Abstract
Local application of lithium or aspirin with biological scaffold has been identified as a potent means to improve bone formation. In this study, lithium and aspirin modified calcium phosphate cement (Asp-Li/CPC) was prepared, and the feasibility of this biological scaffold in the treatment of osteoporotic bone defect was observed in vivo and in vitro. In vitro experiments confirmed that Asp-Li/CPC had better ability to promote MC3T3-E1 cells differentiation into osteoblasts, osteoblast mineralization and viability, and promote cell expression of ALP, OP, RUNX-2, OC and COL-1 protein than simple CPC or lithium modified CPC by MTT, Alizarin red staining and Western blot evaluation. In vivo experiments confirmed that Asp-Li/CPC presented the strongest effect on bone regeneration and bone mineralization through the comparison with CPC group and Li/CPC group with X-ray images, Micro-CT and Histological evaluation. RT-qPCR analysis showed that Asp-Li/CPC, Li/CPC group and CPC group demonstrated increased BMP2, Smad1, OPG than the OVX group (P<0.05), while Asp-Li/CPC exhibited decreased TNF-α, IFN-γ and RANKL than the OVX group (P<0.05). Experiments in vivo and in vitro show that Asp-Li/CPC is a scheme for rapid repair of femoral condylar defects, and these effects may be achieved by inhibiting local inflammation and through BMP-2/Smad1 and OPG/RANKL signaling pathway.
Keywords: Calcium phosphate cement, lithium, aspirin, inflammation, osteoporotic bone defect
Introduction
Postmenopausal osteoporosis is an age-related progressive bone disease characterized by bone loss and structural destruction, which increases the risk of fracture in postmenopausal women and has become a worldwide health problem [1]. Osteoporotic fracture is usually related to bone defect, so the clinical treatment of osteoporotic fracture has attracted more attention to the role of bone defect. In the past, autogenous bone transplantation was considered to be the best method for the treatment of bone defects [2]. However, autologous transplants can produce another wound, even more wound sites, greater discomfort, and donor site complications, such as infection, hematoma or laceration [3,4]. Unfortunately, most patients with osteoporosis are elderly people who often have a large number of underlying diseases, and their poor tolerance to anesthesia further restricts the market for autologous bone transplantation [5]. Therefore, it poses higher challenges for clinicians to deal with these diseases.
In recent years, with the development of bioengineering materials, the application of calcium phosphate cement (CPC) scaffolds in clinics has brought new options for clinicians to treat osteoporotic bone defects [6]. CPC has been widely used to repair bone defects in orthopaedics and dentistry because of its similar composition, bone conductivity and good biological activity [7]. Owing to the lack of osteoinductive ability of CPC, it is undesirable to be used in osteoporosis patients with significantly impaired bone repair ability, so new requirements are put forward for this kind of materials [8]. The incorporation of metal elements into materials can change the biological characteristics of materials, so in recent years, many different kinds of metal ions have been combined into calcium phosphate materials and achieved good results in animal and cell experiments. Lithium (Li) is widely used in the treatment of bipolar disorder. Previous studies have shown that Li may play an important role in bone metabolism [9,10]. Experiments in vivo and in vitro have confirmed that Li can inhibit the activity of osteoclasts by reducing the formation, differentiation, proliferation and function of osteoclasts, stimulate bone formation by reducing apoptosis of osteoblasts, and stimulate osteoblasts to affect bone turnover by activating Wnt/β-catenin signal pathway [10,11]. Because of a good promoting effect on bone remodeling, lithium is often added to hydroxyapatite, calcium phosphate, bioactive glass and other materials to improve the biological activity and osteogenic ability of raw materials, so as to achieve the effect of rapid repair of bone defects [12-14]. Aspirin (Asp) is a well-known nonsteroidal anti-inflammatory drug that has long been used as treatment of common pain, inflammation and tissue damage [15]. In recent years, ASP has received more and more attention in the field of bone repair [16] because it can improve the differentiation of bone marrow mesenchymal stem cells into osteoblasts and enhance the function of osteoblasts. Previous studies suggested that aspirin had the potential to promote bone regeneration via the inhibition of immune cells under inflammatory conditions [17,18].
In view of the different mechanisms of action of Li regulating bone metabolism and Asp improving osteogenic microenvironment to promote bone defect repair, we, inspired by these above investigations, propose a hypothesis that bone repair can be effectively improved by using a lithium-doped CPC (Li/CPC) modified with aspirin (Asp-Li/CPC). Therefore, the purpose of our current study is to observe whether local administration lithium and Asp can enhance the efficacy of CPC in the treatment of osteoporotic bone defect.
Materials and methods
Experimental animals
Health adult female Sprague-Dawley rats (n = 50), weighing (200-250 g, Central Laboratory of Yijishan Hospital), were used in this study. All rats were maintained in a temperature-controlled environment (22 ± 3°C), 55 ± 5% relative humidity under a 12 h light/dark cycle and humidity and fed standard laboratory food and water. Animal care and all experimental procedures were carried out in accordance with guidelines under the Animal Experimentation of Wannan Medical College.
Preparation of CPC, Li/CPC, Asp-Li/CPC scaffold
Li/CPC was prepared in accordance with previous research [19]. CPC was mixed with TTCP (Sigma-Aldrich, Saint Louis, MO, USA) and DCPA (Sigma-Aldrich) at a ratio of 1:1 as a powder phase. A liquid phase (ph = 4) constitutes Lithium chloride (100; Sigma-Aldrich) and 20% wt of citric acid. Powder and liquid phases mixed at a ratio of 0.3 ml/g were cast into 3D-printed wax models for scaffold production and were downscaled to a diameter of 2.5 mm and a height of 4 mm. The pure CPC ceramic was named CPC, and the Li/CPC with 1.0 wt.% Li designated as Li/CPC. To prepare Asp-Li/CPC scaffold, 0.1 ml of phosphate-buffered saline solution (PBS, pH 7.5) containing 100 µg of aspirin was dropped onto one freeze-dried Li/CPC scaffold, followed by incubation at 4°C overnight. The resulting scaffold was used without washing as the Asp-Li/CPC. Scaffolds were lyophilized and stored at -20°C until further use. The doses and patterns of Li and ASP were determined according to previous experiments [19,20]. 40 scaffolds were prepared from each group for the following experiments.
Cell culture, treatment and evaluation
MC3T3-E1 cells, an osteoblast precursor cell line derived from mouse, were purchased from the Institute of Biochemistry and Cell Biology, CAS (Shanghai, China). MC3T3-E1 cells were cultured at 37°C and in an atmosphere of 5% CO2 in DMEM containing 10% FBS. Thereafter, β-glycerophosphate (10 mM), L-ascorbic acid (50 μg/mL) and dexamethasone (10 nM) were added into DMEM to induce osteogenic differentiation. After reaching confluence, the cells were seeded into leachate of CPC, Li/CPC, Asp-Li/CPC scaffolds treatment or no leachate of scaffold treatment (Control). The leaching solution was the liquid produced by the same amount of material in PBS for 24 hours. In the cell experiment, the extract of 1 ml was added to the culture medium.
