Abstract
Coronavirus SARS-CoV-2 is a novel coronavirus and the seventh that can infect human beings and result in severe and acute respiratory syndrome and deaths. Currently, the world is undergoing a global health emergency due to the SARS-CoV-2 pandemic. As of May 18, SARS-CoV-2 has spread to over two hundred countries and infected more than 4.8 million people, resulting in over 300,000 deaths since the first case of a novel pneumonia (COVID-19) patient was discovered in Wuhan, China at the end of December 2019. Currently, there are no effective and/or approved targeting drugs for it though various supportive therapy drugs such as small molecule drugs, vaccines, antibodies and even Chinese herb medicines have been used in the treatment of the first-line patients. However, certain drugs such as remdesivir and S416 are under clinical investigation and may become therapeutic drugs. In this article, we review and discuss SARS-CoV-2, its person-to-person transmission, genomics and proteomics, and the potential for drug development.
Keywords: SARS-CoV-2, coronavirus, genomics, proteomics, epidemiology, remdesivir, S416, outbreak
Introduction
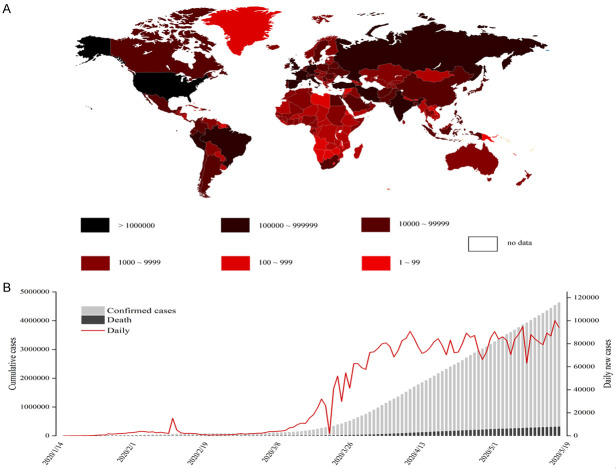
In December 2019, an unidentified pneumonia patient was first discovered in Wuhan, China [1,2]. Subsequently, a novel coronavirus was isolated from early pneumonia patients, and named SARS-CoV-2 by the World Health Organization (WHO) on February 11. By the end of February 2020, the majority of the patients were still located in China (Figure 1A). Unfortunately, by now more than 200 countries have reported confirmed cases. WHO declared the novel coronavirus pneumonia (COVID-19) as a global pandemic on March 11. According to incomplete statistics, as of May 18, more than 4.84 million cases were diagnosed globally and 317,391 cases had died, with the mortality rate approximated at 6.56% (Figure 1B). The main reason for the large number of deaths is that there is no specific drug for the treatment of the coronavirus.
Figure 1.
Statistical results of confirmed cases worldwide. A. Global distribution of confirmed cases. The shades of color represent different numbers of infected cases. The darker the color, the more confirmed cases. B. As of May 18, the number of confirmed cases in various countries around the world, after February 9 (not shown in Figure 1), the number of newly diagnosed cases in other countries in the world surpassed that of China, and this proportion has gradually increased since then.
Fortunately, the results of clinical phase III trials of remdesivir showed that compared with placebo, it can significantly reduce the mortality and shorten the recovery time in adults hospitalized with COVID-19 [3].
SARS-CoV-2 is an enveloped, positive-strand RNA virus, and is the largest RNA virus (with the genome of 27~31.5 kb in size) discovered so far and belongs to Nidovirales, Coronaviridae. The Coronaviridae subfamily has been divided into four genera named Alphacoronvirus (αCoV), Betacoronavirus (βCoV), Gammacoronavirus (γCoV) and Deltacoronavirus (δCoV) [4,5]. SARS-CoV-2 is a βCoV [6].
In this review, we have systematically summarized the epidemiology, morphology, genomics, proteomics, and the present research development of drugs of SARS-CoV-2.
Epidemiological investigation and analysis of SARS-CoV-2
Infectious source
The main sources of infection of COVID-19 were patients. In addition, asymptomatic COVID-19 carriers were also able to spread the virus. Some patients with asymptomatic infection have been detected with SARS-CoV-2 pathogenicity in their respiratory tracts [7]. Due to their physical characteristics, they do not show obvious clinical symptoms, but they can carry the virus themselves, and the virus can be transmitted to others [7]. Unlike patients, asymptomatic infections have no clinical symptoms and are difficult to detect in a timely manner. Therefore, if timely isolation and control measures cannot be taken for asymptomatic carriers, it may lead to a large number of leaks and transmission. Besides patients and asymptomatic infections, patients in the incubation period may also spread the virus. Together with the isolation and treatment of patients, the identification of asymptomatic infections must be a high priority.
