Abstract
Background: As the U.S. population grows older and more diverse, self-management needs are increasingly complicated. In order to deliver effective personalized interventions to those suffer from chronic conditions social determinants of health must be considered. Therefore, psychosocial phenotyping holds strong promise as a tool for tailoring interventions based on precision health principles. Purpose: To define psychosocial phenotyping and develop a research agenda that promotes its integration into chronic disease management as a tool for precision self-management interventions. Methods: Since psychosocial phenotyping is not yet used in interventions for self-management support, we conducted a literature review to identify potential phenotypes for chronic disease self-management. We also reviewed policy intervention case reports from the Centers for Medicare and Medicaid Services to examine factors related to social determinants of health in people with chronic illnesses. Finally, we reviewed methodological approaches for identifying patient profiles or phenotypes. Results: The literature review revealed areas within which to collect data for psychosocial phenotyping that can inform personalized interventions. The findings of our exemplar cases revealed that several environmental or key SDOH such as factors realted with economic stability and neighborhood environment have been closely linked with the success of chronic disease management interventions. We elucidated theory, definitions, and pragmatic conceptual boundaries related to psychosocial phenotyping for precision health. Conclusions: Our literature review with case example analysis demonstrates the potential usefulness of psychosocial phenotyping as a tool to enhance personalized self-management interventions for people with chronic diseases, with implications for future research.
Keywords: Psychosocial phenotyping, precision health, multiple chronic disease, self-management, social determinants of health
Introduction
According to a 2011 CDC report, 6 out of 10 US adults suffer from chronic diseases, at an estimated annual healthcare cost of 3.3 trillion dollars [1]. Changes in demographics, lifestyle, and environmental factors, as well as medical successes, have transformed once terminal diagnoses into chronic conditions. To effectively manage chronic diseases such as diabetes or hypertension, self-management and lifestyle modifications are important. Self-management has been defined as “the ability of the individual, in conjunction with family, community, and healthcare professionals, to manage symptoms, treatments, lifestyle changes, and psychosocial, cultural, and spiritual consequences of health conditions (particularly chronic diseases)” [2]. It is well-known that self-management and changes in health behavior are challenging for many people [3]. Effective chronic disease management requires significant self-management skills, including not only changes in health behavior but also maintenance, problem solving, and resource utilization, all of which must be integrated into patients’ daily lives in order to achieve benefits for health [4].
Individual differences play an important role in the adoption and maintenance of health behavior changes for self-management. For many decades, theory-based tailoring has been the focus of efforts to personalize interventions to improve patients’ self-management success. However, in 2015, the Precision Medicine Initiative ushered in a new era in the use of personalized medicine [5]. This research effort is leading to new approaches in pharmacology [6,7] and clinical medicine [8] that leverage extensive genetic, genomic, and clinical data for individualized care. In cancer management, for example, individualized treatment plans enabled by advances in the availability of data and in analytics are increasingly available [9].
Given the societal need to address chronic diseases and psychosocial challenges, new efforts to provide self-management support for these individuals might utilize precision self-management plans. We propose that rather than supplanting theory-based intervention tailoring with data-driven precision approaches, these two paradigms should work in concert. Existing theoretical evidence can be combined with novel insights gained from machine learning and big data to improve self-management science. While precision approaches have thus far focused on individual factors, including genetics, clinical diagnoses, psychology, and behavior, many health behavior theories that can be applied to self-management address the collective function of intra- and interpersonal factors that take place within family, community, and society such as social determinants of health (SDOH).
Thus there is a distinct need to obtain comprehensive information about individuals to create meaningful profiles, or phenotypes, that can inform the personalization of self-management interventions. In the context of chronic disease management, however, the science of phenotyping is in its infancy. For genetic phenotyping, we currently use genome sequencing; for clinical phenotyping, we use electronic health record (EHR) data. While there is some effort to merge individuals’ clinical data and genetics, e.g., eMerge [10], to provide insights for effective treatment, there are few approaches that include psychosocial factors that influence self-management behaviors.
Here we present a framework for synchronizing these seemingly divergent paradigms via “psychosocial phenotyping”. First we define psychosocial phenotyping; then we review the literature to identify the constructs that should be included in this new type of phenotyping; and finally we discuss the implications of this approach for self-mangement interventions, including methodologies, data sources, and implications for health disparities and ethics. Furthermore, we offer methodological insights and discuss the potential interplay between psychosocial phenotyping and other emerging approaches in self-management science such as digital phenotyping, health equity in the context of population science, and ethical implications.
Psychosocial phenotyping definition
Precision medicine efforts to personalize healthcare for individual patients have focused primarily on using genomic data or selective biomarkers to identify molecularly selective therapeutic targets [11]. More recently, data from clinical records to identify individual differences in prognoses [12,13] have been used. Although these efforts to integrate multiple sources of data into patient care are increasing, precision medicine approaches are used almost exclusively in treatment, despite their potential applicability in prevention and in behavioral interventions. There is extensive evidence from self-management science and behavioral research that different people respond to behavioral interventions in different ways [14]. Individual characteristics such as gender, personality, and cultural and contextual factors influence educational and behavioral interventions. Moreover, growing evidence indicates that environmental factors and SDOH exert strong influences on the prevention and management of chronic diseases.
Phenotyping based on genetic and clinical data has been used to identify characteristics to inform response platforms for treatment options in precision medicine. Phenotypes, sets of observable characteristics that reflect the interaction of an organism’s genes and environment, present an apt analogy for characterizing the combinations of attitudes, social influences, and personal agency that mediate individuals’ chronic disease management. However, these data are not typically represented in current approaches to precision health. We propose that psychosocial phenotyping can propel the use of precision health in self-management science for the future of chronic disease management.
Psychosocial phenotypes have been defined on the basis of the “psychological and social characteristics” of patients’ obtained from EHRs [15]. More recently, however, a patient’s psychosocial phenotype has been defined as “the combination of psychological and social characteristics that explain variations in behavioral response to an intervention” [16]. The National Institute of Diabetes and Digestive and Kidney Diseases refers to a related concept, the behavioral and psychological phenotype, as “a pattern of behavior or psychological characteristics that are measurable/quantifiable and distinct (explains individual variation)” [17]. All of these definitions are similar, but it is important that research on psychosocial phenotypes and psychosocial phenotyping methods focus not only on factors solely internal to the patient but also on external, structural factors that interact with the individual and influence the individual’s response to self-management interventions.
Based on prior work in the field [16,17], we propose a working definition of psychosocial phenotyping in the context of self-management science as a methodology to identify patterns of measurable, quantifiable, distinct behaviors or psychological characteristics that can explain individual variation in the context of the self-management of chronic conditions. This definition is based on the following theoretical premises: (1) as a variable, the psychosocial phenotype must represent the complex interplay between psychological and social determinants of health; (2) phenotyping is a valid, replicable way to identify the behavioral and/or psychological expressions (phenotypes) that meaningfully explain individual variability in behavioral or clinical outcomes in response to self-management interventions; and (3) the identification of phenotypes should improve the matching or tailoring of interventions or suggest novel targets for more efficacious individual and population-level approaches for the self-management of chronic conditions [18].
Genetic or genomic phenotyping has been validated using well-established sequencing protocols for the analysis of big data accumulated over the years. In this paper, we aim to develop an agenda for future research in psychosocial phenotyping for the self-management of chronic disease management on the population level. To fully understand and validate psychosocial phenotyping for use, multiple sources of large, diverse sets of data are necessary-self-reported data, community level data, data collected in clinical settings, and digital traces of behavior. Moreover, a theoretical framework and consensus regarding important factors for psychosocial phenotyping are needed. Finally, common data elements for psychosocial phenotyping must be identified, especially those that are potentially useful in the context of self-management behaviors of those with chronic illnesses.
Literature review method
We conducted an integrated literature review to identify the most common and measurable phenotypic characteristics of individuals that are predictive of individual variation in self-management processes and outcomes. By reviewing current psychosocial phenotyping efforts and including exemplars of psychosocial phenotyping methods in relation to chronic disease management, we were able to establish a basis for psychosocial phenotyping as well as the obvious gaps in the literature. We then present a framework to guide future research.
