Abstract
Objective: The objective is to compare the effect of general anesthesia (GA) and monitored anesthesia care (MAC) on clinical outcomes in patients with endovascular therapy for vertebrobasilar occlusion stroke. Methods: 139 patients undergoing endovascular therapy for vertebrobasilar stroke, were recruited. The patients were randomized into GA group and MAC group (about 1:1 ratio). GA group received general anesthesia and MAC group received monitored anesthesia care during endovascular therapy. The primary outcome measure was the shift in the degree of disability among the 2 groups as measured by the modified Rankin scale score (mRS) at 90 days (80-100 days). Secondary end points included infarct volume and related complications. Results: The patients were assigned randomly (about 1:1 allocation) to GA group (n=72) and MAC group (n=67). The primary outcome of functional independence measured by 90-day mRS score was not significantly different between the 2 groups (median (IQR), 2 (1-3) vs. 3 (1-4); P=0.316). Final infarct volume was smaller in the GA group than in the MAC group (median (IQR), 27.60 (13.75-83.52) vs. 33.60 (26.85-92.95); P=0.045). There were no differences with statistical significance in rates of successful reperfusion (modified Thrombolysis in Cerebral Ischemia (mTICI) 2b-3) between 2 groups (73.61% vs. 76.12%; P=0.734). Early neurological outcomes measured by the 24-hour National Institutes of Health Stroke Scale scores (NIHSS) showed that 11 (interquartile range (IQR), 3-22) in GA group and 11 (interquartile range (IQR), 7-25) in MAC group, but were not statistically significant. There was no statistical difference in postoperative complications between the two groups. Conclusion: For patients who underwent endovascular therapy for vertebrobasilar occlusion strok caused by occlusions in the posterior circulation, MAC appears to be as effective as GA. However, MAC is associated with bigger final infarct volume. Future studies are warranted to confirm our findings.
Keywords: General anesthesia, monitored anesthesia care, endovascular therapy, vertebrobasilar stroke
Introduction
Endovascular therapy (ET) is increasingly used in the treatment for patients with acute ischemic stroke (AIS) and, has been considered the gold standard for anterior circulation occlusions presenting in the early time window (within 6 hours after symptom onset) [1-3]. However, the clinical outcomes of ET vary from patient to patient, depends on both patient factors (age, comorbidities, severity of stroke), as well as procedural factors (time window, operational details) [4,5]. Previous studies have demonstrated that anesthetic approach also have an effect on clinical outcomes, but which anesthetic approach results in the best clinical outcomes remains unclear [6-8]. Both general anesthesia (GA) and conscious sedation/monitored anesthesia care (MAC) during ET have been implemented in clinical trials, and several retrospective studies reported that patients treated with general GA may have worse outcomes than those treated with MAC, but these results may have various bias, including selection bias, recall bias and confounding bias [9-11]. Furthermore, the ultimate selection of anesthetic approach during ET for AIS should be individualized on the basis of clinical characteristics, including risk factors of patient, tolerance of the procedure, and so on [12,13].
In addition, most studies focused on patients with anterior circulation strokes, and there are no prospective studies on patients presenting with posterior circulation strokes to date [14]. Cerebral infarction in the blood supply areas of vertebrobasilar artery and posterior cerebral artery is collectively referred to as posterior circulation infarction, accounting for about 20%-25% of patients with ischemic stroke [15]. Previous studies have suggested that posterior circulation cerebral infarction is associated higher risk of recurrence, poor prognosis and less tolerance to anesthesia [16-19]. The optimal anesthetic approach for the patients presenting with posterior circulation strokes remains unknown. It is the first time, to our best knowledge, using a prospective cohort of patients to evaluate the safety and effectiveness between GA and MAC during ET for treating vertebrobasilar occlusion strokes. In the present prospective cohort study, we sought to test the discrepancies of safety, efficiency, clinical outcomes between the use of GA and MAC during ET.