To figure out the host of COVID-19, various methods such as deep learning algorithms were conducted. It was reported that SARS-CoV-2 was most closely related to bat-SL-CoVZC45 and bat-SL-CoVZXC21, with the similarity of 87.6% and 87.5% respectively [8]. SARS-CoV-2 was highly similar throughout the genome to Bat-CoV RaTG13 and the identity of whole genome sequence is 96.2% [9]. Based on the above results, some researchers predicted bats were the natural hosts of SARS-CoV-2 [8,9]. It is generally believed that the intermediate host of SARS-CoV-2 would be wild animals, and humans are infected due to their close contact with these (killing and/or eating). Some scientists speculate that mink and pangolin may have been the intermediate hosts of this virus based on the mode of virus infection and multiple sequence alignments. However, there is still no clear answer for the intermediate host of SARS-CoV-2 [10].
Transmission routes
The main routes of transmission of SARS-CoV-2 are droplet transmission and close contact transmission. These and other potential routes of COVID-19 transmission are discussed below.
I) Droplet transmission: The main transmission route of COVID-19, it is a respiratory disease that can easily spread to each other when sneezing or talking face to face; II) Intimate contact transmission: People can be infected by SARS-CoV-2 when they have direct physical contact with patients; III) Aerosol transmission: A densely populated and confined environment meets the conditions for aerosol transmission. These include confined space, extended time periods and high concentrations of virus in the air. Droplets mixed in the air form aerosols which cause infection after inhalation [11]. The risk of COVID-19 aerosol transmission can be effectively reduced by strengthening ventilation and environmental disinfection; IV) Fecal (urine)-oral transmission: There is no direct evidence that COVID-19 can be transmitted via the fecal (urine)-oral route. One Study have shown that 4 out of 62 stool samples (6.5%) were positive for Novel Coronavirus, and another 4 patients were positive for SARS-CoV-2 in the gastrointestinal tract, saliva, and urine. Another study showed positive results in oral swabs and fecal swabs by RT-PCR and serological test, indicating COVID-19 has the risk of fecal (urine)-oral transmission, and corresponding preventive work needs to be done; V) Mother-to-child transmission: A baby born to a COVID-19 patient was diagnosed with the virus only 30 h after his birth. This case indicates that SARS-CoV-2 may have a vertical transmission route from mother to child. Although the virus was positive in some patients’ blood, the placental barrier would play an important role in avoiding virus infection to the baby, which means the concentration of COVID-19 has to be higher than a threshold [12]. Therefore, researchers estimated the transmission was still caused by contact.
Susceptible population
Among known cases, the youngest patient was a baby who was born just 30 h while the oldest patient was over 100 years old. Therefore, all age groups are susceptible to infection. It has been found that viral load and contact are two important factors for infection of COVID-19 [13]. The retrospective study indicated that the infection rate of medical staffs in Wuhan was 4.05%. Among medical workers, the infection rate has been seen as high as 29% (40/138). The number of infected males was statistically different in females. The data shows that men are more likely to be infected by COVID-19. Like SARS-CoV and MERS-CoV, SARS-CoV-2 infects human respiratory epithelial cells by S-protein interacting with human ACE2 [14,15]. However, the expression of ACE2 in male cells is higher than that in female cells, which may lead to male infection more easily. The fatality rate was approximate 1.4% in the early stage of COVID-19 outbreak in China, which was lower than that of SARS and MERS. But the current fatality rates in several countries are over 7%.
Transmission dynamics
A study of about 425 cases showed 55% of the early cases (before Jan 1, 2020 - when the South China seafood Market was closed) were related to the market [16]. In subsequent cases of infection, the ratio decreased to 8.2%. The average incubation period of 425 patients involved in the study was 5.2 days (95% CI: 4.1-7.0). Every 7.4 days, the number of people infected with COVID-19 doubled. Recent epidemiological studies have reported that the average incubation period was shorter the previously indicated: 4.8 days (IQR: 3.0-7.2) [17]. Besides, 2.09% of the patients were medical stuffs, and only 1.18% of patients (13/1099) had a direct contact with wildlife. However, 31.30% had been to Wuhan and 71.80% had contact with people from Wuhan, indicating human-to-human transmission. 483 cases (43.95%) were local residents of Wuhan while 26.00% had not recently travelled to Wuhan or contacted with people from Wuhan.