MTT detection
After 3 days’ treatment with leachate, 0.02 ml of MTT solution (5 mg/ml) was added to each well and the plates were incubated at 37°C for 4 h to estimate cell viability. After that, the DMSO was added to dissolve formazan crystals. The absorbance value was measured at 570 nm using a plate reader (Bio-Rad Inc, Hercules, CA, USA).
Alizarin red staining
To detect mineralization (calcium deposits), 3 weeks after induction with leachate, cells were fixed with 4% paraformaldehyde, and stained with Alizarin Red S (2%; Sigma-Aldrich) and the samples were observed by inverted phase contrast microscopy (Eclipse TE2000-U; Nikon, Japan).
Western blot analysis
To detect the expression levels of osteogenesis-related proteins, we performed western blot analyses. After 72 h treatment, the treated cells were harvested and the RIPA lysis buffer and BCA protein assay (Beyotime, Shanghai, China) were used to extract and determine the total proteins. SDS-polyacrylamide gel electrophoresis (10%) was performed to separate the sample protein (20 µg/lane). Then, the proteins were transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). After blocking with 5% bovine serum albumin (Solarbio, Beijing, China) at room temperature for 2 h in Tris-buffered saline and Tween 20 (TBST), the membranes were probed with the primary antibodies, like these against the following proteins: alkaline phosphatase (ALP) (1:1000, ab33923), osteopontin (OP) (1:1000, ab139434), runt-related transcription factor 2 (RUNX-2), osteocalcin (OC) (1:1000, ab139412), type I collagen (COL-1) (1:1000, ab78034) (all from Abcam, Cambridge, UK), and GAPDH (1:1000, TA-08; ZSGB-Bio, Beijing, China), and incubated at 4°C overnight. The appropriate secondary antibody was applied (1:2000; horseradish peroxidase anti-mouse and horseradish peroxidase anti-rabbit) at room temperature for 1 hour. Quantitative analysis of protein expression was accomplished by scanning autoradiogram and densitometry (Image J, NIH).
Surgery and treatment
A bilateral ovariectomized (OVX) or sham operation was performed using standard methods as previous reports described [21,22]. To put it simply, after routine anesthesia, the rats were fixed in prone position and bilateral ovaries were cut off and sutured layer by layer in OVX group under aseptic condition, while rats in Sham group underwent back incision to remove 1 g of para-ovarian fat without ovariectomy. The operated rats were allowed for 12 weeks to conduct osteoporosis. The females of each five randomly selected OVX rats and the five sham-operated ones were harvested for bone mineral density (BMD) evaluation by micro computed tomography (Micro-CT) and hematoxylin-eosin (HE) staining to confirm the establishment of osteoporosis. Afterwards, all OVX animals were randomly divided into four experimental groups: OVX group, CPC group, Li/CPC and Asp-Li/CPC group. There were 10 rats in each group. A 2.5 mm femoral cylindrical defect in OVX rats was selected as a critical-sized defect according to the previously reported methods [23,24]. The rats in the CPC group, Li/CPC and Asp-Li/CPC group were filled with CPC, Li/CPC, and Asp-Li/CPC respectively. The rats were intraperitoneally injected with 20 mg/kg calcein (CA, Sigma, USA) on the 13th and 3rd day before the end of the experiment respectively. At 8 weeks postoperatively, all of the defected rats were euthanized by the over-dose administration of anesthetic agents. The defected femur was dissected and fixed in 10 wt% neutral phosphate-buffered formalin solution to assess bone regeneration.
Micro-CT scanning and X-ray testing
Bone radiographs of defected femur were detected by Dual-energy X-ray Absorptiometry (DXA) scans (Kubetic) on the lateral radiographs of distal femur. For microarchitecture, trabecular bone architecture of defected area was determined by Micro-CT (Micro-CT μm 100, SCANCO Medical, Switzerland). The central 2.5 mm-diameter and external 5 mm-length region of the defect was defined as volume of interest (VOI) to analyze the bone defect healing. Trabecular bone volume per tissue volume (BV/TV), trabecular number (Tb.N), tracecular separation (Tb.Sp) and trabecular thickness (Tb.Th) of VOI were measured as previously described [6,25].
Histological examination
Following Micro-CT scanning and X-ray testing, some femur specimens were decalcified and stained with hematoxylin and eosin (HE) for histopathological examination. The specimens were then dehydrated, embedded in paraffin following standard procedures, and cut into 5 μm ultrathin sections following transition. Subsequently, the section was stained with HE and observed by optical light microscope (Olympus, Japan). Other femur specimens were dehydrated by alcohol and embedded in PMMA. The specimens were cut into 200 μm thick sections using a microtome (Leica Microsystems Ltd, Wetzlar, Germany) and were subsequently ground and polished to a thickness of 40-50 μm as previously described [23].
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was prepared and isolated using Trizol reagent (Invitrogen Corp., Carlsbad, CA, USA) as previously described [26,27]. Briefly, tissue samples of defect area collected by hollow drill were homogenized using a mortar and pestle with liquid nitrogen, using 5 ml of TRIzol per 50-100 mg of tissue. Insoluble material was removed from the homogenate by centrifugation for 10 min at 10,000 rpm at 4°C. RNA was reverse transcribed into cDNA using Primer Script RT reagent kit (Takara, Dalian, China) following standard procedures of the manufacturer’s instructions. The quantitative real-time PCR was performed using the Applied Biosystems 7500 Sequence Detection System (ABI, Vernon, CA, USA) using oligonucleotide primer sequences summarized in Table 1. The expressions of the tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), bone morphogenetic protein 2 (BMP-2), small mothers against deca-pentaplegic homologs-1 (Smad1), osteoprotegerin (OPG), and receptor activator of nuclear factor kappa-B ligand (RANKL) were normalized to a housekeeping gene, GAPDH.
Table 1.
Nucleotide sequences for real-time RT-PCR primers
| Genes | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| RANKL | AGCCGAGACTACGGCAAGTA | AGACCACCTGACCCAGTCC |
| TNF-α | AAGTTCCCAAATGGCCTCCCTCTCATC | GGAGGTTGACTTTCTCCTGGTATGAGA |
| OPG | ACACACCAACTGCAGCTCAC | TGTCCACCAGAACACTCAGC |
| IFN-γ | TGCATCTTGGCTTTGCAGCTCTTCCTCATGGC | TGGACCTGTGGGTTGTTGACCTCAAACTTGGC |
| Smad1 | GTGGAAACAGGGCGACGAAG | AGGGAGCGAGGAATGGTGAC |
| BMP-2 | ACGACGGTAAAGGACATC | ATGGTTGGTGGAGTTCAG |
| GAPDH | ACATTGTTGCCATCAACGAC | TACTCAGCACCAGCATCACC |
Statistical analysis
Measurement data was described as mean ± standard deviation (mean ± SD). Analysis was performed with commercially available statistical software (SPSS 19.0; SPSS Inc., Chicago, IL, USA). A one-way analysis of variance (ANOVA) and post-hoc Tukey test were carried out for comparisons between different groups. For all analyses, statistical significance was defined as P<0.05.