Because there is no scientific consensus regarding how to best derive psychosocial phenotypes, it is important to consider avenues traditionally used to target, account for, and better understand variations in response to interventions. Prior literature has explored myriad reliable psychosocial/nongenomic factors potentially significant for the development of composite phenotypes. By examining the literature for the types of factors that have been assessed and finding ways to integrate them within advances in data science, it may be possible to arrive at a synergy between traditional and contemporary methods.
We specifically reviewed evidence on chronic disease management in the context of metabolic, cardiovascular, and respiratory conditions as well as diabetes mellitus. We searched the English-language literature in PubMed and Web of Science through July 2019 using the following search terms and Medical Subject Headings: chronic disease, diabetes, hypertension, heart failure, asthma, COPD, obesity, intervention adherence, intervention compliance, intervention persistence, education, behavior, profile, characteristics, pheynotype, factor, determinant, predictor, correlate. Included studies were review articles-literature reviews of both psychosocial factors AND phenotypes/profiles, according to titles/abstracts-that examined at least two psychosocial factors for participants but not for the interventions themselves. Studies were excluded if they focused on pharmacological management, clinical phenotyping, pain, or genetic disorders, or if they were done with animal subjects. Articles were then reviewed by the authors to ensure that they met selection criteria, with any disagreements resolved via group consensus.
Selection of “exemplar” SDOH cases
To select pertinent cases that illustrate the promise of psychosocial phenotyping, we also considered several policy intervention case reports from the Centers for Medicare and Medicaid Services (CMS) that address social determinants of health (SDOH) of people with chronic illnesses and demonstrate the clinical utility of personalized interventions informed by social needs rather than simply by medical diagnosis [19-22]. Among them, we chose the best examples for each of the domains of factors that emerged from our initial literature review. The selected studies were reviewed and discussed.
Results
Search results, characteristics, and factor domains
Our search yielded 28,379 studies that were reduced to 105 through title and abstract screening. Full text screening further reduced those studies to 24, which consisted of systematic reviews and meta-analyses, the majority of which were published in the last 5 years (71%, n = 17). See Figure 1 for study selection flowchart. Eleven of the 24 studies (46%) were meta-analyses. All chronic conditions represented by the search terms were included in our sample, with cardiovascular disease (38%, n = 9 studies) the most frequent, followed by metabolic syndrome (25%, n = 6) and diabetes (17%, n = 4). The most frequent study outcomes measured participants’ adherence/engagement to interventions (67%, n = 16 studies), and nearly all of the studies evaluated associations between psychosocial factors and the stated outcome(s) of interest (92%, n = 22). The reviewed studies yielded five domains of factors important to the formation of psychosocial phenotypes: demographic, psychological, social, clinical, and environmental. Demographic and psychological characteristics tied as the most frequently included domains (79%, n = 19 studies) and only 3 studies (13%) examined all five domains of factors.
Figure 1.
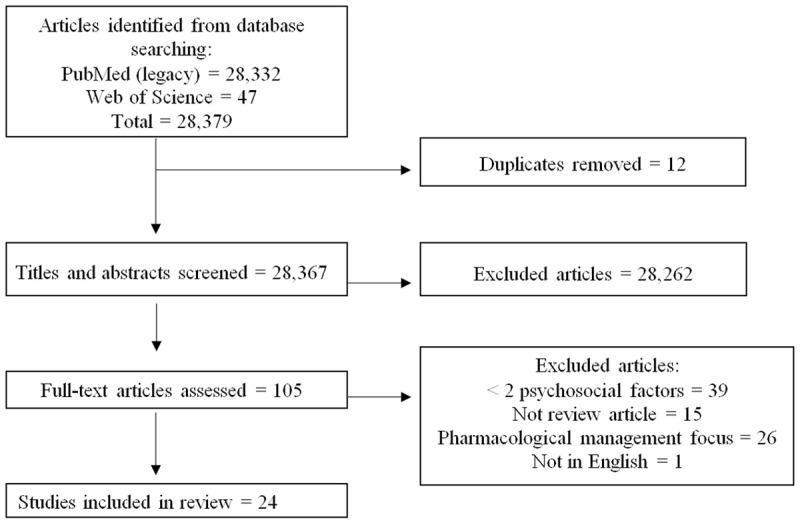
Flow diagram of study selection process.
Demographic characteristics
The majority of the studies (79%, n = 19) included at least two of the commonly examined characteristics of gender, age, racial/ethnicity, marital status, educational attainment, income, and socioeconomic status [4,8,23-34]. Studies also examined specific characteristics that related to their specific research question(s): immigration status [8], number of children living in the home [4], languages spoken [24], insurance type(s) [1,28], religion [25], and other financial indicators such as out-of-pocket spending on prescription drugs or insurance copayments [3,28,29,34]. Of the three studies that did not include demographic characteristics, one was a review paper focused specifically on psychosocial predictors [31], and two were not review papers but described psychosocial phenotypes using other methods: one was a qualitative study [16]; the other, a multidisciplinary workshop [26].
Many of the studies examined in the 24 articles presented individual interventions in which specific demographic or individual characteristics were selected as factors in intervention design. The most frequently used individual characteristics were gender, level of education, access to regular care, and household or individual socioeconomic status. Among demographic factors, gender, age, and education were often linked to clinical outcomes or the process of self-management of chronic illnesses. For example, one meta-analysis [30] found that medication adherence interventions in hypertensive patients were most effective in older women with higher education and moderate to higher income. The authors of that article suggested that characteristics of interventions would need to be adapted for younger men with limited income to increase effectiveness.
Psychological factors
Multiple psychological factors are related to a person’s emotional and mental state. Such factors were as commonly examined in the literature as demographic characteristics (79%, n = 19 studies) [15,16,24-27,29,31-43]. Included psychological factors were as follows: self-efficacy (25%, n = 6) [16,25,27,32,33,36], depression (58%, n = 14) [24,25,27,29,31,33,35,36,38,39,41-44], stress [25,27,36], body image [36], and readiness/stage of change [36]. In 2 studies, self-efficacy [25] and stage of change [24] were derived from theoretical approaches to behavior change.
Several of the meta-analyses indicated that individual levels of self-efficacy explained significant variations in self-management outcomes among populations with chronic illnesses [24,25,28]. Similarly, levels of depression also influenced both processes (self-management behaviors) and outcomes of self-management in many studies [31]. For example, self-efficacy was a strong predictor for dietary adherence among people with Type 2 diabetes in a meta-analysis conducted by Brown et al. Also, people with depression were twice as likely to be observed with medication nonadherence as were people without depression [31].
One novel factor included in two studies [31,33] addressing cardiovascular disease was “distressed” or Type D personality. Type D personality-social inhibition and negative affectivity-was found to be related to medication nonadherence [2,24,45-47] and decreased consulting behavior despite increased symptoms [24,48,49].
Social factors
Ten studies (42%) [2,4,7,8,16,26,31-33,50] addressed factors such as social support (25%, n = 6) [2,4,16,26,31,33]. Other such factors, including social pressure [8], social norms [7], patient-child relationship [32], and social network size [2], were addressed by single studies in the sample. Results from 5 studies examining social support showed that it was associated with the outcome of interest and showed a positive relationship such that increased social support increased self-management outcomes (21%, n = 5) [2,8,24,26,33]. For example, a meta-analysis by Lemstra found that weight loss interventions with social support had higher adherence than did those without social support (RR, 1.29: 95% CI, 1.24-1.34) [35]. The social support in the studies in Lemstra’s meta-analysis included group sessions, peer coaches, social support contracts, and “buddy” programs [35].
Clinical factors
The clinical domain comprised factors related to the chronic disease of interest and any comorbidities and was represented by 14 studies in the sample (58%) [3,5,9,15,16,23,25,28,29,31-34,50]. The most common factors were duration of disease [5,9,25], weight status/BMI [16,23], number of medications [25,33], and comorbidities (25%, n = 6 studies) [3,25,29,31-33]. For example, people with disease conditions such as osteopororsis and hyperlipidemia had the highest rates of nonadherence to medication interventions in comparson with people with diabetes in one meta-analysis [28]. Three studies used clinical factors for their outcome of interest, with two examining hemoglobin A1C [5,9] and one examining cardiorespiratory fitness via maximal or submaximal incremental cardiopulmonary exercise on a treadmill or cycle ergometer [30].