The basic reproduction rate (R0) is another crucial parameter of COVID-19 that is not yet clear. In the early research, R0 was estimated based on the model of 425 cases’ transmission and time fitting. According to the average continuous time interval of 7.5 days (95% CI: 5.3-19), R0 is inferred to be 2.2 (95% CI: 1.4-3.9) [16]. The WHO gave the estimation that the R0 of COVID-19 is 1.4-2.5. In another study, groups from USA and UK. cooperated to determine R0. They assumed the incubation period of COVID-19 was 4.4 days which was similar to SARS. Based on the lift capacity of Wuhan, human-to-human transmission and diagnosed cases before Jan 22, 2020, they drew an epidemic model and estimated the R0 was approximate 3.1113. The authors ascribed the higher R0 to the use of data in the other province besides Hubei. Other researchers in Harvard University and Guangdong CDC gave their predicted R0, 2.0-3.1 and 2.9 respectively [18,19]. A study based on the results of 5 independent models (Exponential growth, Maximum likelihood, Sequential Bayesian, Time-dependent reproduction numbers and SEIR) showed the R0 was 4.38 (95% CI: 3.63-5.13) before the city of Wuhan was administratively closed, 3.41 (95% CI: 3.16-3.65) after Wuhan closed and 3.39 (95% CI: 3.09-3.70) in the whole period [17]. With the development of the epidemic situation, another study used the chain binomial model to analyze the epidemiological data of 8866 patients. The results showed that R0 was 3.77 (95% CI: 3.51-4.05) based on the average incubation period of 5 days and the average continuous time interval of 7 days. It was higher than that of SARS (3.0) and MERS (< 1) [11].
Spread process
Since the first COVID-19 patient was discovered in December 2019, there were 4 stages in the COVID-19 transmission process: local outbreak, community transmission, wide transmission in China and worldwide transmission. The stage of local outbreak took place before Jan 1, 2020. The cases in this stage all linked to the South China Seafood Market. COVID-19 was exposed to more people around Wuhan and other provinces in the community transmission stage. The Spring Festival transportation caused frequent movement of population which led to wild and irresistible transmission of COVID-19 around China. Also contributing were parties and holidays during the Spring Festival, the lack of medical production, like surgical masks and disinfectant, which led to the dire consequences. The number of daily new cases increased dramatically during the whole of January. Fortunately, the Chinese government took strong and insightful measures such as the quarantine of Hubei province and the building of 14 module hospitals including Huoshenshan and Leishenshan hospitals. During this period, many countries provided their generous help. The effect of the struggle by the Chinese people and the help of other countries was obvious. The number of daily new cases decreased gradually following Feb 16, 2020 and the fatality rate was about 2.1% in China. On Feb 26, 2020, the number daily new cases in world other than China was 1027, which was the first day that China contributed less than 50%. The proportion of new cases in China continually decreased in the following days. On Mar 15, 2020, the number total cumulative cases were 153546, and the portion of China started to be less than half. China’s portion decreased gradually in the following days, demonstrating the worldwide spread of COVID-19. Therefore, China will continue to face the serious risk of another outbreak due to case retransmission from outside the country.
The structure of SARS-CoV-2 under Cryo-EM
Like other coronavirus, particles of SARS-CoV-2 are moderately pleiomorphic or roughly spherical, with diameters of about 80-160 nm (Figure 2). The periphery is enveloped by lipid bilayers under Cryo-EM (Figure 2A green arrow), spike proteins of about 23 nm in whole length are embedded in the envelope (red arrow in Figure 2A), and the width of spike’s head is approximately 7 nm (yellow arrow in Figure 2A), under the envelope is the nucleocapsid of the virus [20] (Figure 2A blue arrow). The structure of SARS-CoV-2 mode is shown in Figure 2B. It is vividly named as coronavirus because of its shape which is similar to the crown of medieval European kings.
Figure 2.
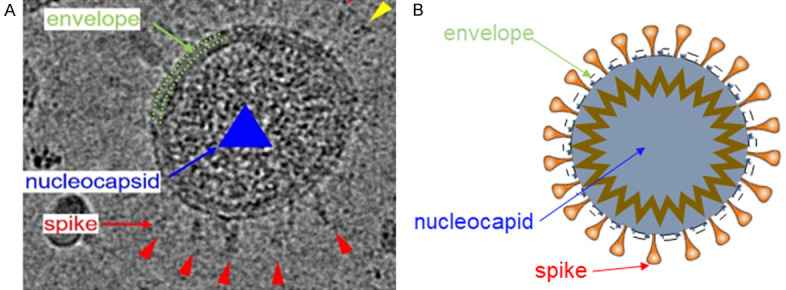
Morphology and mode structure of SARS-CoV-2 by Cryo-EM. A. Morphology of SARS-CoV-2 by Cryo-EM [20]. B. Mode structure of SARS-CoV-2.