Materials and methods
Study design and participants
This study was a single center, prospective, blinded end point evaluation, cohort study, which enrolled patients undergoing endovascular therapy for vertebrobasilar occlusion stroke from February 2017 to November 2019 at Southern Medical University. The primary outcome of this study was modified Rankin scale (mRS) score. PASS 15.0 software was used. Referring to the basis of previous studies, the parameters were set as non-inferiority study, mRS score was improved to 1.91±0.53 and 1.90±0.76, and the non-inferiority margin was 14%, and the test efficiency was 80%, P=0.05 (bilateral). So, the sample size was calculated as 48 cases in each group, 96 cases in total [20]. Consider 20% of shedding cases, 120 patients should be included. In the actual study, we collected 139 cases (>120). Patients were included in this study if they (1) could be treated with ET within 6h after symptoms onset; (2) had a National Institutes of Health Stroke Scale scores (NIHSS) ≥4 at admission and premorbid mRS scores <2; (3) were diagnosed with acute posterior circulation stroke caused by vertebrobasilar occlusion verified by computed tomographic angiography (CTA), magnetic resonance angiography (MRA), digital-subtraction angiography (DSA); (4) were ≥18 years of age. Patients were excluded from this study if they (1) has an increased risk of suffering from bleeding, including platelet count <100×109/L, and a history of surgery and substantive organ biopsy within 1 month; (2) had a life expectancy less than 90 days; (3) had contraindications of ET, including arteriovenous malformation or concomitant aneurysm; (4) had incomplete information or the follow-up was lost; (5) were intubated at presentation or with a premorbid mRS score of more than 2 (score range: 0-6, with a lower score indicating independent living) as well as those who had a Glasgow Coma Scale (GCS) score lower than 9 (score range: 3-15, with a lower score indicating lower levels of consciousness). The flowchart (Figure 1) displays the number details of patients included and excluded. Patients were assigned randomly to GA group and MAC group (about 1:1 allocation). This study was approved by the Ethics Committee of Southern Medical University. Patients or their next of kin were then required to give written informed consent to fulfill the trial. No data monitoring committee was involved.
Figure 1.
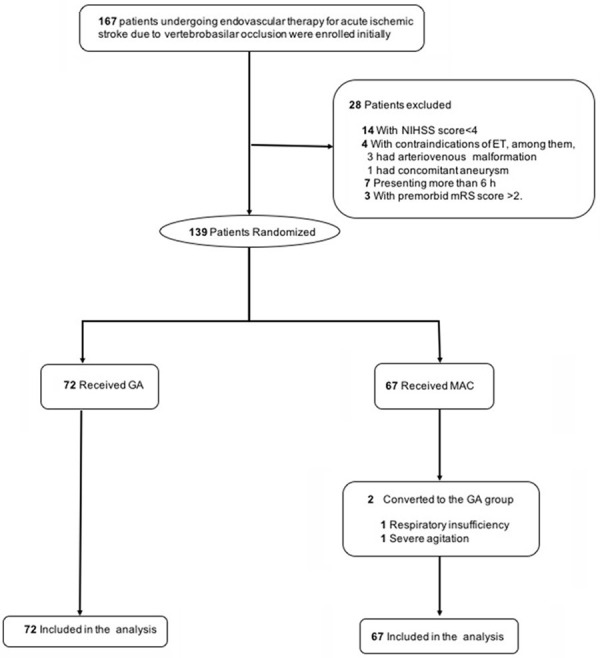
Flowchart of the study sample. GA: general anesthesia; MAC: monitored anesthesia care.
Anesthesia and thrombectomy
GA and MAC were both standard anesthetic procedures during EVT at our institution prior to trial initiation. For patients in the GA group, rapid sequence intubation with Suxamethonium (CARBOMER INC, USA. Bolus 0.5-1 mg/kg), Alfentanil (Nhwa Pharmaceutical Co., Ltd, China. Bolus 0.02-0.03 mg/kg) and Propofol (EMMX Biotechnology LLC, USA. Bolus 1-5 mg/kg followed by 2-10 mg/kg/h) was performed. Endotracheal intubation was followed by mechanical ventilation. Anesthesia was maintained with Propofol (EMMX Biotechnology LLC, USA. 2-10 mg/kg/h) and remifentanil (National Pharmaceutical Industry Co. Ltd., China. 0.2-1 μg/kg/min). In the neuro-interventional suite, patients in the MAC group received a fentanyl bolus (Nhwa Pharmaceutical Co., Ltd, China.) of 25-50 μg, which was repeated as necessary. A Propofol (EMMX Biotechnology LLC, USA) infusion of 1-4 mg/kg/hr. was initiated and adjusted as required. The patient’s sedation was controlled to a Ramsay sedation score of 4 (patient asleep, shows brisk responses to light glabellar tap or loud auditory stimulus) or 5 (patient asleep, shows sluggish response to light glabellar tap or loud auditory stimulus).
A radial artery catheter was used during the thrombectomy to measure the invasive arterial blood pressure including systolic blood pressure (SBP), and mean arterial pressure (MAP). Decreases in blood pressure were treated with vasopressors (ephedrine/phenylephrine) to maintain blood pressure within recommended limits (SBP>140 mmHg, MAP>70 mmHg). Reperfusion was evaluated by an independent radiologist according to the modified Thrombolysis in Cerebral Ischemia (mTICI) scale score (score range: 0-3, 1 for minimal reperfusion, 2a for less than 50% of the affected vascular territory reperfused, 2b for greater than 50% reperfusion, and 3 for complete reperfusion). The mTICI 2b or 3 was considered as successful reperfusion [21].