Results
Animals
Five rats died from the establishment ovariectomy or bone defect model. One rat was infected after surgery, 3 rats were anesthetized accidentally, and 1 rat was operated accidentally. The survived 45 rats were included for further analysis.
Osteoporosis animal model validation
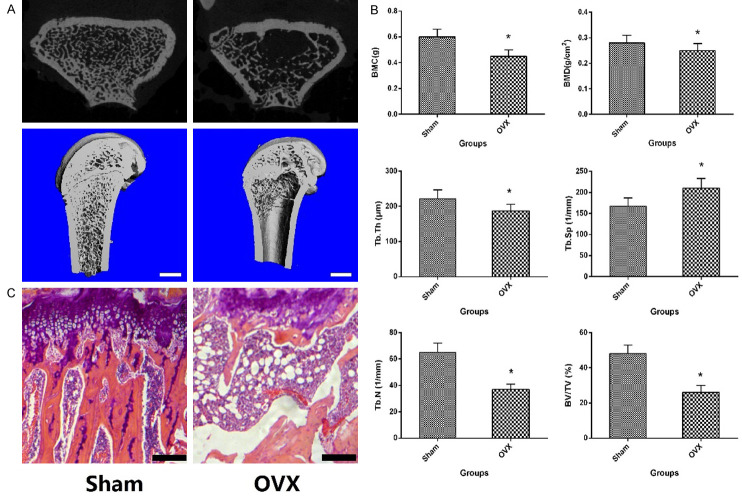
In order to determine the success of the osteoporosis model induced by ovariectomy, 5 rats in each group were randomly killed 3 months after bilateral ovariectomy for Micor-CT and histological examination. The results of histological and Micro-CT performed on the distal metaphysis of femur are presented in Figure 1A-C. Compared with the Sham group, the quantity and quality of trabecular bone were significantly lower than those in the OVX group. Compared with the sham group, rats in the OVX group exhibited a significant decrease in BMD (P<0.05), BMC (P<0.05), BV/TV (P<0.05), Tb.N (P<0.05) and Tb.Th (P<0.05), as well as a significant increase in Tb.Sp (P<0.05). Thus, statistically, there were obviously significant differences between OVX and the Sham group (P<0.05). Histological and imaging results demonstrated that osteoporosis occurred in the bones of ovariectomized rats, and the model was successfully established in this study.
Figure 1.
The Micro-CT 3D reconstruction of trabecular bone at femoral metaphyseal and quantitative results expressed as BMD, BMC, BV/TV, Tb.N, Tb.Sp and Tb.Th (A, B), as well as historical analysis (C) of femoral metaphysis. *P<0.05 vs. Sham group, the scale bar represents 1 mm.
Osteoblastic viability
In order to determine the effect of biomaterials on MC3T3-E1 cells activity, this study further conducted MTT experiments. As shown in Figure 2, the cell viability of Asp-Li/CPC group evaluated by MTT was significantly higher than that of Control group (P<0.05), CPC group (P<0.05) and Li/CPC group (P<0.05). The results show that the three kinds of leachate of biomaterial can increase the biological activity of cells, and the leachate of Asp-Li/CPC scaffold demonstrates the best effect in the three groups.
Figure 2.
MTT assay of cell viability with different treatment. *P<0.05 vs. Control group, #P<0.05 vs. CPC, &P<0.05 vs. Li/CPC group.
Alizarin red staining
In order to observe the effect of different biomaterials on osteogenic mineralization of MC3T3-E1 cells, the cellular alizarin red staining experiment was further carried out in this study. Alizarin red staining with quantification of area in osteogenic differentiation of MC3T3-E1 cells is shown in Figure 3. The mineralized nodules (number per well) and mineralized area (%) of Asp-Li/CPC group were significantly higher than Control group (P<0.05), CPC group (P<0.05) and Li/CPC group (P<0.05). The results showed that the leachate of three kinds of biological material can increase cell biological activity, and the leachate of Asp-Li/CPC scaffold demonstrates the best effect in the three groups. These results suggest that the ability of composite biomaterials Asp-Li/CPC to promote osteoblast mineralization is better than that of CPC and Li/CPC.
Figure 3.
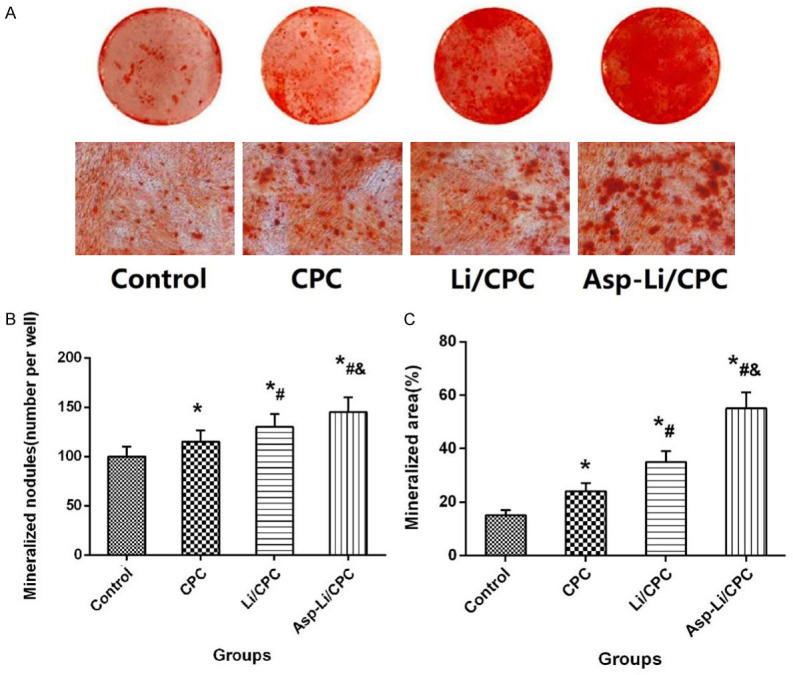
(A) Representative images of alizarin red staining, Mineralized nodules (number per well, B) and Mineralized area (%, C) of different group. *P<0.05 vs. Control group, #P<0.05 vs. CPC, &P<0.05 vs. Li/CPC group, the scale bar represents 1 mm.