Genomics and proteomics analysis of SARS-CoV-2
Genomics
The genome of SARS-CoV-2 is about 29.8 kb in size, with two flanking untranslated regions (UTR) and 14 open reading frames (ORFs) which encode 27 proteins. Located at the 5’-terminus of the genome, the orf1ab and orf1a genes encode the polyprotein pp1ab and pp1a respectively, which constitute 15 non-structural protein (nsps), including nsp1 to nsp16 apart from nsp11. Four structural proteins (M, N, E, and S) and eight accessory proteins (3a, 3b, 7a, 7b, 8b, 9b, p6, and orf14) are encoded at the 3’-terminus of the genome (Figure 3) [21].
Figure 3.
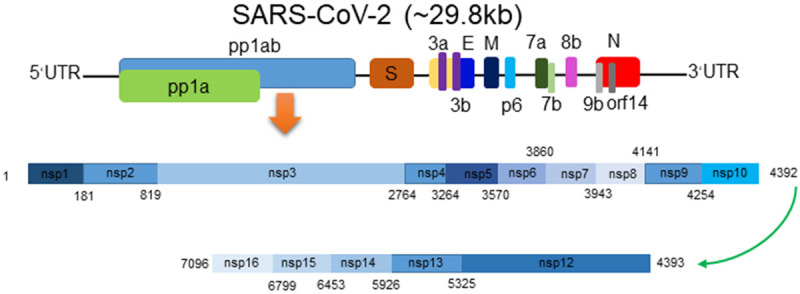
SARS-CoV-2 genome structure and its encoded proteins [21]. The genome of SARS-CoV-2 which is a positive-strand RNA virus encodes 15 non-structural proteins, 4 structural proteins, and 8 accessory proteins.
The results of genomic analysis showed that the sequence homology of SARS-CoV-2 and two other coronaviruses (bat-SL-CoVZC45 and bat-SL-CoVZXC21) collected in Zhoushan, Eastern China in 2018 was the highest, exceeding 87%. The sequence identities of SARS-CoV-2 in five gene regions (E, M, N, 7, 14) are more than 90%. The highest homology of E gene was 98.7%, and the lowest homology of S gene was only 75% [8]. The sequence identities of SARS-CoV-2 with the other two Coronavirus (SARS-CoV and MERS-CoV) were 79% and 50%, respectively [21].
Proteomics
Most of the coding proteins of SARS-CoV-2 are highly consistent with those of the bat-derived coronavirus. Most of the proteins encoded by SARS-CoV-2, bat-SL-CoVZC45 and bat-SL-CoVZXC21 have similar lengths, with few insertions or deletions [22]. The structural proteins of SARS-CoV-2 are the same as other coronaviruses, including spike (S) protein, envelope (E) protein, membrane (M) protein and nucleocapsid (N) protein. Among them, E, M and N proteins have more than 90% genetic similarity with SARS-CoV, but the S protein shows the largest difference at 76%. However, the protein structure of SARS-CoV-2 and MERS-CoV are quite different, the biggest similarity is in E protein, which is only 30%, and the similarities of M, N and S proteins are less than 10% [23].
It is worth noting that although SARS-CoV-2 is close to bat-SL-CoVZC45 and bat-SL-CoVZXC21 at the whole gene level, the external subdomain of its S protein receptor binding domain (RBD) is closer to SARS-CoV. Among the 14 amino acid sequences of S protein RBD in SARS-CoV, SARS-CoV-2 has 8 strictly conserved residues and 6 amino acid mutations, which may affect its selectivity and permeability.
Both SARS-CoV-2 and SARS-CoV enter host cells through the receptor angiotensin converting enzyme II (ACE2) [9,24], and the cellular protease TMPRSS2 is used to activate the S protein. Whether the other two receptors of SARS-CoV (DC-SIGN and L-SIGN) are the receptors of SARS-CoV-2 has not been confirmed. Dipeptide peptidase 4 (DPP4), the main receptor of MERS-CoV, however, has been proved not to be the receptor of SARS-CoV-2.