Outcomes and imaging analysis
The primary outcome measure was the shift in the degree of disability among the 2 groups as measured by the mRS score at 90 days (80-100 days). Secondary end points included infarct volume and related complications. The cerebral infarct volume was calculated by the Pullicino formula (length * width * layer number/2) based on the cranial CT or MRI scan within 48 hours after AIS [22]. Safety end points were 90-day mortality, vessel injury, and any parenchymal hematoma according to the European Cooperative Acute Stroke Study. All of imaging outcomes were obtained by imaging doctors and laboratory doctors who were blinded to this study. The mRS scores at 90 days (80-100 days) after the stroke and NIHSS score were assessed by a registered nurse who was unaware of the randomization by telephone.
Statistical analysis
Two patients received MAC initially were converted to GA due to respiratory insufficiency and severe agitation. Intention-to-treat (ITT) analysis was used in the analysis, so the 2 patients were still included in MAC group when analyzing. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 18.0 (IBM Armonls, NY, USA). Continuous variables were recorded as mean values ± standard deviation (x̅ ± sd), and categorical variables were expressed by proportions (%). The unpaired 2-tailed Student t test or Mann-Whitney U test were performed to compare the mean values or data distribution of continuous variables. And categorical variables were compared with the χ2 (chi-square) test or Fisher exact test, as appropriate. A P value of <0.05 was considered statistically significant.
Results
167 patients undergoing ET for AIS due to vertebrobasilar occlusion were enrolled initially, 28 patients who were not fulfill the inclusion criteria were excluded. A total of 139 patients were included in this study finally. Then the patients were assigned randomly (about 1:1 allocation) to GA group (n=72) and MAC group (n=67). Baseline characteristics were well balanced between the 2 groups, including age, sex, stroke risk factors, level of stoke, NIHSS scores, and ET technical approach, SBP, MAP and HR (Table 1). The x̅ ± sd of intraoperative MAP was (96.25±15.97) mmHg in GA group and (102.06±18.03) mmHg in MAC group, indicating intraoperative MAP was lower in GA than MAC with statistical significance (P=0.046; Figure 2).
Table 1.
Baseline demographic, clinical, and treatment data
| Variable | GA (n=72) | MAC (n=67) | P |
|---|---|---|---|
| Age | 72.1±6.8 | 71.9±7.5 | 0.897 |
| Male (%) | 38 (52.78) | 34 (50.75) | 0.811 |
| Premorbid mRS score (%) | 0.540 | ||
| 0 | 55 (76.39) | 50 (74.63) | |
| 1 | 10 (13.89) | 11 (16.42) | |
| 2 | 5 (6.94) | 6 (8.96) | |
| 3 | 2 (2.78) | 0 (0.00) | |
| Platelets, median (IQR) | 217 (174-231) | 209 (163-223) | 0.155 |
| Hypertension (%) | 34 (47.22) | 31 (46.27) | 0.910 |
| Atrial fibrillation (%) | 24 (33.33) | 27 (40.30) | 0.395 |
| Smokers (%) | 21 (29.17) | 19 (28.36) | 0.961 |
| Diabetes (%) | 12 (16.67) | 9 (13.43) | 0.595 |
| Dyslipidemia (%) | 23 (31.94) | 26 (38.81) | 0.398 |
| Thrombectomy procedure (%) | 0.924 | ||
| Stent retriever | 15 (20.83) | 14 (20.90) | |
| ADAPT technique | 27 (37.50) | 26 (38.81) | |
| Both | 15 (20.83) | 12 (17.91) | |
| SBP (mmHg) | |||
| Baseline | 162.76±17.58 | 165.78±18.97 | 0.333 |
| Intraoperative | 155.96±14.13 | 153.13±11.80 | 0.205 |
| MAP (mmHg) | |||
| Baseline | 114.11±16.57 | 111.43±19.93 | 0.389 |
| Intraoperative | 96.25±15.97 | 102.06±18.03 | 0.046 |
| Intraoperative MAP decreased by more than 40% from baseline | 4 (5.56) | 3 (4.48) | 1.000 |
| Heart rate (bpm) | |||
| Baseline | 92.71±15.20 | 96.19±12.25 | 0.141 |
| Intraoperative | 76.83±10.28 | 79.40±9.91 | 0.136 |
| Intraoperative HR decreased by more than 30% from baseline | 10 (13.89) | 6 (8.96) | 0.362 |
| Onset-to-door time ((OTD), min) | 142.3±39.3 | 129.6±47.3 | 0.086 |
| Procedure time (min) | 130.4±43.6 | 143.3±45.7 | 0.091 |
| SpO2 (%) | 97.07±3.12 | 96.10±3.32 | 0.080 |
| Intraoperative agitation (%) | 6 (8.33) | 8 (11.94) | 0.480 |
| Use of vasoactive drugs (%) | 10 (13.89) | 6 (8.96) | 0.362 |
Note: GA: general anesthesia; MAC: monitored anesthesia care; mRS: modified Rankin scale.
Figure 2.