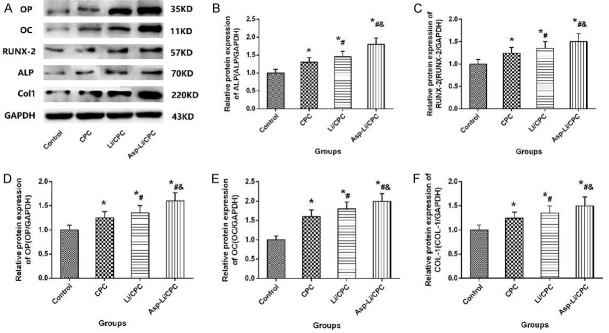
Osteogenic protein expression of MC3T3-E1 cells
Osteoblasts are regulated by proteins expressed by a variety of osteoblast-related genes in the process of osteoblast proliferation and differentiation, and the expression of these proteins can reflect the biological characteristics of osteoblasts in the culture system. In order to observe the effects of different biomaterials on MC3T3-E1 cells into osteoblasts, western blotting experiments were carried out to determine the expression of related proteins in each group. Protein expression in osteogenic differentiation of MC3T3-E1 cells with different intervention treatments is illustrated in Figure 4. The osteogenic protein expressions such as ALP, OP, RUNX-2, OC and COL-1 of Asp-Li/CPC group were significantly higher than Control group (P<0.05), CPC group (P<0.05) and Li/CPC group (P<0.05). The results showed that the leachate of three biological materials increased proteins expressed by a variety of osteoblast-related genes, and the leachate of Asp-Li/CPC scaffold demonstrated the best effect in the three groups. These results indicate that the composite biomaterial Asp-Li/CPC has the ability to promote the expression of genes related to the regulation of osteogenesis better than CPC and Li/CPC.
Figure 4.
(A) Representative images of Osteogenic Protein Expression and Osteogenic Protein Expression for OP (B), ALP (C), RUNX-2 (D), OC (E) and COL-1 (F) of MC3T3-E1 cells; *P<0.05 vs. Control group, #P<0.05 vs. CPC, &P<0.05 vs. Li/CPC group.
X-ray analysis
In order to understand the effect of three kinds of biomaterials implanted into the defect area on bone repair, we examined the defect area by X-ray to determine the healing condition. X-ray images demonstrated the repair effect of defect for different treatment as shown in Figure 5. The unrepaired region of Asp-Li/CPC group was significantly lower than OVX group (P<0.05), CPC group (P<0.05) and Li/CPC group (P<0.05). The X-ray images directly and objectively showed that the effects of the three biological materials in repairing bone defects were better than those of the blank control group. Compared with the repair effect of the three materials, it was found that the effect of Asp-Li/CPC scaffold in repairing osteoporotic bone defect reached the expected effect. X-ray results show that the composite biomaterial Asp-Li/CPC is better than CPC and Li/CPC in repairing osteoporotic bone defects from a macro perspective.
Figure 5.
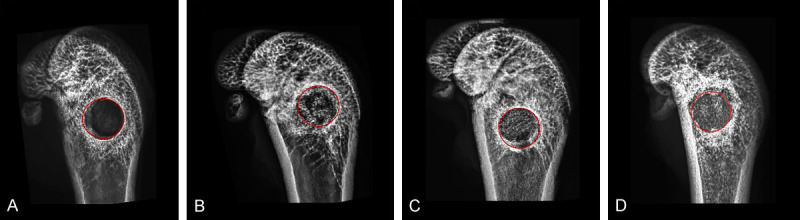
X-ray images of Bone regeneration in defected area. OVX group (A), CPC group (B), Li/CPC group (C) and Asp-Li/CPC group (D). Red circle in the figure are presented as defect area, the scale bar represents 1 mm.
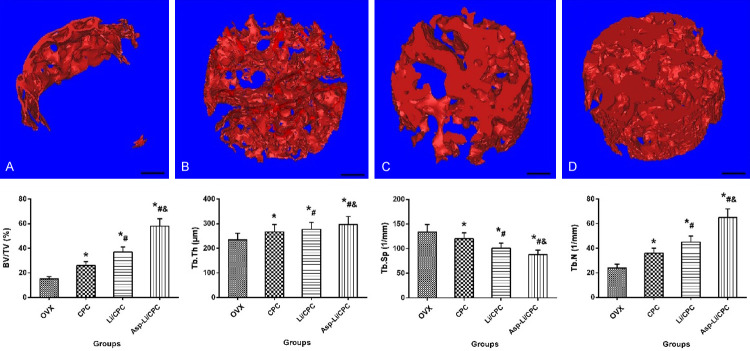
Micro-CT evaluation
In order to more clearly and intuitively understand the effects of three kinds of biomaterials implanted into the defect area on bone repair, we performed Micro-CT examination on the defect area to determine the healing and observe the microscopic condition of bone defect healing. The 3D reconstruction of the defect area clearly depicted the effects of different treatments on bone regeneration qualitatively (Figure 6). The quantitative results were expressed as BV/TV, Tb.N, Tb.Sp and Tb.Th. Compared with OVX group, CPC group and Li/CPC group, Asp-Li/CPC revealed the highest values of BV/TV (P<0.05), Tb.N (P<0.05), and Tb.Th (P<0.05), as well as the lowest value of Tb.Sp (P<0.05). Compared with the repair effect of the three materials, it was found that Asp-Li/CPC biological scaffold quickly and effectively promoted bone reconstruction in the defect area. Micro-CT results show that the composite biomaterial Asp-Li/CPC is better than CPC and Li/CPC in repairing osteoporotic bone defects from a microscopic perspective.
Figure 6.
The 3D reconstruction of Bone regeneration and Quantitative results expressed as BV/TV, Tb.N, Tb.Sp and Tb.Th in defected area. (A) (OVX group), (B) (CPC group), (C) (Li/CPC group) and (D) (Asp-Li/CPC group). *P<0.05 vs. OVX group, #P<0.05 vs. CPC, &P<0.05 vs. Li/CPC group, the scale bar represents 1 mm.
Histological analysis
In order to further understand the effects of three kinds of biomaterials implanted into the defect area on bone repair from the microscopic level, we performed histological examination to determine the healing and observe the reduction of bone defect materials. Histological images recorded bone repair and biomaterial residue of defect for different treatment are presented in Figure 7. At 8 weeks after defect surgery, a large amount of bone tissue filled the defect area in the CPC group, Li/CPC group and Asp-Li/CPC group, but no bone formation seemed to be observed in the OVX group. In the three groups of biomaterial implantation, compared with CPC group and Li/CPC group, Asp- Li/CPC group presented the strongest effect on bone regeneration and biomaterial degradation (P<0.05). Compared with the repair effect of the three materials, it was found that Asp-Li/CPC biomaterials degraded in time to make room for new bone formation, and accelerated bone formation to achieve the effect of rapid bone repair. Histological analysis results show that the composite biomaterial Asp-Li/CPC is better than CPC and Li/CPC in repairing osteoporotic bone defects from a histological micro-level.
Figure 7.