Environmental and SDOH-related factors
The final domain of factors that emerged from the literature was the environment, which was least frequently assessed (33%, n = 8 studies) [1,3,4,7,23,31,34]. Environment was most often operationalized in the assessment of healthcare settings (17%, n = 4 studies) [1,3,4,31], with a few studies including more specific factors such as neighborhood environment [7,23] and community population type such as urban versus rural [31,34]. Our sample shows a clear gap in the traditional literature in addressing environmental factors within the context of chronic disease management.
Results from the exemplar case studies found that in addition to safety of the home and neighborhood environment, evolving literature on SDOH includes factors related to food security and access to transportation [51]. Because of information constraints in traditional research or literature regarding broad social factors, we examined recent efforts by the CMS to address SDOH. Many SDOH demonstration intervention projects are still in planning or undergoing implementation, so that full evaluation reports are not yet available, but preliminary reports on these programs do provide insight for identifying important social factors that may be useful in expanding our understanding of SDOH in those with chronic diseases. Overall, early reports indicate that programs addressing SDOH tend to produce better health outcomes, better health equity, and more economic strategies than do traditional programs based on medical diagnosis [52]. For example, under a 2012 Medicaid demonstration waiver, the state of Oregon introduced a coordinated care model in which organizations experimented with reimbursement for a wide variety of flexible services that supplement covered benefits, in order to address SDOH that affect individuals’ care processes. Examples of reimbursed flexible services provided by Oregon care organizations include (1) items (or devices) helpful for managing specific chronic conditions; (2) assistance with food and nutrition; (3) classes or memberships to promote wellness; (4) temporary housing and environmental improvements; (5) transportation; and (6) care coordination or case management programs [21]. Precise cost effectiveness data are not yet available, but this SDOH program for a CMS population as well as other health care plans that recognize SDOH are rapidly being expedited.
According to a recent evaluation of approaches to SDOH across 17 states, commonly reported topics for SDOH screening were housing instability and food insecurity [22]. Many of these programs are based on the definitions and framework for SDOH given in Healthy People 2020, which refer specifically to (1) economic stability (employment, food insecurity, housing instability, poverty); (2) education (early childhood education and development, enrollment in higher education, high school graduation, language and literacy); (3) social and community context (civic participation, discrimination, incarceration, social cohesion); (4) health and health care (access to health care, access to primary care, health literacy); and (5) neighborhood and built environment (access to foods that support healthy eating, crime and violence, environmental conditions, quality of housing) [53].
A psychosocial phenotyping approach that encompasses all the major factor domains including environmental and SDOH can hold the greatest potential in eliciting factors of most significance to chronic disease self-management. Specifically, the demographic, psychological, social and environmental factors discussed above are the likely candidates for inclusion in psychosocial phenotypes and deserve further exploration. See Table 1.
Table 1.
Characteristics of included studies
| First author, Year | Study design | Chronic disease focus | Sample characteristics | Selected psychosocial factors & outcomes addressed | Factors linked to outcomes | Domains addressed |
|---|---|---|---|---|---|---|
| 1. Alramadan, 2018 | SR | T2DM | ● 13 studies | Factors: | A1c: | Demographics √ |
| ● Arabian Gulf Council countries | ● Diet | - Longer T2DM duration | Psychological √ | |||
| ● Med adherence | - Anxiety | Social | ||||
| ● Self-mgmt | - Depression | Clinical √ | ||||
| ● Attitudes toward T2DM | - Lower education level | Environmental | ||||
| ● Anxiety | - Poor med adherence | |||||
| ● Depression | ||||||
| ● T2DM duration | ||||||
| ● Education | ||||||
| ● Gender | ||||||
| Outcome: | ||||||
| ● A1c | ||||||
| 2. Brown, 2016 | SR/MA | T2DM | ● 739 research reports | Factors: | A1c: | Demographics √ |
| ● 533,445 participants | ● Stress | + Dietary adherence | Psychological √ | |||
| ● Mean age 58.9 ± 6.2 years | ● Depression | + Coping | Social | |||
| ● 52% of studies in the US | ● Anxiety | + Stress | Clinical √ | |||
| ● 73% published | ● Self-efficacy | Adherence behaviors: | Environmental | |||
| ● 11% attrition rate | ● Coping | + Self-efficacy | ||||
| ● Diet adherence | Fasting blood glucose: | |||||
| ● Exercise adherence | - No associations | |||||
| ● Med adherence | ||||||
| ● Diabetes duration | ||||||
| Outcomes: | ||||||
| ● A1c | ||||||
| ● Fasting blood glucose | ||||||
| ● Adherence behaviors | ||||||
| 3. Bryan, 2017 | NIDDK workshop and exemplars | Physical activity and sedentary behavior | ● Not applicable | Factors: | None assessed | Demographics |
| ● Reinforcing value | Psychological √ | |||||
| ● Affective response | Social | |||||
| Outcome: | Clinical | |||||
| ● None assessed | Environmental | |||||
| 4. Burgermaster, 2018 | Qualitative | Obesity prevention | ● 18 children | Factors: | 4 Psychosocial phenotypes related to outcomes identified: | Demographics |
| ● 5th grade | ● Self-efficacy | + Activated-successful at behavior changes | Psychological √ | |||
| ● 88% racial/ethnic minority | ● Self-regulation | - Inspired-motivated but not successful at behavior changes | Social √ | |||
| ● Neighborhood environment | + Reinforced-experienced at target health behaviors | Clinical | ||||
| ● Social norms | - Indifferent-uninterested in behavior changes | Environmental √ | ||||
| ● Skills-nutrition label reading | ||||||
| Outcome-increased: | ||||||
| ● Fruit & veggies | ||||||
| ● Exercise | ||||||
| Outcome-decreased: | ||||||
| ● Sugary drinks | ||||||
| ● Processed snacks | ||||||
| 5. Burgess, 2017 | SR | Obesity | ● 24 total studies | Factors: | Predictors of adherence: | Demographics √ |
| ● 17 of 24 identified predictors of behavior change | ● Self-efficacy | + Mood | Psychological √ | |||
| ● Psychiatric disorders | + Male | Social √ | ||||
| ● Stress | + Older age | Clinical | ||||
| ● Anxiety | + Early weight loss success | Environmental | ||||
| ● Depression | Barriers to behavior change: | |||||
| ● Social pressure | + Physical limitations | |||||
| ● Immigrant status | + Low income | |||||
| Outcomes: | + Poor motivation | |||||
| ● Predictors of adherence | + Social pressures | |||||
| ● Barriers to behavior change | ||||||
| 6. Cheen, 2019 | SR/MA | Chronic diseases | ● 33 studies in SR | Factors: | Med non-adherence: | Demographics √ |
| ● 79% cohort studies in SR | ● Age | + Chronic disease type-osteoporosis | Psychological | |||
| ● 31 studies in MA | ● English speaking | + Younger age | Social | |||
| ● 519,971 participants in MA | ● Insurance type | + Number of co-medications | Clinical | |||
| ● Medicare status | + High co-payment | Environmental √ | ||||