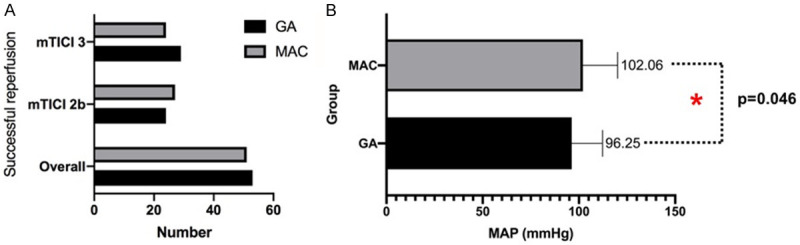
Bar graphs comparing the rates. A: Successful reperfusion after ET; B: Intraoperative mean arterial pressure for the GA (black) vs. MAC (gray) groups. GA: general anesthesia; MAC: monitored anesthesia care; mTICI: modified Thrombolysis in Cerebral Ischemia. * indicates that there was a statistical difference between two groups with P=0.046.
The primary outcome of functional independence measured by 90-day mRS score was not significantly different between the 2 groups (median (IQR), 2 (1-3) vs. 3 (1-4); P=0.316; Table 2 and Figure 3). No significant difference for the primary outcome was observed between the treatment groups in the subgroup analyses, except the subgroup with gender (P<0.05; Table 3 and Figure 4). Final infarct volume was smaller in the GA group than in the MAC group (median (IQR), 27.60 (13.75-83.52) vs. 33.60 (26.85-92.95); P=0.045). There were no differences with statistical significance in rates of successful reperfusion (mTICI 2b-3) between 2 groups (73.61% vs. 76.12%; P=0.734). Early neurological outcomes measured by the 24-hour NIHSS score showed that 11 (interquartile range (IQR), 3-22) in GA group and 11 (interquartile range (IQR), 7-25) in MAC group, but were not statistically significant. The median (IQR) of length of hospital stay was 17 (11-24) d after GA and 16 (9-21) d after MAC, but there was no statistically significant difference between the two groups (P=0.095). There was no statistical difference in postoperative complications between the two groups (Table 2).
Table 2.
Clinical outcomes and complications
| Outcome | GA (n=72) | MAC (n=67) | P |
|---|---|---|---|
| Successful reperfusion (%) | 53 (73.61) | 51 (76.12) | 0.734 |
| mTICI 2b | 24 (33.33) | 27 (40.30) | 0.395 |
| mTICI 3 | 29 (40.28) | 24 (35.82) | 0.589 |
| Acute infarct volume, median (IQR), mL | 12.10 (8.40-22.38) | 12.70 (5.85-25.75) | 0.848 |
| Final infarct volume, median (IQR), mL | 27.60 (13.75-83.52) | 33.60 (26.85-92.95) | 0.045 |
| Infarct volume growth, median (IQR), mL | 12.90 (6.35-59.40) | 29.00 (15.80-69.90) | 0.017 |
| 90-d mRS score, median (IQR) | 2 (1-3) | 3 (1-4) | 0.316 |
| NIHSS score in 24 h, median (IQR) | 11 (3-22) | 11 (7-25) | 0.073 |
| Length of hospital stay, median (IQR), d | 17 (11-24) | 16 (9-21) | 0.095 |
| Complications (%) | 26 (36.11) | 16 (23.88) | 0.117 |
| Hypertension or hypotension (>180 mmHg or <120 mmHg) | 16 (22.22) | 11 (16.42) | 0.387 |
| Pneumothorax | 8 (11.11) | 3 (4.48) | 0.148 |
| Pneumonia | 2 (2.78) | 2 (2.99) | 1.000 |
Note: GA: general anesthesia; MAC: monitored anesthesia care; mTICI: modified Thrombolysis in Cerebral Ischemia; NIHSS: National Institutes of Health Stroke Scale scores.
Figure 3.
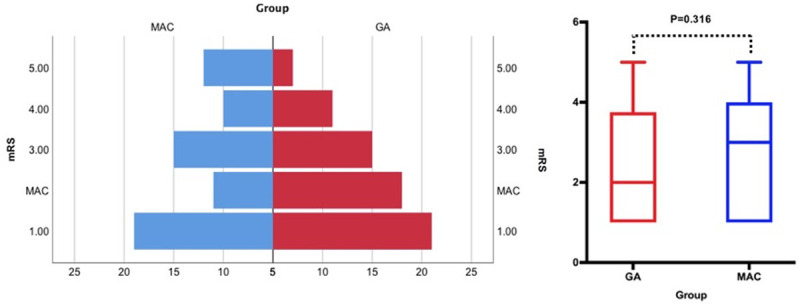
Shift analysis as assessed by the Mann-Whitney U-test, P=0.316. MAC: monitored anesthesia care; GA: general anesthesia.
Table 3.