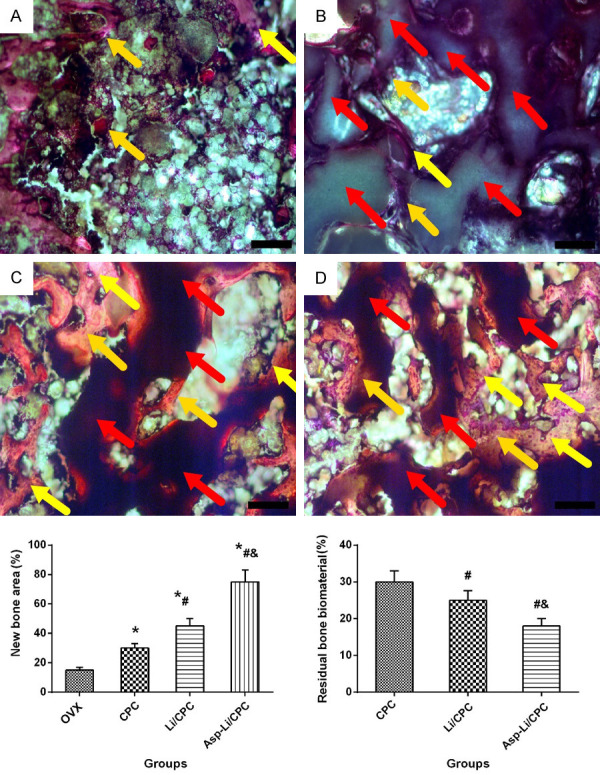
Bone regeneration and biomaterial degradation of defected area. *P<0.05 vs. OVX group, #P<0.05 vs. CPC, &P<0.05 vs. Li/CPC group (at 10× magnification; the yellow arrows indicate bone tissues, and the red arrows indicate bone biomaterials).
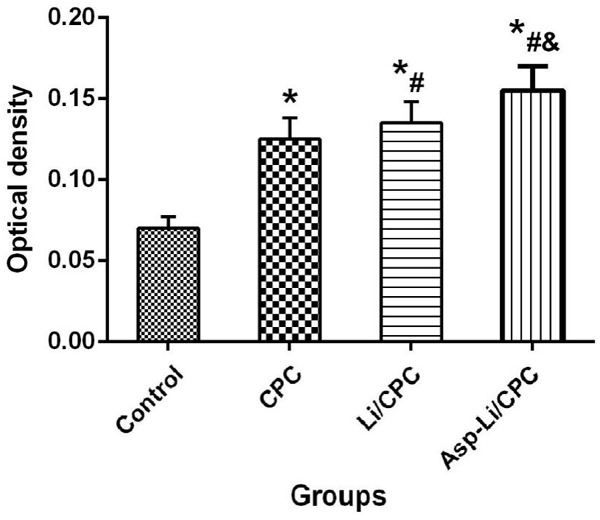
Fluorescent analysis
In order to further understand the effect of three kinds of biomaterials implanted into the defect area on bone tissue mineralization from the microscopic level, we carried out histological examination to determine the mineralization of the defect area. In fluorescent analysis (Figure 8), Asp-Li/CPC showed the largest calcein green-marked defect area (P<0.05), and Asp-Li/CPC exhibited the highest values of relative bone mineralization (green/green marked defect area) (P<0.05) compared with that of the OVX group, CPC group and Li/CPC group. Regarding repair effect of the three materials, it was found that Asp-Li/CPC biomaterials can promote the mineralization of new bone tissue in the defect area. Fluorescent analysis results show that the composite biomaterial Asp-Li/CPC is better than CPC and Li/CPC in repairing osteoporotic bone defects from a new bone tissue mineralization angle.
Figure 8.
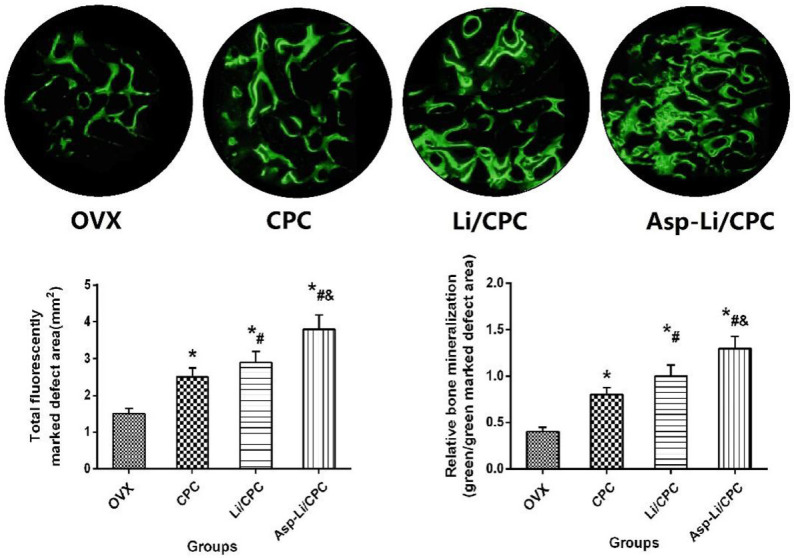
Total fluorescently marked defect area (mm2) and relative bone mineralization (green/green marked defect area) after treatment. *P<0.05 vs. OVX group, #P<0.05 vs. CPC, &P<0.05 vs. Li/CPC group (at ×200 magnification).
RT-qPCR analysis
It is well known that the process of bone repair is regulated by many factors and signal pathways, including bone morphogenetic protein 2 (BMP-2)/small mothers against deca-pentaplegic homologs-1 (Smad1) and osteoprotegerin (OPG)/receptor activator of nuclear factor kappa-B ligand (RANKL) signaling pathway [28,29], inflammatory factor such as tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) and osteogenic marker: Alkaline phosphatase (ALP), osteopontin (OP), runt-related transcription factor 2 (RUNX-2), osteocalcin (OC), type I collagen (COL-1) [30]. Because these signaling pathways and factors play an important role in the bone repair process, we used RT-qPCR to check the gene expression of these factors in the defect area. Gene expression of defect area bone tissue after different treatment is presented in Figure 9. Eight weeks after defect surgery, Asp-Li/CPC, Li/CPC group and CPC group showed increased BMP2, Smad1, OPG than the OVX group (P<0.05), while Asp-Li/CPC exhibited decreased TNF-α, IFN-γ and RANKL than the OVX group (P<0.05). Moreover, Asp-Li/CPC treatment presented the strongest effect on TNF-α, IFN-γ, BMP2, Smad1, OPG and RANKL than other treatment groups (P<0.05). Compared with the repair effect of the three materials, it was found that local use of Asp-Li/CPC biomaterials not only activated the BMP-2/Smad1 and OPG/RANKL signaling pathways, but also inhibited local inflammation, so as to achieve the effect of rapid completion of bone repair. RT-qPCR analysis results show that the composite biomaterial Asp-Li/CPC is better than CPC and Li/CPC in repairing osteoporotic bone defects from a gene regulation level.
Figure 9.