| ● Distrust of meds | ||||||
| ● Care setting | ||||||
| ● Alcohol consumption | ||||||
| ● Chronic dx type | ||||||
| Outcome: | ||||||
| ● Med non-adherence | ||||||
| 7. Choi, 2017 | SR/MA | T2DM | ● 33 studies in SR | Factors: | Med engagement: | Demographics √ |
| ● 79% in US | ● Age | - Women | Psychological √ | |||
| ● 22 studies in MA | ● Gender | - Depression | Social | |||
| ● MA included age, gender, depression and costs only | ● Depression | - Out-of-pocket costs | Clinical √ | |||
| ● Out-of-pocket costs | + Older age | Environmental √ | ||||
| ● Comorbidities | ||||||
| ● Glycemic control | ||||||
| ● Pharmacy type | ||||||
| Outcome: | ||||||
| ● Med engagement | ||||||
| 8. Conn, 2015 | SR/MA | HTN | ● 101 studies | Factors: | Med adherence: | Demographics √ |
| ● 88% published | ● Setting | + Female | Psychological | |||
| ● 8.1% attrition | ● Discipline | + Older age | Social √ | |||
| ● Dose | + Moderate to high income | Clinical | ||||
| ● Duration | + Duration of intervention | Environmental √ | ||||
| ● Social support for adherence | ||||||
| ● Socioeconomic status | ||||||
| ● Age | ||||||
| ● Gender | ||||||
| Outcome: | ||||||
| ● Med Adherence | ||||||
| 9. Crawshaw, 2016 | SR/MA | Acute coronary syndrome | ● 17 total studies | Factors: | Med non-adherence: | Demographics |
| ● 7,401 participants | ● Depression | + Depression | Psychological √ | |||
| ● Mean age 61.8 ± 4.5 years | ● Distressed personality type | + Distressed personality type | Social √ | |||
| ● Social network size | + Treatment beliefs | Clinical | ||||
| ● Social support | - Social support | Environmental | ||||
| ● Treatment beliefs | ||||||
| Outcome: | ||||||
| ● Med non-adherence | ||||||
| 10. Fuentes, 2019 | Cohort, Creation of two psycho-social profiles to explore associations with socio- economic variables | Obesity | ● 4,519 participants | Factors included in psychosocial profiles: | Psychosocial profiles (adverse and favorable profiles) and socioeconomic variables: | Demographics √ |
| ● Paris, France | ● Weight dissatisfaction | - No associations | Psychological √ | |||
| ● Mean age 53.3 | ● Weight locus of control | Social | ||||
| ● 67.1% employed | ● Perceptions of body | Clinical √ | ||||
| ● 21% obese | ● Quality of life | Environmental √ | ||||
| ● Self-efficacy | ||||||
| Outcomes: | ||||||
| Socioeconomic variables | ||||||
| ● Childhood socioeconomic status | ||||||
| ● Socioeconomic status | ||||||
| ● Neighborhood socioeconomic level | ||||||
| 11. Gundlapalli, 2013 | Develop algorithms for psychosocial phenotyping | Not applicable | ● Veterans Affairs data | Developed lexicon for homelessness: | Psychosocial concepts identified: | Demographics √ |
| ● Pulled from 218 standard note titles | ● 300+ terms identified from literature, experts, and medical records | + 58,707 | Psychological √ | |||
| ● Used NLP pipeline v3NLP | Outcomes: | Hit rate: | Social √ | |||
| ● Number of psychosocial concepts identified | + 0.2 concepts per document | Clinical √ | ||||
| ● Hit rate | Precision: | Environmental √ | ||||
| ● Precision | + 80% | |||||
| ● Sensitivity | Sensitivity: | |||||
| + 49% (95% CI 43-55%) | ||||||
| 12. Kessing, 2016 | SR/MA | Heart failure | ● 65 studies | Factors: | Self-care: | Demographics |
| ● 31 studies from US | ● Anxiety | - Depression | Psychological √ | |||
| ● 72% cross sectional | ● Depression | - Self-efficacy | Social | |||
| ● Self-efficacy | - Mental well being | Clinical | ||||
| ● Distressed personality type | Med adherence: | Environmental | ||||
| ● Mental well being | - None significant | |||||
| Outcome: | ||||||
| ● Self-care | ||||||
| ● Med adherence | ||||||
| 13. Krass, 2015 | SR/MA | T2DM | ● 27 studies | Factors: | Med Adherence: | Demographics √ |
| ● 67% cross-sectional | ● Age | - Depression | Psychological √ | |||
| ● 60% in US | ● Education | - Healthcare costs | Social | |||
| ● Health status | Clinical √ | |||||
| ● Socioeconomic status | Environmental | |||||
| ● Diabetes duration | ||||||
| ● Comorbidities | ||||||
| ● Number of meds | ||||||
| ● Types of meds | ||||||
| Outcome: | ||||||
| ● Med adherence | ||||||
| 14. Lemstra, 2016 | SR/MA | Weight loss/obesity | ● 27 studies | Factors: | Weight loss adherence: | Demographics √ |
| ● 6,803 participants | ● Age | + Social support | Psychological √ | |||
| ● 74% RCTs | ● Income | Social √ | ||||
| ● Education | Clinical | |||||
| ● Social support | Environmental | |||||
| ● Depression/mood | ||||||
| ● Weight | ||||||
| ● Smoking status | ||||||
| Outcome: | ||||||
| ● Weight loss adherence | ||||||
| 15. Leung, 2017 | Literature review | Weight mgmt/obesity | ● 19 studies | Factors: | Weight mgmt. adherence: | Demographics √ |
| ● 47% RCTs | ● Self-efficacy | + Older age | Psychological √ | |||
| ● 15 studies BMI ≥30 | ● Depression | + Higher education | Social √ | |||
| ● Stress | - Depression | Clinical √ | ||||
| ● Previous weight loss attempts | - Stress | Environmental | ||||
| ● Age | - Previous weight loss attempts | |||||
| ● Employment status | ||||||
| ● Education | ||||||
| ● BMI | ||||||
| Outcome: | ||||||
| ● Weigh mgmt. adherence | ||||||
| 16. Lewey, 2013 | MA | Statin use | ● 53 total studies | Factors: | Statin adherence: | Demographics √ |
| ● 2,663,638 participants | ● Gender | - Women | Psychological | |||
| ● 55% in US | ● Race | - Nonwhite race | Social | |||
| Outcome: | Clinical | |||||
| ● Statin adherence | Environmental | |||||
| 17. Mann, 2010 | SR/MA | Statin use | ● 22 total studies | Factors: | Med adherence: | Demographics √ |
| ● 2,663,638 participants | ● Race | - Lower and oldest age | Psychological √ | |||
| ● 55% in US | ● Income | - Women | Social | |||
| ● Depression | - Lower income | Clinical √ | ||||
| ● Dementia | + Diagnosis of diabetes or HTN | Environmental | ||||
| ● Health beliefs | ||||||
| ● Disease severity | ||||||
| ● Comorbidities | ||||||
| ● Costs | ||||||
| Outcome: | ||||||
| ● Med adherence | ||||||
| 18. Ofori-Asenso, 2018 | SR/MA | Statin use | ● 45 total studies | Factors: | Nonadherence: | Demographics √ |
| ● 1,842,054 participants | ● Age | + Smoking status | Psychological √ | |||
| ● 53% in Europe | ● Gender | + Women | Social | |||
| ● Smoking status | + Depression | Clinical √ | ||||
| ● Comorbidities | - History of CVD | Environmental | ||||
| ● Copayment | Discontinuation: | |||||
| ● Depression | + Smoking status | |||||
| Outcome: | + Low income | |||||
| ● Nonadherence | - History of CVD | |||||
| ● Discontinuation | - Comorbidities of diabetes or HTN | |||||
| 19. Ombrellaro, 2018 | SR/MA | Cardio-respiratory fitness | ● 15 studies in SR | Factors: | Cardiorespiratory fitness: | Demographics √ |
| ● 3 studies in MA | ● Education | + High education | Psychological | |||
| ● 93% cross-sectional | ● Socioeconomic status | Social | ||||
| ● 40% in US | Outcome: | Clinical | ||||
| ● Cardiorespiratory fitness | Environmental | |||||
| 20. Oosterom-Calo, 2013 | SR | Heart failure | ● 11 studies | Factors: | Med adherence: | Demographics √ |
| ● 64% in US | ● Age | + Hospital setting | Psychological √ | |||
| ● Education | Social √ | |||||
| ● Social support | Clinical √ | |||||
| ● Setting | Environmental √ | |||||
| ● Comorbidities | ||||||
| ● Functional status | ||||||
| ● Depression | ||||||
| Outcome: | ||||||
| ● Med adherence | ||||||
| 21. Ritz, 2013 | Literature review | Asthma | ● 34 studies | Factors: | None assessed | Demographics √ |