Primary outcome as the improvement of mRS score in prespecified subgroups
| Subgroup | No | Changes in mRS score | |||
|---|---|---|---|---|---|
|
|
|
||||
| GA | MAC | GA | MAC | Difference (95% CI) | |
| Age | |||||
| ≤70 | 45 | 39 | -2.15 (-2.74 to -1.56) | -2.51 (-3.06 to -1.97) | -0.56 (-0.40 to 0.28) |
| >70 | 27 | 28 | -2.16 (-2.66 to -1.66) | -2.32 (-2.98 to -1.66) | -0.08 (-0.81 to -0.66) |
| Sex | |||||
| Male | 38 | 34 | -2.37 (-2.88 to -1.85) | -2.44 (-3.06 to -1.82) | 0.35 (-0.57 to 1.28) |
| Female | 34 | 33 | -1.91 (-2.47 to -1.45) | -2.42 (-2.00 to -1.86) | -0.79 (-1.56 to -0.02) |
| NIHSS | |||||
| >17 | 24 | 30 | -2.04 (-2.53 to -1.55) | -2.33 (-2.96 to -1.70) | -0.29 (-1.21 to 0.63) |
| ≤17 | 48 | 37 | -2.21 (-2.72 to -1.69) | -2.51 (-3.07 to -1.95) | -0.30 (-1.21 to 0.62) |
| Hypertension | |||||
| Yes | 34 | 31 | -2.15 (-2.69 to -1.60) | -2.58 (-3.13 to -2.03) | -0.42 (-1.04 to 0.20) |
| No | 38 | 36 | -2.16 (-2.70 to -1.62) | -2.31 (-2.93 to -1.69) | -0.14 (-0.94 to 0.66) |
| DHR | |||||
| >30% | 10 | 6 | -2.60 (-3.62 to -1.58) | -2.33 (-4.86 to 0.21) | -1.00 (-3.20 to 1.20) |
| ≤30% | 62 | 61 | -2.08 (-2.49 to -1.67) | -2.44 (-2.86 to -2.03) | -0.23 (-0.80 to 0.34) |
| SR | 53 | 51 | -2.19 (-2.59 to -1.78) | -2.37 (-2.85 to -1.89) | -0.25 (-0.95 to 0.44) |
Note: GA: general anesthesia; MAC: monitored anesthesia care; NIHSS: National Institutes of Health Stroke Scale scores.
Figure 4.
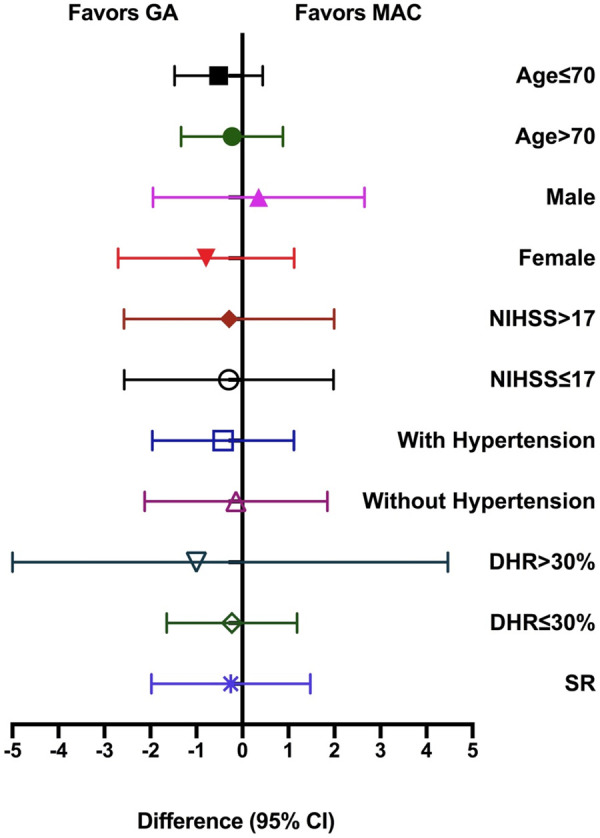
Primary outcome as the Improvement of mRS Score in Prespecified Subgroups. No significant difference for the primary outcome was observed between the treatment groups in the subgroup analyses, except the subgroup with gender (female). GA: general anesthesia; MAC: monitored anesthesia care; NIHSS: National Institutes of Health Stroke Scale scores.