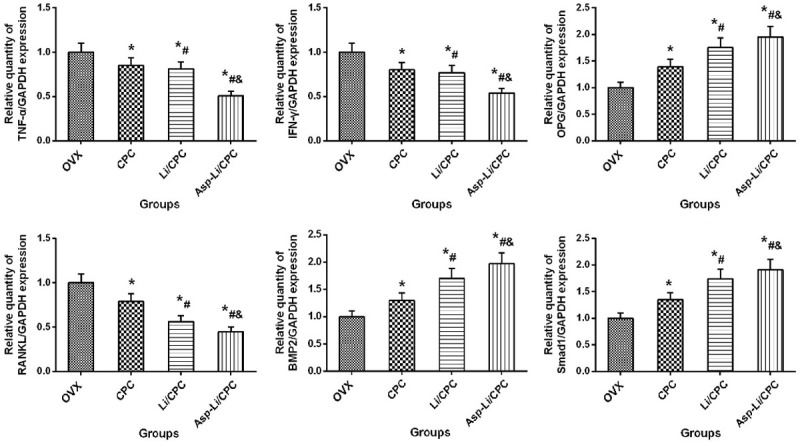
Gene expression of defect area bone tissue after different treatment. *P<0.05 vs. OVX group, #P<0.05 vs. CPC, &P<0.05 vs. Li/CPC group.
Discussions
Nowadays, due to the low regeneration ability of bone remodeling imbalance compared with normal bone, and the lack of bone grafts specially designed for osteoporotic defects, the treatment of osteoporotic defects is still a tricky problem for clinicians. In our previous or other studies, the local application of aspirin was considered to be a good material to support bone defect repair and osseointegration [23,27]. Lithium also shows strong biological and osteogenic activity in bone defect repair and osseointegration [12-14]. It can be concluded that osteoporosis impairs the ability to repair bone defects, even with the use of pure calcium phosphate biomaterials is difficult to achieve desirable result like CPC shown in current study [24]. According to the requirements of osteogenic biomaterials, on the basis of previous studies, Asp and Li were creatively introduced to improve the surface physical, chemical and biological properties of CPC scaffolds, in order to meet the requirements of osteogenic characteristics and osteogenic microenvironment of traditional biomaterials replacement and repair. The results of this study also confirm the hypothesis in the preface, namely, ASP and Li modified CPC can quickly complete bone regeneration of osteoporotic bone defects, which has obvious advantages over pure CPC or Li/CPC.
In the current research, MC3T3-E1 cells cultured in the leachate of different biological scaffolds induction medium have exhibited the highly osteogenic differentiation ability. When cultured with leachate of Asp-Li/CPC scaffolds, mouse osteoblastic MC3T3-E1 cells showed increased differentiation and mineralization by MTT and Alizarin Red S (RES) staining. Moreover, the leachate of Asp-Li-loaded CPC scaffolds obviously promote adherence, proliferation, alkaline phosphatase activity, or mineralization of on growth MC3T3-E1 pre-osteoblasts compared to other control groups. As we all know, during bone resorption by osteoclasts, many cytokines such as ALP, OP, COL-1, RUNX-2 and OC are activated and released from the bone matrix to recruit Bone marrow mesenchymal stem cells and induce differentiation into mature osteoblasts in coupling bone formation with resorption [30]. When MC3T3-E1 osteoblasts are exposed to leachate of Asp-Li/CPC scaffold culture media, the expressions of ALP, OP, COL-1, RUNX-2 and OC are increased when compared with other groups. In our current research, empty defects of OVX group expose poor potential in osteoporotic bone, which were similar to previous studies [31,32]. Imaging and histological scanning revealed the presence of perforations in different biological scaffolds accelerated early bone formation and defect bridging. In the study, we used a composite scaffold with incorporation of CPC and sustained release of lithium to repair femoral metaphysis bone defects in OVX rats, demonstrating that the bioactivity of lithium released from the scaffolds was well maintained, which improves new bone formation and the healing of defects. Even compared with Li/CPC group, bone defects treated with Asp-Li/CPC scaffolds demonstrate a great variation in healing tendency. The Asp-Li/CPC scaffold can achieve satisfactory curative effect in osteoporotic bone defect in vivo and in vitro.
Why lithium and aspirin modified CPC scaffolds can achieve satisfactory results in osteoporotic bone defects? We think that this result is related to the continuous effect of local Asp and Li applied to defected area after Asp-Li/CPC scaffold implant and the local degradation of Asp-Li/CPC scaffold creates a more harmonious environment for bone repair. A potential bioactive Lithium element therapy is emerging with the potential to improve bone regeneration in defects to enhance healing in many cell and animal researches [33,34]. Previous research has shown that lithium promotes the differentiation of osteoblast precursors into osteoblasts and may have an inhibitory effect on osteoclasts [33,34]. Asp is a standard anti-inflammatory drug that has been studied for its ability to promote osteogenic differentiation in a relatively bone-specific manner [16]. Many studies aimed at investigating the local application of Asp on bone repair have been conducted using animal models in recent years [17,35,36].
Lithium play an important role in regulating the balance between bone resorption and formation and aspirin present anti-inflammatory activity. It has previously been demonstrated that BMP-2/Smad1 and OPG/RANKL signaling pathway play the critical role in the interaction between osteoblasts and osteoclasts [28,29]. Proinflammatory T cells secrete cytokines, including IFN-γ and TNF-α, which can inhibit impede stem cell transplantation-based bone repair, but aspirin can promote bone formation through inhibition the production of IFN-γ and TNF-α [17]. In this study, RT-PCR was used to detect the expression of target genes. Examinations of all the specimens indicated that there were substantially higher levels of Smad1, BMP-2 and OPG gene, but lower RANKL gene in Li/CPC than CPC and control group. Since Li was not only activate BMP-2/Smad1 signaling pathway in osteoblasts derived from BMP-2, the results suggest that OPG/RANKL signaling pathway remains mediated in these osteoblasts and promote further proliferation and differentiation of osteoblasts. Our results are similar to those previously reported studies [3,37]. RT-qPCR results also show that Asp-Li/CPC group had the lowest levels of IFN-γ and TNF-α gene, which were all lower than CPC, Li/CPC and control group. In view of the results presented above, we believe that these satisfactory results, in combination with all experimental and analysis data presented above, strongly support the promote bone repair mechanism for the excellent Li promoting osteogenesis, inhibiting osteoclastogenesis and Asp inhibiting local inflammation.
As far as we know, this is the first study to evaluate local use of Lithium and aspirin to improve the osteogenic properties of CPC to meet the requirements of osteoporotic bone defect repair. However, there are some limitations to note with the current study. Firstly, the characterization of different biomaterials and the release characteristics of Asp and Li were not further observed. Secondly, the effects of various biomaterials on osteoclasts were not observed from the biological characteristics of osteoclasts. Finally, in this study, the femoral condyle defect of ovariectomized rats was selected as the model, whether it is suitable for large segment defect of femur and skull defect is not known, and the study time is 8 weeks, and the curative effect before and after 8 weeks is not known.