| ● Race | Psychological √ | |||||
| ● Parent-child relationship | Social √ | |||||
| ● Family conflicts | Clinical √ | |||||
| ● Anxiety | Environmental √ | |||||
| ● Depression | ||||||
| ● Urban environments | ||||||
| ● Illness beliefs | ||||||
| ● Physical activity level | ||||||
| ● Comorbidities | ||||||
| Outcome: | ||||||
| ● None assessed | ||||||
| 22. Sedlar, 2017 | SR | Heart failure | ● 30 studies | Factors: | Self-care: | Demographics √ |
| ● 50% cross-sectional | ● Age | - Depression | Psychological √ | |||
| ● Health-related quality of life | Social | |||||
| ● Gender | Clinical √ | |||||
| ● Education | Environmental | |||||
| ● Depression | ||||||
| ● Left ventricular ejection fraction | ||||||
| Outcome: | ||||||
| ● Self-care | ||||||
| 23. Wu, 2008 | Literature review | Heart failure | ● 50 studies | Factors: | Med adherence: | Demographics √ |
| ● 50% cross-sectional | ● Income | + Social support | Psychological √ | |||
| ● Race | - Forgetfulness | Social √ | ||||
| ● Comorbidity | Clinical √ | |||||
| ● Depression | Environmental | |||||
| ● Forgetfulness | ||||||
| ● Multiple meds | ||||||
| ● Social support | ||||||
| Outcome: | ||||||
| ● Med adherence | ||||||
| 24. Zeber, 2013 | SR | Chronic diseases | ● 24 studies | Factors: | Initial med adherence: | Demographics √ |
| ● 50% cross-sectional | ● Drug class | - Poor treatment response | Psychological | |||
| ● Age | - High copayments | Social | ||||
| ● Socioeconomic status | - Middle and older age | Clinical √ | ||||
| ● Urban residency | - Comorbidities of long-term diseases like diabetes or psoriasis | Environmental √ | ||||
| ● Copayment | Overall med adherence: | |||||
| ● Treatment response | - Younger age | |||||
| ● Comorbidities | - Poor social support | |||||
| Outcome: | ||||||
| ● Initial med adherence | ||||||
| ● Overall med adherence |
Discussion
Knowledge gaps and future directions
Overall, psychosocial phenotypes and phenotyping present an evolving science with room for theoretical refinement, including useful definitions and pragmatic conceptual boundaries. As for cross-validation and replication, psychosocial phenotyping is still in an exploratory phase. There is certainly a need for psychosocial phenotyping, which shows promise as a tool to enhance personalized self-management intervention strategies for people with chronic diseases.
Theory
Whereas the first wave of precision medicine research has focused on gathering genomic and biological data, truly personalized care will be possible only when an intervention or care practice integrates psychological, behavioral, environmental, social, and cultural determinants along with biological determinants [54]. For psychosocial phenotyping methods to actualize the full potential of precision health and advance self-management science as well as health equity research, a concerted effort among researchers to develop and refine a theoretical framework to guide this line of inquiry is needed. The role of big data in precision health is critical, and precison health models must provide a theoretical basis with pertinent multilevel conceptual domains as well as guide data collection from multiple sources.
Our literature review shows that theoretical models that integrate biological, social, psychological, and environmental determinants and are operationalized empirically to guide precision health interventions are rare. At present, many current precision health interventions that use genomic and biological data are predominantly guided by narrow disease-specific theoretical frameworks. Fortunately, however, several conceptual and theoretical perspectives and models in public and behavioral health hold strong potential to guide precision health interventions: the ecological model [55], the precede-proceed model [56], and the chronic care model [57,58]. Those models have been used to provide a theoretical orientation for multilevel projects rather than as empirical theoretical models to guide testable hypotheses. With rapid advances in technology and data science, however, such models can guide precision health interventions.
The development of theoretical models to guide precision health interventions or to support personalized healthcare decision-making should integrate ways to collect “genomic, biological, behavioral, environmental and other data on individuals on individuals” [59]. The integration of these dimensions into theoretical models and the accumulation of these data will enable useful psychosocial phenotyping to advance personalized health through self-management science, patient-centered care, and policy [54].
Methodology and measurement
Powerful methods for data capture and processing can help realize the promise of psychosocial phenotyping. Data sources should provide information that can encapsulate individuals’ internal characteristics such as health status, demographics, and self-management behaviors, as well as external characteristics such as social support or access to healthcare resources, among others. Technological advances have enabled the exploration of available, powerful personal devices and wearables with GPS units to implement personalized intervention with real-time assessment [59]. After the collection of multi-dimensional data, analytic tools must be sophisticated enough to provide a composite of the individual’s internal and external characteristics in order to develop a psychosocial profile for self-management. Our review suggests that four common methodological tools hold the potential to extract psychosocial phenotypes relevant to self-management.
Meta-analyses and related analytic tools
In the absence of well-validated psychosocial phenotyping in many populations, diseases, or environmental contexts, meta-analyses of patient characteristics that have been examined as mediators or predictors of chronic disease self-management behaviors can be an important methodology to inform the development of psychosocial phenotypes. Meta-analyses can include large enough numbers of patients for big data analysis and can be used to find patterns that inform the conceptualization of psychosocial phenotypes. Brown et al. [25] and Cheen et al. [28] conducted meta-analyses of studies that addressed predictors of disease-related behaviors and health outcomes in order to discover the relative impacts of certain psychosocial characteristics on disease-related self-management behaviors and related health outcomes in comparison with others (see Table 2). Psychosocial determinants such as depression, coping, or self-efficacy were found to have large effects on self-management behaviors such as physical activity or dietary adherence for diabetes [28] or heart failure [33]. Certain behaviors predictive of health outcomes such as dietary and medication adherence were strongly related to improved glycemic control, and glucose self-monitoring was related to fasting blood glucose [25]. In another systematic review of 53 studies (n = 2,663,638), Lewey and colleagues found that female gender and non-white race had higher odds of medication nonadherence. Such findings can inform interventions to improve medication adherence and self-care behaviors in especially vulnerable populations. A use case scenario with psychosocial phenotypes derived from these meta-analyses is provided in Table 2, along with benefits and limitations of using the methodology of meta-analyses for deriving psychosocial phenotypes.
Table 2.