Discussion
ET has been increasingly used for the treatment of AIS in recent years, and is currently performed by multiple disciplines, without consensus about standardized anesthesia approaches protocols, as a result of an ongoing controversy about the safety and effectiveness between GA and MAC [23,24]. Some observational studies have compared the clinical outcomes of GA and MAC for the treatment of AIS. Most of these studies have been retrospective analyses, demonstrating controversial results without consensus, given their intrinsic selection bias. Some previous studies suggested that GA was inferior to MAC during ET, suggesting that the use of GA during ET was associated with poor clinical outcomes (mRS≥3), higher respiratory complications and higher mortality [25,26]. But the generalizability of their conclusion is limited only to the single thrombectomy device proposed by the authors. Moreover, numerous studies have demonstrated better clinical outcomes with the use of MAC. For example, Mc Donald et al. demonstrated that conscious sedation was associated with a survival benefit by the use of Market Scan database [27]. However, it was voluntary that participating in the commercial database, and the authors did not take into consideration the significant unmeasured confounding caused by self-selection. The Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MRCLEAN) study, reported that there was no different with statistical significance regarding the procedural times between GA and MAC group [28]. Contrary to these studies, a randomized clinical trial, General or Local Anesthesia in Intra Arterial Therapy (GOLIATH), have showed signals in favor of the use of GA for the primary end point of 90-day mRS scores, and the GA group had better improvement in NIHSS scores and smaller infarct growth. Similarly, in the Sedation versus Intubation for Endovascular Stroke Treatment (SIESTA) study, the GA group had a higher rate of successful reperfusion (mTICI 2b and 3 with an absolute difference of 8.5%) [29]. In this single-center, randomized, prospective, blinded end point cohort study, the primary outcome of mRS at 90 days (80-100 days) was not different with statistical significance between the GA and MAC group (2 (1-3) vs. 3 (1-4)), which demonstrate that MAC is as effective as GA during ET. In addition, we found comparable rates of reperfusion in the GA and MAC groups. However, the infarct volume growth and final infarct volume were higher in GA with statistical significance, which was hypothesized to be the result of procedural delays and increased door-to-groin puncture times.
Many published series have excluded patients with vertebrobasilar occlusion strokes because a significant proportion of those patients undergoing angiography suite were already intubated [30,31]. Consequently, the data are scarce regarding the safety and practicability of MAC for the treatment of patients presenting with vertebrobasilar occlusion strokes, only limited to nonrandomized observational studies [32-34]. Although these studies demonstrated that patients treated with MAC behaved similarly to those treated with GA with similar clinical outcomes and complications, they failed to balance the differences in the NIHSS scores between the GA group and MAC group. NIHSS scores at 24 hours (median (IQR): 11 (3-22) vs. 11 (7-25)) were not significantly higher in the GA cohort. In addition, our study had been well balanced regarding the NIHSS score prospectively, which made our founding more reliable. Some studies evaluated the feasibility of MAC for ET either in anterior or posterior circulations and found that MAC group had a lower incidence of complications and mortality [35,36]. These studies failed to compare the results of GA in emergency situations, especially in the presence of severe brainstem ischemia.
The intraoperative blood pressure level of during ET is closely related to the prognosis of patients. Lower blood pressure before recanalization may reduce the perfusion of ischemic brain tissue and aggravate brain injury. GA group had a more frequent blood pressure drop with worse ET outcomes according to previous study [37]. Intraoperative MAP decreases of more than 40% is an independent risk factor for poor prognosis [38]. GOLIATH trials showed that blood pressure was lower significantly in the GA group. In line with the GOLIATH study, our study also found that intraoperative MAP was lower in GA than MAC with statistical significance. What need to be concerned about is that prevention of hypotension can improve nervous system prognosis. In addition, we had also analyzed the primary outcome as the improvement of mRS score in prespecified subgroups, including age, sex, NIHSS with or without over 17, hypertension, DHR and SR. No significant difference for the primary outcome was observed between the treatment groups in the subgroup analyses, except the subgroup with gender (female). For subgroup of female, the changes in mRS score were -1.91 (-2.47 to -1.45) in GA and -2.42 (-2.00 to -1.86) in MAC, and the difference (95% CI) was -0.79 (-1.56 to -0.02) with P value lower than 0.05.
This study has several limitations. The primary limitation is that this study was performed at a single center, limiting its generalizability to other centers that use different anesthesia approaches and ET. However, we standardized anesthesia protocol and ET procedure used in this study as much as possible. Another limitation of this study, like other studies, is the relatively small sample size. Third, current definitions of GA andMAC are heterogeneous, and allow for various choices of drugs and measures. In addition, only one prognostic time point was observed, and no other prognostic indicators were observed.
For patients who underwent endovascular therapy for vertebrobasilar occlusion stroke, MAC appears to be as effective as GA. However, MAC is associated with bigger final infarct volume. Future studies are warranted to confirm our findings.
Acknowledgements
This work was supported by the Guangzhou Science and Technology Project (201904010389) and National Natural Science Foundation of China (62076253).
