossavi M, Parsamanesh N, Bahrami A, Atkin S, Sahebkar A. Role of the NLRP3 inflammasome in cancer. Mol Cancer. 2018;17:158. doi: 10.1186/s12943-018-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Escobar G, Barbarossa L, Barbiera G, Norelli M, Genua M, Ranghetti A, Plati T, Camisa B, Brombin C, Cittaro D, Annoni A, Bondanza A, Ostuni R, Gentner B, Naldini L. Interferon gene therapy reprograms the leukemia microenvironment inducing protective immunity to multiple tumor antigens. Nat Commun. 2018;9:2896. doi: 10.1038/s41467-018-05315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen X, Fu X, Zhao W, Ho W, Xing S, Zhao Z. Loss of tyrosine phosphatase SHP2 activity promotes growth of colorectal carcinoma HCT-116 cells. Signal Transduct Target Ther. 2020;5:83. doi: 10.1038/s41392-020-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