Case Scenarios for Deriving Psychosocial Phenotypes from Selected Meta Analyses
| Analytic Approach | Data Source | Use Case Scenario | Pros | Cons |
|---|---|---|---|---|
| NLP-based algorithm on EHR records | a) EHR, n = 316,355 documents | Terms related to homelessness phenotype that provided direct evidence of actual (e.g. sleeping in park), at risk (e.g. doubled up) or needs (e.g. needs socks) related to homelessness was extracted from the EHR | Sheer volume and variety of available data through EHRs | i) Potential inaccuracies in data quality and accuracy |
| a) Gundlapalli (2013) | ii) Static data that fails to capture of the dynamic evolution of health status of an individual temporally | |||
| Meta-Analysis | a) 31 studies, n = 519,971 | a) Phenotype for medication non-adherence included younger age, higher number of concurrent medications, orthopedic practitioner specialty and higher co-payment | i) Marked increases in statistical power; | i) Restriction of variables to those that were measured by instruments in the included studies, but fail to capture unmeasured covariates that can act of potential confounders |
| a) Cheen (2019): Prevalence and factors contributing to medication non-adherence | b) 759 studies, n = 533,445 | b) Phenotype for improved glucose control included self-efficacy, coping, dietary adherence and medication adherence | ii) Greater heterogeneity in subject demographics; | ii) Pooling of data may introduce uncertainty due to potential sampling errors or unmeasured covariates (Lemstra, et al., 2016) |
| b) Brown (2016): Predictors of diabetes outcomes | iii) Ppportunity to test hypotheses not considered in the original studies; and | iii) Failure to capture temporal influence of psychosocial factors due to static data measurement. (Lueng, et al., 2015) | ||
| iv) Increased efficiency in both time and money incorporating the vast historical information already available | ||||
| Population-level rich questionnaires | a) RECORD questionnaire with 6460 participants aged 30-79 years living in the Paris region between 2011 and 2014 | i) Phenotype for adverse weight profile - negative body image, underestimation of the impact of weight in quality of life, low weight-related self-efficacy, and weight-related external locus of control; | i) Combination of multiple dimensions of socioeconomic characteristics in current socioeconomic status, economic status in childhood, and education status in the residential neighborhood allowed to assess the overall impact of a family of psychosocial mechanisms on obesity simultaneously | i) Eligibility to complete the survey was restricted to employed individuals which could have excluded more socio-economically deprived individuals |
| a) Fuentes (2020) | ii) Phenotype for favorable weight profile-positive body image, high self-efficacy, and internal locus of control | ii) Recall bias of participants to answer life-course related questions | ||
| iii) Large proportion of observations with missing values (32%). | ||||
| Qualitative | a) Interviews with 18 students participating in a school-based behavior change interventions | i) Activated psychosocial phenotype - successful behavior-changers with strong internal supports | Rich, and nuanced details that identify psychosocial characteristics of varying responses to behavioral interventions | Timing of the interviews precludes prospective identification of psychosocial phenotypes to assess their influence on intervention results |
| a) Burgermaster (2018) | ii) Indifferent psychosocial phenotype - uninterested in behavior change and only did target behaviors if family insisted |
Structured and unstructured EHR documentation
EHRs are emerging as repositories of individuals’ data that can facilitate the derivation of psychosocial phenotypes for self-management. The proliferation of EHR usage makes it possible to gather data on thousands of patients, and the growing sophistication and robustness of analytic tools for natural language processing (NLP) and machine learning enable researchers to use EHRs as a viable data source to derive psychosocial phenotypes. Gundlapalli et al. [15] have used NLP on a large corpus of free-text clinical data including provider notes to unlock rich information to identify psychosocial phenotypes [15]. See Table 2 for a use case scenario with a derived phenotype, as well as benefits and limitations of using EHR records for deriving psychosocial phenotypes.
Qualitative data
Qualitative methodologies provide nuanced, rich analyses of patients’ contextual factors and can also be valuable for deriving psychosocial phenotypes related to self-management. For example, in the systematic review conducted by Burgess et al. [27], qualitative articles revealed factors relevant to individuals’ psychosocial contexts such as environmental, societal, and social pressures; negative thoughts/moods; socioeconomic constraints; and lack of enjoyment of exercise as barriers to behavior change. Burgermaster et al. [16] employed case-ordered meta-matrices to identify salient psychosocial phenotypes that helped in the derivation of four psychosocial phenotypes of responses to behavioral interventions to prevent childhood obesity (see Table 2).
Factor and cluster analysis
Factor and cluster analysis of large amounts of both structured and unstructured data can also serve as a tool for identifying and refining psychosocial phenotypes. Fuentes et al. employed multilevel population surveys administered to a city population cohort that included not only socioeconomic and demographic characteristics but also psychological health evaluations and health-related behavioral, psychological, cognitive, and attitudinal characteristics to derive psychosocial profiles for obesity [32]. The data from this large epidemiological cohort of adults enabled the use of sophisticated analytic methods such as factor analysis and cluster analysis to derive clear obesity-related psychosocial profiles. An interesting finding from this study was the clear relationship between the unified psychosocial profile and depression and gender but not other socioeconomic dimensions.
Emerging data types
Digital health data for psychosocial phenotyping is becoming ubiquitous owing to the proliferation of smart phones and the wide use of social media. Social media are increasingly becoming an avenue for gathering data on not only individuals’ health behaviors but also their attitudes toward those behaviors. Twitter tweets and Facebook posts and related responses can provide a cumulative understanding of trends in health behaviors at least among those who are active on social media. Thus, McIver et al. [60] characterized the profiles of individuals who posted in groups about sleep by identifying patterns in their tweets on Twitter. The use of social media as an additional data source to develop meaningful psychosocial phenotypes is still in its infancy and it is important to consider that the profiles developed from social media may not provide completely accurate representations of individuals.
Ethical implications
Psychosocial phenotyping based on social and behavioral data does have ethical ramifications with respect to the collection, storing, and analysis of such data. Implicit biases may result from overrepresentation of certain social or behavioral characteristics in a dataset and may exacerbate disparities. Datasets oftenunderrepresent minority communities due to disparities in healthcare access [61]. Incomplete data or data varying in accuracy may lead to confounding or misleading interpretations which could affect the utility of psychosocial phenotyping. Given the sensitive nature of social and behavioral data, it is imperative that researchers take steps to protect the privacy and confidentiality of individuals’ data and prevent misuse for commercial or legal purposes [62]. Finally, providing participants with informed consent that enables them to understand how their sensitive social and behavioral data will be protected, deidentified, and used is an important ethical consideration that will require continued and sustained efforts [62].
Health disparities implications
The U.S. population is growing older and becoming more diverse. As the population ages, so do its needs for care. The frequency and burden of chronic diseases are rising, and many subgroups within the increasingly diverse U.S. population are experiencing health disparities despite efforts to redress such gaps. We spend 1.5 trillion dollars on management and care of chronic conditions, yet more than half of our population lacks adequate disease manageme. For example, the recent COVID-19 pandemic has revealed that ethnic minorities from resource-scarce communities are more susceptible to COVID-19, with deaths disproportionately high among African Americans and other ethnic minority groups [23]. This unfortunate susceptibility among underserved populations is likely related to poor management of chronic diseases. Insufficient community health infrastructures create challenges in accessing care, as well as an inefficient environment for self-management support, leading to poor management of chronic diseases [23,50].
Limitations
The findings of this study have several important limitations, given the evolving nature of psychosocial phenotyping. First, we had to examine proxy variables rather than well-established phenotypes, because the science of psychoscial phenotyping has not yet matured. Second, our analysis is largely focused on the findings of meta-analyses rather than individual studies. Emerging data from digital platforms have the potential to provide equally strong insights, as do structured data from research projects that we could not include in this analysis.
Nevertheless, this paper suggests important implicatons for future research and practice in implementing precision health strategies for the self-management of chronic illnesses. Given that current psychosocial phenotyping is predominantly exploratory, with large volumes of unstructured data, our review suggests the need for a theoretically guided psychosocial phenotyping algorithm with adequate levels of efficiency and scientific sophistication. Ultimately, methodological insights will be translated in novel research to determine phenotypic characteristics of people with chronic conditions and deliver personalized clinical management and self-management interventions.
Conclusion
To fulfill the promises of precision health practice in the coming years requires a concerted effort in both research and practice. With this paper, we hope to raise awareness about the wide range of issues related to the application of precision health principles within self-management across various populations with chronic conditions. For the research community, we have suggested directions for future research, data collection, and methodology to advance self-management science by maximizing benefits of precision health and advanced technology. We hope that this paper will stimulate scientific discussions about the use of psychological, behavioral, social, and environmental information via useful phenotyping for effective personized self-management interventions in people with chronic conditions. Factors empirically validated as facilitating optimal management of chronic conditions can be used by researchers as common data elements to enrich a collective data pool [63] that will ultimately inform algorithms for valid psychosocial phenotyping and delivery of highly personalized interventions.
We also hope to stimulate a discussion of policy regarding future investment in the collection of pertinent data and the creation of infrastructures to support personalized interventions in populations with chronic conditions and/or limited resources. Current technology equips us to integrate large volumes of structured and unstructured data from multiple sources for phenotyping and subsequent personalized intervention delivery. This will rquire significant societal investment. The success of precision medicine in cancer treatment using genetic information to find precise therapies is based on years of societal investment in basic and clinical research, on the collection of significant amounts of data, on the building of technological infrastructures, and on the development of treatments. Thought leaders in behavioral medicine and population health are now calling for such investments “to provide patient-centered, personalized care, informed by the best combination of genomic, biological, behavioral, and social-environmental information” [54].