At the amino acid level, SARS-CoV-2 and SARS-CoV are also similar, but there are still some differences. For example, the 8a protein is not existent in SARS-CoV-2; the 8b protein has 84 amino acids in SARS-CoV, but 121 amino acids in SARS-CoV-2; the 3b protein has only 22 amino acids in SARS-CoV-2, but 154 amino acids in SARS-CoV [21]. The orf8 protein of SARS-CoV-2 does not contain any known functional domain or motif, while an aggregation motif VLVVL (75-79 amino acids) was found in orf8b of SARS-CoV, which can activate NLRP3 inflammasome in macrophages [25].
Progress in drug research of SARS-CoV-2
The COVID-19 is a sudden-onset infectious disease. Unfortunately, there are still no effective drugs for treating it. As our own professional experience confirms, it is a long process from research and development to broad availability of an innovative drug, so the fastest path is to screen out potentially effective therapeutic drugs including vaccines from already approved listed drugs (Table 1).
Table 1.
The drugs for SARS-CoV-2 in clinical research
| Drugs | Targets | Ref. |
|---|---|---|
| Vaccines | S protein | [29] |
| Disulfiram | papain-like proteases | [26] |
| Thiopurine analogues | papain-like proteases | [33,34] |
| Lopinavir | 3C-like proteases | [35-38] |
| Ritonavir | 3C-like proteases | [35-38] |
| ASC09F | 3C-like proteases | - |
| Darunavir | 3C-like proteases | [39] |
| Favipiravir | RdRp | [42] |
| Ribavirin | RdRp | [41] |
| Remdesivir | RdRp | [43,44] |
| Arbidol | unknown | - |
| Oseltamivir | unknown | - |
| Corticosteroids | host | [16,53] |
| Chloroquine | host | [44] |
| IFN-α2b | host | [35,41,51] |
| S416 | host’s DHODH | [48] |
| Tocilizumab | IL-6 | [52] |
| Kevzara | IL-6 | - |
At present, drug targets for SARS-CoV-2 are mainly divided into two categories: one approach targets viral proteins, such as spike protein, papain like protease, 3C like protease, RNA dependent RNA polymerase, while other approaches are based on host targeting [26,27].
Development for different types of SARS-CoV-2 vaccines
Currently, more than 70 kinds of SARS-CoV-2 vaccines under development worldwide contains live-attenuated vaccines, subunit vaccines, mRNA vaccines, DNA vaccines, live-vector vaccines and peptide vaccines, some of them have entered clinical trials [28]. For instance, one adenovirus type-5 (Ad5) vectored COVID-19 vaccine expressing S protein now has been in clinical phase III trials, because the vaccine showed good tolerance in clinical phase I trials, and produced immune response to SARS-CoV-2 in humans [29]. Unfortunately, however, another vaccine from Oxford University have already failed, six vaccinated monkeys were infected with SARS-CoV-2 once again, although antibodies were produced.
A problem in vaccine development is that SARS-CoV-2 only induces mild disease in transgenic animals expressing human ACE2, while wild-type mice cannot be infected, so it is difficult to establish an animal model of SARS-CoV-2 vaccine for the toxic tests. Besides, the S protein of SARS-CoV-2 not only plays an important role in receptor binding but also exhibits other biological activity which may cause severe liver damage and leads to antibody-dependent enhancement (ADE). To fight the coronavirus pandemic which has occurred every decade in 21th century, an effective vaccine may be needed [30].
Viral S protein
Coronaviruses enter the cell by the spike (S) protein binding to the receptor ACE2, but the S proteins need to be cleaved before they can function. It is found that SARS-CoV-2 has a new Furin Protease cleavage site, so the development of Furin protease inhibitor is of great significance for the treatment of COVID-19 [31,32]. But unfortunately, no drug for this target is currently entering clinical trials.
Non-structural proteins of SARS-CoV-2
The non-structural proteins of coronavirus are significant for its. At present, the main drugs targeting non-structural proteins are disulfiram, lopinavir, ritonavir, asc09f, darunavir, favipiravir, ribavirin, penciclovir, remdesivir, galidesivir, and S416 [26].
Disulfiram is a drug approved for the treatment of alcoholism. It has been shown that disulfiram can effectively inhibit the papain-like proteases activity of MERS-CoV and SARS-CoV [26]. In addition, drugs targeting papain-like proteases also include thiopurine analogues [33,34]. However, SARS-CoV-2-related drugs currently being developed for this target are mainly focused on pre-clinical research.
Targets for drugs such as lopinavir, ritonavir, ASC09F, and darunavir are 3C-like proteases. These drugs affect viral replication by targeting 3C-like proteases [35-38]. Lopinavir, ritonavir and darunavir have been approved for HIV treatment [39]. ASC09F for HIV is in clinical research. Unfortunately, recent research by academician Wang Chen et al. showed that lopinavir and ritonavir, which have previously entered clinical phase III, were not effective in treating severe patients of COVID-19. The results have been published in NEJM [40]. Deliravir and ASC09F are still in clinical phase III trials of COVID-19.