Disclosure of conflict of interest
None.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 4.Smith WS. Endovascular stroke therapy. Neurotherapeutics. 2019;16:360–368. doi: 10.1007/s13311-019-00724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues FB, Neves JB, Caldeira D, Ferro JM, Ferreira JJ, Costa J. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ. 2016;353:i1754. doi: 10.1136/bmj.i1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang A, Abramowicz AE. Role of anesthesia in endovascular stroke Therapy. Curr Opin Anaesthesiol. 2017;30:563–569. doi: 10.1097/ACO.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen LK, Simonsen CZ, Rasmussen M. Anesthesia practice for endovascular therapy of acute ischemic stroke in Europe. Curr Opin Anaesthesiol. 2019;32:523–530. doi: 10.1097/ACO.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 8.Rössel T, Paul R, Richter T, Ludwig S, Hofmockel T, Heller AR, Koch T. Anästhesiologisches management bei endovaskulären gefäßeingriffen (management of anesthesia in endovascular interventions) Anaesthesist. 2016;65:891–910. doi: 10.1007/s00101-016-0241-9. [DOI] [PubMed] [Google Scholar]
- 9.Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36:525–529. doi: 10.3174/ajnr.A4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abou-Chebl A, Zaidat OO, Castonguay AC, Gupta R, Sun CH, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Linfante I, Dabus G, Malisch TW, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Nguyen TN, Taqi M, Abraham MG, Janardhan V, Shaltoni H, Novakovic R, Yoo AJ, Chen PR, Britz GW, Kaushal R, Nanda A, Issa MA, Nogueira RG. North American SOLITAIRE stent-retriever acute stroke registry: choice of anesthesia and outcomes. Stroke. 2014;45:1396–1401. doi: 10.1161/STROKEAHA.113.003698. [DOI] [PubMed] [Google Scholar]
- 11.Bekelis K, Missios S, MacKenzie TA, Tjoumakaris S, Jabbour P. Anesthesia technique and outcomes of mechanical thrombectomy in patients with acute ischemic stroke. Stroke. 2017;48:361–366. doi: 10.1161/STROKEAHA.116.015343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CZ, Gelb AW. Anesthesia management for endovascular treatment. Curr Opin Anaesthesiol. 2014;27:484–488. doi: 10.1097/ACO.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 13.Goyal N, Malhotra K, Ishfaq MF, Tsivgoulis G, Nickele C, Hoit D, Arthur AS, Alexandrov AV, Elijovich L. Current evidence for anesthesia management during endovascular stroke therapy: updated systematic review and meta-analysis. J Neurointerv Surg. 2019;11:107–113. doi: 10.1136/neurintsurg-2018-013916. [DOI] [PubMed] [Google Scholar]
- 14.Jadhav AP, Bouslama M, Aghaebrahim A, Rebello LC, Starr MT, Haussen DC, Ranginani M, Whalin MK, Jovin TG, Nogueira RG. Monitored anesthesia care vs intubation for vertebrobasilar stroke endovascular therapy. JAMA Neurol. 2017;74:704–709. doi: 10.1001/jamaneurol.2017.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparaco M, Ciolli L, Zini A. Posterior circulation ischaemic stroke-a review part i: anatomy, aetiology and clinical presentations. Neurol Sci. 2019;40:1995–2006. doi: 10.1007/s10072-019-03977-2. [DOI] [PubMed] [Google Scholar]
- 16.Go S. Posterior circulation ischemic stroke. Mo Med. 2015;112:192–196. [PMC free article] [PubMed] [Google Scholar]
- 17.Sato R, Semba T, Saya H, Arima Y. Concise review: stem cells and epithelial-mesenchymal transition in cancer: biological implications and therapeutic targets. Stem Cells. 2016;34:1997–2007. doi: 10.1002/stem.2406. [DOI] [PubMed] [Google Scholar]
- 18.Markus HS, van der Worp HB, Rothwell PM. Posterior circulation ischaemic stroke and transient ischaemic attack: diagnosis, investigation, and secondary prevention. Lancet Neurol. 2013;12:989–998. doi: 10.1016/S1474-4422(13)70211-4. [DOI] [PubMed] [Google Scholar]
- 19.Caplan L. Posterior circulation ischemia: then, now, and tomorrow. The thomas willis lecture-2000. Stroke. 2000;31:2011–2023. doi: 10.1161/01.str.31.8.2011. [DOI] [PubMed] [Google Scholar]
- 20.Simonsen CZ, Yoo AJ, Sørensen LH, Juul N, Johnsen SP, Andersen G, Rasmussen M. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75:470–477. doi: 10.1001/jamaneurol.2017.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers G, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonagh DL, Olson DM, Kalia JS, Gupta R, Abou-Chebl A, Zaidat OO. Anesthesia and sedation practices among neurointerventionalists during acute ischemic stroke endovascular therapy. Front Neurol. 2010;1:118. doi: 10.3389/fneur.2010.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina CA, Selim MH. General or local anesthesia during endovascular procedures: sailing quiet in the darkness or fast under a daylight storm. Stroke. 2010;41:2720–2721. doi: 10.1161/STROKEAHA.110.595447. [DOI] [PubMed] [Google Scholar]
