Abstract
First-generation immunological checkpoint inhibitors, such as CTLA-4, PD-L1 and PD-1 exhibit significant advantages over conventional cytotoxic drugs, such as oxaliplatin and 5-FU, for the treatment of colorectal cancer. However, these inhibitors are not ideal due to their low objective response rate and the vulnerability of these treatment methods when faced with emerging drug resistant cancers. This study summarizes the immunological characteristics of colorectal cancer treatment, and analyzes the ways in which OX40 may improve the efficacy of these treatments. Activation of the OX40 signaling pathway can enhance the activity of CD4+/CD8+ T cells and inhibit the function of Treg. Simultaneously, OX40 can directly inhibit the expression of Foxp3, affect the inhibitory function of Treg, and inhibit the immunosuppressive factors in the tumor microenvironment so as to reverse immune escape and reverse drug resistance. Therefore, OX40 is an important target for treating colorectal cancer in “cold tumors” with less immunogenicity.
Keywords: OX40, immune escape, colorectal cancer, microenvironment
Introduction
Colorectal cancer is tumor malignancy that occurs in the colon or rectum and is one of the top three causes of morbidity and top five causes of mortality worldwide [1]. Mortality in colorectal cancer is usually due to systemic metastasis of the cancer, as a result of treatment failing to address weak immunogenicity-related “cold tumor” immune escape and extremely efficient drug-resistant mutations of the cancer. The current approach for drug improvement is passive and inefficient. Newly developed drugs tend to disorder immune environments and contribute to the development of drug resistant tumors [2]. Optimal treatment strategies simultaneously address each of these factors by facilitating the efficient detection and killing of non-drug resistant tumor cells by immune cells. Research on tumor immunotherapy has revealed that cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies and programmed death 1/programmed death ligand (PD-1/PD-L1) antibodies are effective targets for the treatment of colorectal cancer [3]. Additionally, solid tumors studies have revealed that combined application of checkpoint inhibitors, CTLA-4 and PD-1, yield significantly decreased chances of drug-resistant cancer development [4]. The emergence of drug resistant first-generation immunological checkpoint inhibitors urgently prompts the need for new immunomodulatory antibodies that break down tumor cell-mediated immune tolerance through multiple signaling pathways. Therefore, the immune costimulatory molecule OX40 (CD134) is a promising novel target for colorectal cancer immunotherapy.
First, this study reviews fundamental immunology concepts. The adaptive immune system is responsible for distinguishing tumor cells from normal cells. Tumor specific antigens present as peptides on MHC class I molecules for recognition by cytotoxic CD8+ T cells (or MHC class II molecules for recognition by CD4+ T cells). In order to trigger the CD8+ T cell response, tumor cell antigens are processed by dedicated antigen presenting cells (APC), such as dendritic cells (DCs) and CD4+ helper T cell antigens, are presented to CD8+ T cells [5,6]. The formation of tumor immunosuppressive microenvironments is attributed to two processes. Tregs, myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM) are critical for the creation of tumor immunosuppressive microenvironments. TAMs play a critical role in this process by releasing immunosuppressive cytokines, such IL-10, IL-4, TGF-β, vascular endothelial growth factor (VEGF) and arginase. Another method of immune suppression by tumors is achieved through the upregulation of immunosuppressive receptors or ligands, such as CTLA-4, PD-1 and PD-L1. These factors interact to create immunosuppressive microenvironments. For example, vascular endothelial growth factor receptor (VEGFR) signaling can enhance the PD-L1/PD-L2 pathway [7,8].
Previously used cytotoxic drugs
Oxaliplatin: Oxaliplatin is a standard first-line treatment drug for colorectal cancer [9,10]. Oxaliplatin treats colorectal cancer by activating the immune system APCs; this process is independent of T cell activation or MHC upregulation. Previous studies have found that co-culture of colorectal cancer cells in an oxaliplatin supernatant promotes the maturation of DCs and increases the proliferation of T cells [11]. However, oxaliplatin-associated death of colorectal cancer cells results in the production of HMGB1, which induces immunogenicity-related cell death in various colon cancer cell lines. Treatment with cisplatin has not been found to produce these outcomes as this process of immunogenicity-related cell death relies on TLR-4 [12,13]. Other studies have validated the role of oxaliplatin as an immunogenic cell death-inducing factor. Oxaliplatin mediates the expression of calcium net protein and HMGB1, which neutralize antibodies to eliminate the expression of the HMGB1/calcium net protein/immunogenic cell death. This also confirms the importance of the HMGB1-TLR4 control shaft in oxaliplatin-mediated immune function [14]. Secondly, oxaliplatin reverses immunosuppression created by tumor growth. This could be mediated through PD-1/PD-L1, following DC exposure to oxaliplatin, resulting in enhanced stimulation of T cells. During this process, there are no changes to the expression levels of MHC or costimulatory molecules, but the expression of PD-L2 and PD-L1 are decreased. This leads to elevated antigen-specific proliferation and enhances the recognition of tumor cells by T cells [15]. Joint treatment of colorectal cancer patients with IL-12 and oxaliplatin stimulate T lymphocyte and NK cell proliferation, which balances the ratio between effector cells and regulation/suppression cells. This increase in the CD8+/Tregs ratio and reduction in MDSC abundance enhances the immune response against colorectal cancer and eliminates liver metastases [16].