The target of drugs such as fapilavir, ribavirin, penciclovir, redecvir, and galidivir is RNA-dependent RNA polymerase (RdRp). These drugs are nucleoside analogs that block viral RNA synthesis by targeting RdRp, and ultimately inhibit viral replication. Fapilavir, ribavirin, and penciclovir were approved for the treatment of influenza, hepatitis C virus (HCV)/respiratory syncytial virus (RSV), and herpes simplex virus (HSV), respectively [41,42]. At present, only papavivir and ribavirin are conducting clinical research on COVID-19. Galidivir was originally developed for the treatment of HCV but pre-clinical studies have shown excellent antiviral activity against SARS-CoV-2 [26,43,44].
Previously, a patient with SARS-CoV-2 infection in the United States recovered under the treatment of remdesivir, so it has attracted considerable attention. Remdesivir (Figure 4A), also known as GS-5734, is the monophosphoramide prodrug of GC-441524, which is a C-adenosine nucleoside analogue. Studies show that remdesivir targets RNA-dependent RNA polymerase and therefore has broad-spectrum antiviral activity against Ebola-Kikwit, Ebola-Makona, Sudan virus, Bundibugyo virus, Marburg virus, MERS-CoV in vitro (EC50 = 0.02 µM-10 µM) [45]. In Vero E6 cells, the EC50 of SARS-CoV-2 = 1.76 µM [44].
Figure 4.
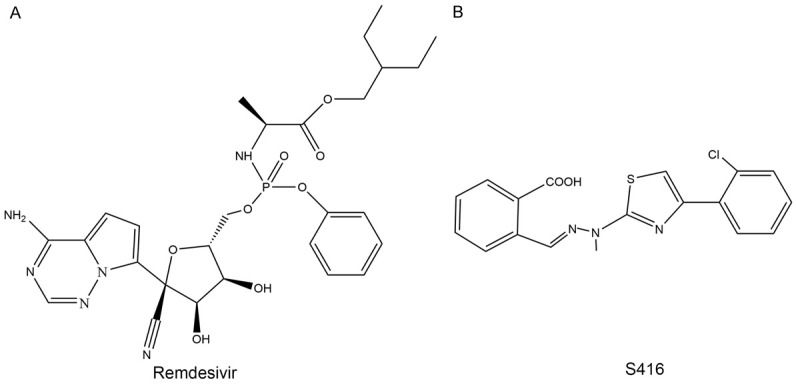
Small molecule compounds Remdesivir and S416. Remdesivir targets viral RNA-dependent RNA polymerase (A), S416 targets the host’s DHODH (B) [48], both of them have broad-spectrum antiviral activities.
There are also drugs that target SARS-CoV-2, but whose targets are unknown. Examples include arbidol and oseltamivir, both of which are used for the treatment of influenza. Abidol inhibits influenza infection by binding influenza virus’s hemagglutinin to affect its membrane fusion with host cells. Oseltamivir is a neuraminidase inhibitor that affects virus-infected cells by inhibiting the neuraminidase activities of the influenza viruses. Neither of these drug targets exists in the SARS-CoV-2. Although the mechanism against the novel coronavirus is still unclear, these two drugs both have significant effects in the treatment of COVID-19, and phase IV clinical studies of patients have been conducted [26,46,47].
Targeting human related proteins
Recently, Rui Xiong reported that a small molecule compound named S416 (Figure 4B) inhibited SARS-CoV-2 more effectively than remdesivir, with EC50 = 17 nM in Vero E6 cells, much less than 1.76 µM. But the target of S416 is inconsistent with that of remdesivir. It targets the host’s DHODH, a rate-limiting enzyme in pyrimidine synthesis in vivo, which blocks viral RNA replication by efficiently inhibiting pyrimidine synthesis, but has low toxicity to cells. It is currently effective against influenza viruses (EC50 = 0.01-0.06 µM), SARS-CoV, MERS-CoV, Ebola (EC50 = 0.018 µM), Zika virus (EC50 = 0.021 µM) and circulating SARS-CoV-2 [48].