Given the increasing need of self-management support within various populations, precision interventions based on meaningful and practical psychosocial phenotyping will enhance the effectiveness of such programs, reduce financial and social costs, and improve the quality of life of the most vulnerable. Moreover, clinical practice equipped with useful phenotyping and technology-guided interventions will be an extremely powerful tool to improve health equity in populations with complex social needs and limited resources.
Acknowledgements
The study was supported by two grants from the National Institute of Nursing Research, the Center for Transdisciplinary Collaborative Research in Self-management Science (P30, NR015335) and Precision Health Intervention Methodology Training in Self-Management of Multiple Chronic Conditions (T32NR019035) at The University of Texas at Austin School of Nursing. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other supporters.
Disclosure of conflict of interest
None.
References
- 1.National Center for Health Statistics (US) Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville. 2016 [PubMed] [Google Scholar]
- 2.Richard AA, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh. 2011;43:255–264. doi: 10.1111/j.1547-5069.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 3.Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care. 2016;39:2089–2095. doi: 10.2337/dc16-0346. [DOI] [PubMed] [Google Scholar]
- 4.Schulman-Green D, Jaser S, Martin F, Alonzo A, Grey M, McCorkle R, Redeker NS, Reynolds N, Whittemore R. Processes of self-management in chronic illness. J Nurs Scholarsh. 2012;44:136–144. doi: 10.1111/j.1547-5069.2012.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, Devilee P, Meindl A, Couch FJ, Southey M, Goldgar DE, Evans DG, Chenevix-Trench G, Rahman N, Robson M, Domchek SM, Foulkes WD. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–2257. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwaederle M, Zhao M, Lee JJ, Lazar V, Leyland-Jones B, Schilsky RL, Mendelsohn J, Kurzrock R. Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: a meta-analysis. JAMA Oncol. 2016;2:1452–1459. doi: 10.1001/jamaoncol.2016.2129. [DOI] [PubMed] [Google Scholar]
- 8.EpiPM Consortium. A roadmap for precision medicine in the epilepsies. Lancet Neurol. 2015;14:1219–1228. doi: 10.1016/S1474-4422(15)00199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams J, Conley B, Mooney M, Zwiebel J, Chen A, Welch JJ, Takebe N, Malik S, McShane L, Korn E, Williams M, Staudt L, Doroshow J. National cancer institute’s precision medicine initiatives for the new national clinical trials network. Am Soc Clin Oncol Educ Book. 2014:71–76. doi: 10.14694/EdBook_AM.2014.34.71. [DOI] [PubMed] [Google Scholar]
- 10.Williams MS, Taylor CO, Walton NA, Goehringer SR, Aronson S, Freimuth RR, Rasmussen LV, Hall ES, Prows CA, Chung WK, Fedotov A, Nestor J, Weng C, Rowley RK, Wiesner GL, Jarvik GP, Del Fiol G. Genomic information for clinicians in the electronic health record: lessons learned from the clinical genome resource project and the electronic medical records and genomics network. Front Genet. 2019;10:1059–1059. doi: 10.3389/fgene.2019.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyne GO, Takebe N, Chen AP. Defining precision: the precision medicine initiative trials NCI-MPACT and NCI-MATCH. Curr Probl Cancer. 2017;41:182–193. doi: 10.1016/j.currproblcancer.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Wei WQ, Leibson CL, Ransom JE, Kho AN, Caraballo PJ, Chai HS, Yawn BP, Pacheco JA, Chute CG. Impact of data fragmentation across healthcare centers on the accuracy of a high-throughput clinical phenotyping algorithm for specifying subjects with type 2 diabetes mellitus. J Am Med Inform Assoc. 2012;19:219–224. doi: 10.1136/amiajnl-2011-000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson AE, Kerr WT, Thames A, Li T, Xiao J, Cohen MS. Electronic health record phenotyping improves detection and screening of type 2 diabetes in the general United States population: a cross-sectional, unselected, retrospective study. J Biomed Inform. 2016;60:162–168. doi: 10.1016/j.jbi.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald S, Davidson K. Using N-of-1 methods to study or change health-related behaviour and outcomes: a symposium summary. European Health Psychologist. 2016;18:38–42. [Google Scholar]
- 15.Gundlapalli AV, Redd A, Carter M, Divita G, Shen S, Palmer M, Samore MH. Validating a strategy for psychosocial phenotyping using a large corpus of clinical text. J Am Med Inform Assoc. 2013;20:e355–e364. doi: 10.1136/amiajnl-2013-001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgermaster M, Contento I, Koch P, Mamykina L. Behavior change is not one size fits all: psychosocial phenotypes of childhood obesity prevention intervention participants. Transl Behav Med. 2018;8:799–807. doi: 10.1093/tbm/ibx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Diabetes and Digestive and Kidney Diseases: Ancillary Studies to Identify Behavioral and/or Psychological Phenotypes Contributing to Obesity (R01 Clinical Trial Optional) Retrieved Sep 2, 2020 from https://grants.nih.gov/grants/guide/pa-files/PAR-16-304.html.
- 18.Hickey KT, Bakken S, Byrne MW, Bailey DCE, Demiris G, Docherty SL, Dorsey SG, Guthrie BJ, Heitkemper MM, Jacelon CS, Kelechi TJ, Moore SM, Redeker NS, Renn CL, Resnick B, Starkweather A, Thompson H, Ward TM, McCloskey DJ, Austin JK, Grady PA. Precision health: advancing symptom and self-management science. Nurs Outlook. 2019;67:462–475. doi: 10.1016/j.outlook.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb LM, Quiñones-Rivera A, Manchanda R, Wing H, Ackerman S. States’ influences on medicaid investments to address patients’ social needs. Am J Prev Med. 2017;52:31–37. doi: 10.1016/j.amepre.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb L, Ackerman S, Wing H, Manchanda R. Understanding medicaid managed care investments in members’ social determinants of health. Popul Health Manag. 2017;20:302–308. doi: 10.1089/pop.2016.0092. [DOI] [PubMed] [Google Scholar]
- 21.Kushner J, McConnell KJ. Addressing Social determinants of health through medicaid: lessons from oregon. J Health Polit Policy Law. 2019;44:919–935. doi: 10.1215/03616878-7785823. [DOI] [PubMed] [Google Scholar]
- 22.Chisolm DJ, Brook DL, Applegate MS, Kelleher KJ. Social determinants of health priorities of state Medicaid programs. BMC Health Serv Res. 2019;19:167. doi: 10.1186/s12913-019-3977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abedi V, Olulana O, Avula V, Chaudhary D, Khan A, Shahjouei S, Li J, Zand R. Racial, economic and health inequality and COVID-19 infection in the United States. medRxiv. 2020 doi: 10.1007/s40615-020-00833-4. 2020.04.26.20079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alramadan MJ, Afroz A, Hussain SM, Batais MA, Almigbal TH, Al-Humrani HA, Albaloshi A, Romero L, Magliano DJ, Billah B. Patient-related determinants of glycaemic control in people with type 2 diabetes in the gulf cooperation council countries: a systematic review. J Diabetes Res. 2018;2018:9389265. doi: 10.1155/2018/9389265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SA, García AA, Brown A, Becker BJ, Conn VS, Ramírez G, Winter MA, Sumlin LL, Garcia TJ, Cuevas HE. Biobehavioral determinants of glycemic control in type 2 diabetes: a systematic review and meta-analysis. Patient Educ Couns. 2016;99:1558–1567. doi: 10.1016/j.pec.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryan AD, Jakicic JM, Hunter CM, Evans ME, Yanovski SZ, Epstein LH. Behavioral and psychological phenotyping of physical activity and sedentary behavior: implications for weight management. Obesity (Silver Spring) 2017;25:1653–1659. doi: 10.1002/oby.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess E, Hassmén P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clin Obes. 2017;7:123–135. doi: 10.1111/cob.12183. [DOI] [PubMed] [Google Scholar]