Conclusions
In the current study, we demonstrated that co-modification of CPC by lithium and aspirin is a scheme for rapid repair of femoral condylar defects, and these effects may be achieved by inhibiting local inflammation and promoting osteogenic activity through BMP-2/Smad1 and OPG/RANKL signaling pathway.
Acknowledgements
This study was supported by a grant from the University Natural Science Research Project of Anhui Province (CN) (grant no. KJ2017A266, KJ2017A117), Funding of “Peak” Training Program and “Panfeng” Innovation Team Project for Scientific Research of Yijishan Hospital, Wannan Medical College (grant no. GF2019G04, PF2019005, GF2019T02 and PF2019007), National Natural Science Foundation of China (82002322), Talented Scholars of Wannan Medical College (YR201917), Young and Middle-aged Key Project of Wannan Medical College (WK2020ZF16), Additive Manufacturing Institute of Anhui Polytechnic University Open Project (Grant No. 2020ybxm06), Wuhu Science and Technology Plan Project (Grant No. 2020ms3-1).
Disclosure of conflict of interest
None.
References
- 1.Liu Y, Wang H, Zhou XZ, Li N, Guo YC, Chen TP. Pentraxin 3 promotes the osteoblastic differentiation of MC3T3-E1 cells through the PI3K/Akt signaling pathway. Biosci Rep. 2020;40:BSR20201165. doi: 10.1042/BSR20201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao ZS, Qiang Z, Tu KK, Huang ZL, Xu HM, Sun T, Lv YX, Cui W, Yang L. Treatment study of distal femur for parathyroid hormone (1-34) and β-tricalcium phosphate on bone formation in critical size defects in rats. J Biomater Appl. 2015;30:484–491. doi: 10.1177/0885328215592854. [DOI] [PubMed] [Google Scholar]
- 3.Tang CY, Chen W, Luo Y, Wu J, Zhang Y, McVicar A, McConnell M, Liu Y, Zhou HD, Li YP. Runx1 up-regulates chondrocyte to osteoblast lineage commitment and promotes bone formation by enhancing both chondrogenesis and osteogenesis. Biochem J. 2020;477:2421–2438. doi: 10.1042/BCJ20200036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tao ZS, Zhou WS, Tu KK, Huang ZL, Zhou Q, Sun T, Lv YX, Cui W, Yang L. Effect exerted by teriparatide upon repair function of β-tricalcium phosphate to ovariectomised rat’s femoral metaphysis defect caused by osteoporosis. Injury. 2015;46:2134–2141. doi: 10.1016/j.injury.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 5.Ghamor-Amegavi EP, Yang X, Qiu J, Xie L, Pan Z, Wang J, Zhang X, Ke X, Zhao T, Zhang L, Gou Z. Composition control in biphasic silicate microspheres on stimulating new bone regeneration and repair of osteoporotic femoral bone defect. J Biomed Mater Res B Appl Biomater. 2020;108:377–390. doi: 10.1002/jbm.b.34396. [DOI] [PubMed] [Google Scholar]
- 6.Tao ZS, Tu KK, Huang ZL, Zhou Q, Sun T, Xu HM, Zhou YL, Lv YX, Cui W, Yang L. Combined treatment with parathyroid hormone (1-34) and beta-tricalcium phosphate had an additive effect on local bone formation in a rat defect model. Med Biol Eng Comput. 2016;54:1353–1362. doi: 10.1007/s11517-015-1402-8. [DOI] [PubMed] [Google Scholar]
- 7.Xu Z, Wang N, Liu P, Sun Y, Wang Y, Fei F, Zhang S, Zheng J, Han B. Poly (Dopamine) coating on 3D-printed poly-lactic-co-glycolic acid/β-tricalcium phosphate scaffolds for bone tissue engineering. Molecules. 2019;24:4397. doi: 10.3390/molecules24234397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Kai Z, Wang D, Tao L, Zhang P, Wang D, Liu D, Sun S, Zhong J. Allogenic chondrocyte/osteoblast-loaded β-tricalcium phosphate bioceramic scaffolds for articular cartilage defect treatment. Artif Cells Nanomed Biotechnol. 2019;47:1570–1576. doi: 10.1080/21691401.2019.1604534. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Cai L, Tang L, Zhang X, Yang L, Zheng K, He A, Boccaccini AR, Wei J, Zhao J. Highly dispersed lithium doped mesoporous silica nanospheres regulating adhesion, proliferation, morphology, ALP activity and osteogenesis related gene expressions of BMSCs. Colloids Surf B Biointerfaces. 2018;170:563–571. doi: 10.1016/j.colsurfb.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Vachhani K, Whyne C, Wang Y, Burns DM, Nam D. Low-dose lithium regimen enhances endochondral fracture healing in osteoporotic rodent bone. J Orthop Res. 2018;36:1783–1789. doi: 10.1002/jor.23799. [DOI] [PubMed] [Google Scholar]
- 11.Arioka M, Takahashi-Yanaga F, Sasaki M, Yoshihara T, Morimoto S, Hirata M, Mori Y, Sasaguri T. Acceleration of bone regeneration by local application of lithium: Wnt signal-mediated osteoblastogenesis and Wnt signal-independent suppression of osteoclastogenesis. Biochem Pharmacol. 2014;90:397–405. doi: 10.1016/j.bcp.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Li D, Zhao J, Yang Z, Kang P. In vivo evaluation of porous lithium-doped hydroxyapatite scaffolds for the treatment of bone defect. Biomed Mater Eng. 2018;29:699–721. doi: 10.3233/BME-181018. [DOI] [PubMed] [Google Scholar]
- 13.Khan PK, Mahato A, Kundu B, Nandi SK, Mukherjee P, Datta S, Sarkar S, Mukherjee J, Nath S, Balla VK, Mandal C. Influence of single and binary doping of strontium and lithium on in vivo biological properties of bioactive glass scaffolds. Sci Rep. 2016;6:32964. doi: 10.1038/srep32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu J, Peng L, Li J, Tao T, Chen Y. Effects of second-generation antiepileptic drugs compared to first-generation antiepileptic drugs on bone metabolism in patients with epilepsy: a meta-analysis. Horm Metab Res. 2019;51:511–521. doi: 10.1055/a-0963-0054. [DOI] [PubMed] [Google Scholar]