At the same time, a series of clinical symptoms such as inflammatory response and cytokine storm have been reported in patients infected with SARS-CoV-2. Corticosteroids, Chloroquine, Interferon α2b (IFN-α2b), Tocilizumab, and Kevzara have entered the clinical research. Corticosteroids are mainly used in anti-inflammatory therapy, and studies have shown that they have therapeutic effects on the clinical response caused by SARS-CoV-2 [49,50]. Chloroquine is mainly used in anti-malarial and autoimmune diseases, and recent studies have shown that it has a broad spectrum of antiviral effects, mainly by regulating the pH in the body to affect viral infections [27,44]. IFN-α2b is an immunomodulator that produces antiviral effects by improving the immunity of patients [35,36,51]. Tocilizumab is the first approved IL-6 blocking antibody that specifically binds membrane IL-6 receptors and soluble IL-6 receptors and inhibits signal transduction. It has been proven safe in the treatment of rheumatoid arthritis [52]. In the United States and European Union, tocilizumab has also been approved to treat severe and life-threatening cytokine release syndrome (CRS) associated with chimeric antigen receptor T cell (CAR-T) therapy. Clinical studies have shown that patients with COVID-19 had a rapid decrease in fever within days after receiving tocilizumab, and 75% of patients (15 of 20) had reduced need for supplemental oxygen. Based on these results, China has recently updated the COVID-19 treatment guidelines and approved the use of tolicizumab as an antibody drug to treat critically ill patients. Similar to tocilizumab, Kevzara is a fully human monoclonal antibody that targets the IL-6 receptor. Sanofi and its partner Regeneron will jointly conduct clinical trials. Regeneron will lead clinical trials in the United States while Sanofi will lead clinical trials outside the United States, including in areas such as Italy that are more affected by COVID-19.
Conclusion
The worldwide pandemic of COVID-19 has caused, and will further cause immeasurable losses to the global economy. Effective medical interventions are in urgent needs. Whether it is remdesivir, reported at the beginning of the epidemic, or S416, which has recently been reported to be more effective, we hope that these two new drugs will achieve good clinical efficacy and bring the epidemic to an end as soon as possible. Another exciting news is that on March 20, 2020, the Angus Cameron team by analyzing global data, found that the higher the outdoor ambient temperature, the lower the incidence of COVID-19! So far, we human beings have never been overwhelmed by a pandemic, nor we will be this time, but we must learn from it. For instance, strengthening the international medical communities to defeat this new challenge.
Acknowledgements
We would greatly appreciate the supports from Shenzhen Science and Technology Program. (Grant No.: KQTD20170810154011370), Xiangtan Institute of Industrial Technology Collaborative Innovation, and Xiangtan Science and Technology Bureau. We also thank Professor John Dunlap for his valuable advice and editing of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Wang G, Jin X. The progress of 2019 novel coronavirus event in China. J Med Virol. 2020;92:468–72. doi: 10.1002/jmv.25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–23. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yavarian J, Rezaei F, Shadab A, Soroush M, Gooya MM, Azad TM. Cluster of Middle East respiratory syndrome coronavirus infections in Iran, 2014. Emerg Infect Dis. 2015;21:362–4. doi: 10.3201/eid2102.141405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–6. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo SH, Liu W, Liu ZJ, Zheng XY, Hong CX, Liu ZR, Liu J, Weng JP. A confirmed asymptomatic carrier of 2019 novel coronavirus (SARS-CoV-2) Chin Med J (Engl) 2020;133:1123–1125. doi: 10.1097/CM9.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Q, Li M, Wang C, Wang P, Fang Z, Tan J, Wu S, Xiao Y, Zhu H. Host and infectivity prediction of Wuhan 2019 novel coronavirus using deep learning algorithm. BioRxiv. 2020 [Google Scholar]
- 11.Yang Y, Lu Q, Liu M, Wang Y, Zhang A, Jalali N, Dean N, Longini I, Halloran ME, Xu B, Zhang X, Wang L, Liu W, Fang L. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. MedRxiv. 2020 [Google Scholar]
- 12.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–9. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci. 2004;61:2738–43. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang MW, Cheng Y, Zhang J, Jiang XM, Wang L, Deng J, Wang PH. Increasing host cellular receptor-angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. J Med Virol. 2020 doi: 10.1002/jmv.26139. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, Lou Y, Gao D, Yang L, He D, Wang MH. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–7. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Read JM, Bridgen JRE, Cummings DAT, Ho A, Jewell CP. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. MedRxiv. 2020 doi: 10.1098/rstb.2020.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019-nCoV. BioRxiv. 2020 doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Yang Y, Gao Y, Shen C, Ju B, Liu C, Tang X, Wei J, Ma X, Liu W, Xu S, Liu Y, Yuan J, Wu J, Liu Z, Zhang Z, Wang P, Liu L. Viral architecture of SARS-CoV-2 with post-fusion spike revealed by Cryo-EM. BioRxiv. 2020 [Google Scholar]