- 25.Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS, Yoo AJ, Hsu DP, Rymer MM, Tayal AH, Zaidat OO, Natarajan SK, Nogueira RG, Nanda A, Tian M, Hao Q, Kalia JS, Nguyen TN, Chen M, Gupta R. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke. 2010;41:1175–1179. doi: 10.1161/STROKEAHA.109.574129. [DOI] [PubMed] [Google Scholar]
- 26.Brinjikji W, Pasternak J, Murad MH, Cloft HJ, Welch TL, Kallmes DF, Rabinstein AA. Anesthesia-related outcomes for endovascular stroke revascularization: a systematic review and meta-analysis. Stroke. 2017;48:2784–2791. doi: 10.1161/STROKEAHA.117.017786. [DOI] [PubMed] [Google Scholar]
- 27.McDonald JS, Brinjikji W, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anaesthesia during mechanical thrombectomy for stroke: a propensity score analysis. J Neurointerv Surg. 2015;7:789–794. doi: 10.1136/neurintsurg-2014-011373. [DOI] [PubMed] [Google Scholar]
- 28.Berkhemer OA, van den Berg LA, Fransen PS, Beumer D, Yoo AJ, Lingsma HF, Schonewille WJ, van den Berg R, Wermer MJ, Boiten J, Lycklama À Nijeholt GJ, Nederkoorn PJ, Hollmann MW, van Zwam WH, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW, Roos YB. The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. Neurology. 2016;87:656–664. doi: 10.1212/WNL.0000000000002976. [DOI] [PubMed] [Google Scholar]
- 29.Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, Nagel S, Klose C, Pfaff J, Bendszus M, Ringleb PA, Kieser M, Möhlenbruch MA, Bösel J. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316:1986–1996. doi: 10.1001/jama.2016.16623. [DOI] [PubMed] [Google Scholar]
- 30.Jumaa MA, Zhang F, Ruiz-Ares G, Gelzinis T, Malik AM, Aleu A, Oakley JI, Jankowitz B, Lin R, Reddy V, Zaidi SF, Hammer MD, Wechsler LR, Horowitz M, Jovin TG. Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke. 2010;41:1180–1184. doi: 10.1161/STROKEAHA.109.574194. [DOI] [PubMed] [Google Scholar]
- 31.Nichols C, Carrozzella J, Yeatts S, Tomsick T, Broderick J, Khatri P. Is periprocedural sedation during acute stroke therapy associated with poorer functional outcomes? J Neurointerv Surg. 2018;10:i40–i43. doi: 10.1136/jnis.2009.001768.rep. [DOI] [PubMed] [Google Scholar]
- 32.Langner S, Khaw AV, Fretwurst T, Angermaier A, Hosten N, Kirsch M. (Endovascular treatment of acute ischemic stroke under conscious sedation compared to general anesthesia-safety, feasibility and clinical and radiological outcome) Rofo. 2013;185:320–327. doi: 10.1055/s-0032-1330361. [DOI] [PubMed] [Google Scholar]
- 33.Li F, Deshaies EM, Singla A, Villwock MR, Melnyk V, Gorji R, Yang ZJ. Impact of anesthesia on mortality during endovascular clot removal for acute ischemic stroke. J Neurosurg Anesthesiol. 2014;26:286–290. doi: 10.1097/ANA.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 34.Davis MJ, Menon BK, Baghirzada LB, Campos-Herrera CR, Goyal M, Hill MD, Archer DP. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116:396–405. doi: 10.1097/ALN.0b013e318242a5d2. [DOI] [PubMed] [Google Scholar]
- 35.Abou-Chebl A, Krieger DW, Bajzer CT, Yadav JS. Intracranial angioplasty and stenting in the awake patient. J Neuroimaging. 2006;16:216–223. doi: 10.1111/j.1552-6569.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 36.Chamczuk AJ, Ogilvy CS, Snyder KV, Ohta H, Siddiqui AH, Hopkins LN, Levy EI. Elective stenting for intracranial stenosis under conscious sedation. Neurosurgery. 2010;67:1189–1193. doi: 10.1227/NEU.0b013e3181efbcac. discussion 1194. [DOI] [PubMed] [Google Scholar]
- 37.Treurniet KM, Berkhemer OA, Immink RV, Lingsma HF, Ward-van der Stam VMC, Hollmann MW, Vuyk J, van Zwam WH, van der Lugt A, van Oostenbrugge RJ, Dippel DWJ, Coutinho JM, Roos YBWEM, Marquering HA, Majoie CBLM MR CLEAN investigators. A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J Neurointerv Surg. 2018;10:107–111. doi: 10.1136/neurintsurg-2017-012988. [DOI] [PubMed] [Google Scholar]
- 38.Löwhagen Hendén P, Rentzos A, Karlsson JE, Rosengren L, Sundeman H, Reinsfelt B, Ricksten SE. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46:2678–2680. doi: 10.1161/STROKEAHA.115.009808. [DOI] [PubMed] [Google Scholar]