- 15.Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 16.Yuan M, Zhan Y, Hu W, Li Y, Xie X, Miao N, Jin H, Zhang B. Aspirin promotes osteogenic differentiation of human dental pulp stem cells. Int J Mol Med. 2018;42:1967–1976. doi: 10.3892/ijmm.2018.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17:1594–601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Pan M, Gao Y, Zhang L, Ge W, Tang P. Dose-dependent roles of aspirin and other non-steroidal anti-inflammatory drugs in abnormal bone remodeling and skeletal regeneration. Cell Biosci. 2019;9:103. doi: 10.1186/s13578-019-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao ZS, Bai BL, He XW, Liu W, Li H, Zhou Q, Sun T, Huang ZL, Tu KK, Lv YX, Cui W, Yang L. A comparative study of strontium-substituted hydroxyapatite coating on implant’s osseointegration for osteopenic rats. Med Biol Eng Comput. 2016;54:1959–1968. doi: 10.1007/s11517-016-1494-9. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Peng X, Qin Y, Wang R, Tang J, Cui X, Wang T, Liu W, Pan H, Li B. Acceleration of bone regeneration by activating Wnt/β-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci Rep. 2017;7:45204. doi: 10.1038/srep45204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao ZS, Zhou WS, Wu XJ, Zhang X, Wang L, Xie JB, Xu ZJ, Ding GZ, Yang M. Prevention of ovariectomy-induced osteoporosis in rats: comparative study of zoledronic acid, parathyroid hormone (1-34) and strontium ranelate. Z Gerontol Geriatr. 2019;52:139–147. doi: 10.1007/s00391-018-1376-x. [DOI] [PubMed] [Google Scholar]
- 22.Tao ZS, Lv YX, Cui W, Huang ZL, Tu KK, Zhou Q, Sun T, Yang L. Effect of teriparatide on repair of femoral metaphyseal defect in ovariectomized rats. Z Gerontol Geriatr. 2016;49:423–428. doi: 10.1007/s00391-015-0949-1. [DOI] [PubMed] [Google Scholar]
- 23.Tao ZS, Wu XJ, Zhou WS, Wu XJ, Liao W, Yang M, Xu HG, Yang L. Local administration of aspirin with β-tricalcium phosphate/poly-lactic-co-glycolic acid (β-TCP/PLGA) could enhance osteoporotic bone regeneration. J Bone Miner Metab. 2019;37:1026–1035. doi: 10.1007/s00774-019-01008-w. [DOI] [PubMed] [Google Scholar]
- 24.Tao ZS, Zhou WS, Wu XJ, Wang L, Yang M, Xie JB, Xu ZJ, Ding GZ. Single-dose local administration of parathyroid hormone (1-34, PTH) with β-tricalcium phosphate/collagen (β-TCP/COL) enhances bone defect healing in ovariectomized rats. J Bone Miner Metab. 2019;37:28–35. doi: 10.1007/s00774-018-0906-3. [DOI] [PubMed] [Google Scholar]
- 25.Tao ZS, Zhou WS, Tu KK, Huang ZL, Zhou Q, Sun T, Lv YX, Cui W, Yang L. Treatment study of distal femur for parathyroid hormone (1-34) and β-tricalcium phosphate on bone formation in critical-sized defects in osteopenic rats. J Craniomaxillofac Surg. 2015;43:2136–43. doi: 10.1016/j.jcms.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Tao ZS, Wu XJ, Yang M, Xu HG. Local administration with silymarin could increase osseointegration of hydroxyapatite-coated titanium implants in ovariectomized rats. J Biomater Appl. 2019;34:664–672. doi: 10.1177/0885328219863290. [DOI] [PubMed] [Google Scholar]
- 27.Tao Z, Zhou W, Wu X, Lu H, Ma N, Li Y, Zhang R, Yang M, Xu HG. Local administration of aspirin improves osseointegration of hydroxyapatite-coated titanium implants in ovariectomized rats through activation of the notch signaling pathway. J Biomater Appl. 2020;34:1009–1018. doi: 10.1177/0885328219889630. [DOI] [PubMed] [Google Scholar]
- 28.Ren C, Gong W, Li F, Xie M. Pilose antler aqueous extract promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells by stimulating the BMP-2/Smad1, 5/Runx2 signaling pathway. Chin J Nat Med. 2019;17:756–767. doi: 10.1016/S1875-5364(19)30092-5. [DOI] [PubMed] [Google Scholar]
- 29.Ge YW, Feng K, Liu XL, Zhu ZA, Chen HF, Chang YY, Sun ZY, Wang HW, Zhang JW, Yu DG, Mao YQ. Quercetin inhibits macrophage polarization through the p-38α/β signalling pathway and regulates OPG/RANKL balance in a mouse skull model. J Cell Mol Med. 2020;24:3203–3216. doi: 10.1111/jcmm.14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva de Oliveira JC, Luvizuto ER, Sonoda CK, Okamoto R, Garcia-Junior IR. Immunohistochemistry evaluation of BMP-2 with β-tricalcium phosphate matrix, polylactic and polyglycolic acid gel, and calcium phosphate cement in rats. Oral Maxillofac Surg. 2017;21:247–258. doi: 10.1007/s10006-017-0624-3. [DOI] [PubMed] [Google Scholar]
- 31.García-García P, Reyes R, Pérez-Herrero E, Arnau MR, Évora C, Delgado A. Alginate-hydrogel versus alginate-solid system. Efficacy in bone regeneration in osteoporosis. Mater Sci Eng C Mater Biol Appl. 2020;115:111009. doi: 10.1016/j.msec.2020.111009. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Li Z, Jiang Y, Liu H, Feng Y, Wang Z, Liu H, Wang J, Yang B, Lin Q. Bioinspired mineral hydrogels as nanocomposite scaffolds for the promotion of osteogenic marker expression and the induction of bone regeneration in osteoporosis. Acta Biomater. 2020;113:614–626. doi: 10.1016/j.actbio.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Pan J, He S, Yin X, Li Y, Zhou C, Zou S. Lithium enhances alveolar bone formation during orthodontic retention in rats. Orthod Craniofac Res. 2017;20:146–151. doi: 10.1111/ocr.12190. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Zhu Y, Lu S, Hu C, Zhong W, Chai Y. Beneficial effects of paeoniflorin on osteoporosis induced by high-carbohydrate, high-fat diet-associated hyperlipidemia in vivo. Biochem Biophys Res Commun. 2018;498:981–987. doi: 10.1016/j.bbrc.2018.03.093. [DOI] [PubMed] [Google Scholar]
- 35.Lei L, Liu Z, Yuan P, Jin R, Wang X, Jiang T, Chen X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J Mater Chem B. 2019;7:2722–2735. doi: 10.1039/c9tb00025a. [DOI] [PubMed] [Google Scholar]
- 36.Du J, Mei S, Guo L, Su Y, Wang H, Liu Y, Zhao Z, Wang S, Liu Y. Platelet-rich fibrin/aspirin complex promotes alveolar bone regeneration in periodontal defect in rats. J Periodontal Res. 2018;53:47–56. doi: 10.1111/jre.12485. [DOI] [PubMed] [Google Scholar]
- 37.Kazemi M, Dehghan MM, Azami M. Biological evaluation of porous nanocomposite scaffolds based on strontium substituted β-TCP and bioactive glass: an in vitro and in vivo study. Mater Sci Eng C Mater Biol Appl. 2019;105:110071. doi: 10.1016/j.msec.2019.110071. [DOI] [PubMed] [Google Scholar]