- 21.Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, Sheng J, Quan L, Xia Z, Tan W, Cheng G, Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–8. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–36. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi CS, Nabar NR, Huang NN, Kehrl JH. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–50. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–90. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Zeng H, Gu J, Li H, Zheng L, Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines (Basel) 2020;8:153. doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L, Jia SY, Jiang HD, Wang L, Jiang T, Hu Y, Gou JB, Xu SB, Xu JJ, Wang XW, Wang W, Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo F, Liao FL, Wang H, Tang HB, Yang ZQ, Hou W. Evaluation of antibody-dependent enhancement of SARS-CoV infection in rhesus macaques immunized with an inactivated SARS-CoV vaccine. Virol Sin. 2018;33:201–4. doi: 10.1007/s12250-018-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia S, Zhu Y, Liu M, Lan Q, Xu W, Wu Y, Ying T, Liu S, Shi Z, Jiang S, Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng KW, Cheng SC, Chen WY, Lin MH, Chuang SJ, Cheng IH, Sun CY, Chou CY. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou CY, Chien CH, Han YS, Prebanda MT, Hsieh HP, Turk B, Chang GG, Chen X. Thiopurine analogues inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biochem Pharmacol. 2008;75:1601–9. doi: 10.1016/j.bcp.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, AlJohani S, Al Harbi S, Kojan S, Al Jeraisy M, Deeb AM, Assiri AM, Al-Hameed F, AlSaedi A, Mandourah Y, Almekhlafi GA, Sherbeeni NM, Elzein FE, Memon J, Taha Y, Almotairi A, Maghrabi KA, Qushmaq I, Al Bshabshe A, Kharaba A, Shalhoub S, Jose J, Fowler RA, Hayden FG, Hussein MA And the MIRACLE trial group. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arabi YM, Asiri AY, Assiri AM, Aziz Jokhdar HA, Alothman A, Balkhy HH, AlJohani S, Al Harbi S, Kojan S, Al Jeraisy M, Deeb AM, Memish ZA, Ghazal S, Al Faraj S, Al-Hameed F, AlSaedi A, Mandourah Y, Al Mekhlafi GA, Sherbeeni NM, Elzein FE, Almotairi A, Al Bshabshe A, Kharaba A, Jose J, Al Harthy A, Al Sulaiman M, Mady A, Fowler RA, Hayden FG, Al-Dawood A, Abdelzaher M, Bajhmom W, Hussein MA the Saudi Critical Care Trials group. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-beta1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21:8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-alpha for Middle East respiratory syndrome. Antivir Ther. 2016;21:455–9. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- 38.Sheahan TP, Sims AC, Leist SR, Schafer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina JM, Squires K, Sax PE, Cahn P, Lombaard J, DeJesus E, Lai MT, Rodgers A, Lupinacci L, Kumar S, Sklar P, Hanna GJ, Hwang C, Martin EA DRIVE-FORWARD trial group. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 96-week results of a randomised, double-blind, non-inferiority, phase 3 trial. Lancet HIV. 2020;7:e16–e26. doi: 10.1016/S2352-3018(19)30336-4. [DOI] [PubMed] [Google Scholar]
- 40.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falzarano D, de Wit E, Rasmussen AL, Feldmann F, Okumura A, Scott DP, Brining D, Bushmaker T, Martellaro C, Baseler L, Benecke AG, Katze MG, Munster VJ, Feldmann H. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–7. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldhill DH, Te Velthuis AJW, Fletcher RA, Langat P, Zambon M, Lackenby A, Barclay WS. The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci U S A. 2018;115:11613–8. doi: 10.1073/pnas.1811345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9:e00221–18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoenen T, Groseth A, Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat Rev Microbiol. 2019;17:593–606. doi: 10.1038/s41579-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 46.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–8. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 48.Xiong R, Zhang L, Li S, Sun Y, Ding M, Wang Y, Zhao Y, Wu Y, Shang W, Jiang X, Shan J, Shen Z, Tong Y, Xu L, Yu C, Liu Y, Zou G, Lavillete D, Zhao Z, Wang R, Zhu L, Xiao G, Lan K, Li H, Xu K. Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. BioRxiv. 2020 doi: 10.1007/s13238-020-00768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou W, Liu Y, Tian D, Wang C, Wang S, Cheng J, Hu M, Fang M, Gao Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N, Mushtaq A. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–32. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm. National Science Review. 2020:6. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–4. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]