- 28.Cheen MHH, Tan YZ, Oh LF, Wee HL, Thumboo J. Prevalence of and factors associated with primary medication non-adherence in chronic disease: a systematic review and meta-analysis. Int J Clin Pract. 2019;73:e13350. doi: 10.1111/ijcp.13350. [DOI] [PubMed] [Google Scholar]
- 29.Choi YJ, Smaldone AM. Factors associated with medication engagement among older adults with diabetes: systematic review and meta-analysis. Diabetes Educ. 2018;44:15–30. doi: 10.1177/0145721717747880. [DOI] [PubMed] [Google Scholar]
- 30.Conn VS, Ruppar TM, Chase JA, Enriquez M, Cooper PS. Interventions to improve medication adherence in hypertensive patients: systematic review and meta-analysis. Curr Hypertens Rep. 2015;17:94. doi: 10.1007/s11906-015-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawshaw J, Auyeung V, Norton S, Weinman J. Identifying psychosocial predictors of medication non-adherence following acute coronary syndrome: a systematic review and meta-analysis. J Psychosom Res. 2016;90:10–32. doi: 10.1016/j.jpsychores.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Fuentes S, Brondeel R, Franco M, Sureda X, Traissac P, Cleary LK, Chaix B. Psycho-social factors related to obesity and their associations with socioeconomic characteristics: the RECORD study. Eat Weight Disord. 2020;25:533–543. doi: 10.1007/s40519-018-00638-9. [DOI] [PubMed] [Google Scholar]
- 33.Kessing D, Denollet J, Widdershoven J, Kupper N. Psychological determinants of heart failure self-care: systematic review and meta-analysis. Psychosom Med. 2016;78:412–431. doi: 10.1097/PSY.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 34.Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2015;32:725–737. doi: 10.1111/dme.12651. [DOI] [PubMed] [Google Scholar]
- 35.Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. 2016;10:1547–1559. doi: 10.2147/PPA.S103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung DY, Bai X, Leung AY, Liu BC, Chi I. Prevalence of medication adherence and its associated factors among community-dwelling Chinese older adults in Hong Kong. Geriatr Gerontol Int. 2015;15:789–796. doi: 10.1111/ggi.12342. [DOI] [PubMed] [Google Scholar]
- 37.Lewey J, Shrank WH, Bowry AD, Kilabuk E, Brennan TA, Choudhry NK. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J. 2013;165:665–678. 678.e661. doi: 10.1016/j.ahj.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother. 2010;44:1410–1421. doi: 10.1345/aph.1P150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ofori-Asenso R, Jakhu A, Curtis AJ, Zomer E, Gambhir M, Jaana Korhonen M, Nelson M, Tonkin A, Liew D, Zoungas S. A systematic review and meta-analysis of the factors associated with nonadherence and discontinuation of statins among people aged ≥65 years. J Gerontol A Biol Sci Med Sci. 2018;73:798–805. doi: 10.1093/gerona/glx256. [DOI] [PubMed] [Google Scholar]
- 40.Ombrellaro KJ, Perumal N, Zeiher J, Hoebel J, Ittermann T, Ewert R, Dörr M, Keil T, Mensink GBM, Finger JD. Socioeconomic correlates and determinants of cardiorespiratory fitness in the general adult population: a systematic review and meta-analysis. Sports Med Open. 2018;4:25. doi: 10.1186/s40798-018-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oosterom-Calo R, van Ballegooijen AJ, Terwee CB, te Velde SJ, Brouwer IA, Jaarsma T, Brug J. Determinants of heart failure self-care: a systematic literature review. Heart Fail Rev. 2012;17:367–385. doi: 10.1007/s10741-011-9292-9. [DOI] [PubMed] [Google Scholar]
- 42.Ritz T, Meuret AE, Trueba AF, Fritzsche A, von Leupoldt A. Psychosocial factors and behavioral medicine interventions in asthma. J Consult Clin Psychol. 2013;81:231–250. doi: 10.1037/a0030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sedlar N, Lainscak M, Mårtensson J, Strömberg A, Jaarsma T, Farkas J. Factors related to self-care behaviours in heart failure: a systematic review of European heart failure self-care behaviour scale studies. Eur J Cardiovasc Nurs. 2017;16:272–282. doi: 10.1177/1474515117691644. [DOI] [PubMed] [Google Scholar]
- 44.Wu JR, Moser DK, Lennie TA, Burkhart PV. Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin North Am. 2008;43:133–153. vii–viii. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Molloy GJ, Randall G, Wikman A, Perkins-Porras L, Messerli-Bürgy N, Steptoe A. Type D personality, self-efficacy, and medication adherence following an acute coronary syndrome. Psychosom Med. 2012;74:100–106. doi: 10.1097/PSY.0b013e31823a5b2f. [DOI] [PubMed] [Google Scholar]
- 46.Williams L, O’Connor RC, Grubb N, O’Carroll R. Type D personality predicts poor medication adherence in myocardial infarction patients. Psychol Health. 2011;26:703–712. doi: 10.1080/08870446.2010.488265. [DOI] [PubMed] [Google Scholar]
- 47.Wu JR, Moser DK. Type D personality predicts poor medication adherence in patients with heart failure in the USA. Int J Behav Med. 2014;21:833–842. doi: 10.1007/s12529-013-9366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiffer AA, Denollet J, Widdershoven JW, Hendriks EH, Smith OR. Failure to consult for symptoms of heart failure in patients with a type-D personality. Heart. 2007;93:814–818. doi: 10.1136/hrt.2006.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelle AJ, Schiffer AA, Smith OR, Widdershoven JW, Denollet J. Inadequate consultation behavior modulates the relationship between type D personality and impaired health status in chronic heart failure. Int J Cardiol. 2010;142:65–71. doi: 10.1016/j.ijcard.2008.12.086. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Fang X, Cai Z, Wu X, Gao X, Min J, Wang F. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among covid-19 patients: a systemic review and meta-analysis. Research (Wash D C) 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Artiga S, Hinton E. Beyond health care: the role of social determinants in promoting health and health equity. Kaiser Family Foundation. 2018 [Google Scholar]
- 52.Adler NE, Glymour MM, Fielding J. Addressing social determinants of health and health inequalities. JAMA. 2016;316:1641–1642. doi: 10.1001/jama.2016.14058. [DOI] [PubMed] [Google Scholar]
- 53.Office of Disease Prevention and Health Promotion: Social Determinants of Health. Healthy People. 2020. from https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health.
- 54.Glasgow RE, Kwan BM, Matlock DD. Realizing the full potential of precision health: the need to include patient-reported health behavior, mental health, social determinants, and patient preferences data. J Clin Transl Sci. 2018;2:183–185. doi: 10.1017/cts.2018.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breslow L. Social ecological strategies for promoting healthy lifestyles. Am J Health Promot. 1996;10:253–257. doi: 10.4278/0890-1171-10.4.253. [DOI] [PubMed] [Google Scholar]
- 56.Green L, Kreuter M. Health promotion planning: an educational and environmental approach. 2nd edition. London: Mayfield Publishing Co; 1991. [Google Scholar]
- 57.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]
- 58.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–544. [PubMed] [Google Scholar]
- 59.Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA. 2016;315:1941–1942. doi: 10.1001/jama.2016.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McIver DJ, Hawkins JB, Chunara R, Chatterjee AK, Bhandari A, Fitzgerald TP, Jain SH, Brownstein JS. Characterizing sleep issues using Twitter. J Med Internet Res. 2015;17:e140. doi: 10.2196/jmir.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollister B, Bonham V. Should electronic health record-derived social and behavioral data be used in precision medicine research? AMA J Ethics. 2018;20:E873–880. doi: 10.1001/amajethics.2018.873. [DOI] [PubMed] [Google Scholar]
- 62.Wagner JK. Ethical and legal considerations for the inclusion of underserved and underrepresented immigrant populations in precision health and genomic research in the United States. Ethn Dis. 2019;29:641–650. doi: 10.18865/ed.29.S3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schairer CE, Rubanovich CK, Bloss CS. How could commercial terms of use and privacy policies undermine informed consent in the age of mobile health? AMA J Ethics. 2018;20:E864–872. doi: 10.1001/amajethics.2018.864. [DOI] [PubMed] [Google Scholar]