5-fluorouracil (5-FU): 5-FU is a basic drug used for the treatment of colorectal cancer [17]. 5-FU specifically has an influence on the immune system aside from its direct cytotoxic effects. 5-FU efficiently and selectively consumes the MDSC of mice in colorectal cancer and increases the expression of IFN-γ produced by tumor-specific T cells [18]. In contrast with oxaliplatin, 5-FU does not induce immunogenic cell death (because it does not upregulate cadherin) and its anti-tumor activity is TLR4 independent. 5-FU effectively activates the immune system by enhancing the inhibition of anti-tumor immune functions [19].
Irinotecan: One of the most important drugs for the treatment of advanced colorectal cancer is irinotecan [20]. Melichar et al. found that irinotecan treatment increased the number of CD4+ and CD8+ cells in peripheral blood of 14 patients with metastatic colorectal cancer [21]. Additionally, Kim et al. demonstrated that irinotecan (as part of a FOLFIRI regimen) inhibited the immunosuppressive environment of tumors to permit the maturation of DCs, by using a DC vaccine transfected with a virus vector overexpressing CEA. Compared with the vaccine alone, the combination of DC vaccine and FOLFIRI enhances tumor-specific immune responses, as the number of CEA-specific IFN-γ secreted lymphocytes increase. Although irinotecan does decrease MDSC and Tregs abundance, additional vaccine doses reverse this effect (Figure 1) [22].
Figure 1.
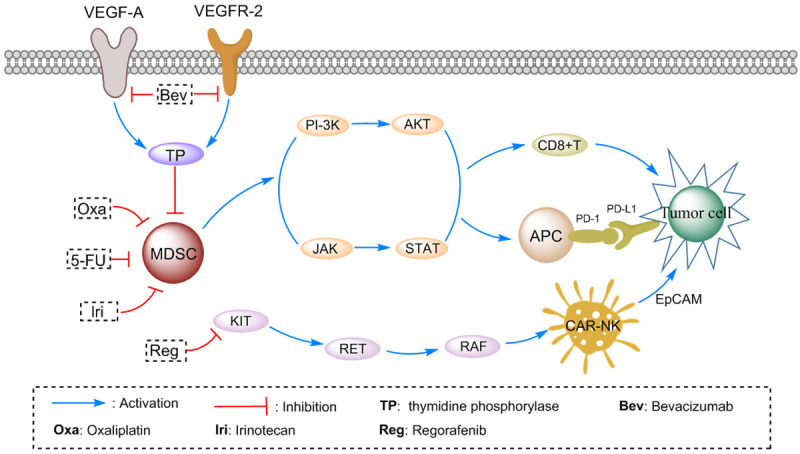
Pathway analysis of Tumor cell and T cells: the pathways that involve MDSC cells, CD8+ T cells, Tumor cells, NK cells and chemotherapy drugs were constructed.
Current individualized anti-VEGF/EGFR drugs
Anti-VEGF therapy: Over the past 10 years, various methods of inhibiting vascular endothelial growth factor (VEGF) have been approved for the treatment of colorectal cancer [23-29]. The main goal of anti-VEGF treatment is the restructuring of the tumor vascular system for enhanced drug delivery [30-32]. However, this therapeutic effect has not been efficiently achieved [33]. Nevertheless, pro-angiogenic factors are critical for the maintenance of the immunosuppressive tumor microenvironment. For example, pro-angiogenic factors stimulate Tregs in addition to producing immunosuppressive cytokines [34-36], thereby inhibiting the function of immune cells [37]. Counteracting these effects through treatment with VEGF facilitates normal immune system regulation and enhanced anti-tumor immune responses in the microenvironment [38,39]. Treatment with bevacizumab enhances the anti-colorectal cancer effect of 5-fluorouracil on the VEGF-A/VEGFR-2 pathway by upregulating thymidine phosphorylase [40]. Additionally, treatment with bee venom peptide interrupts the MAPK signal pathway mediated by VEGFR-2 and COX-2, which in turn effectively inhibits VEGF-A related tumor growth [41]. VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 combined with the high expression CD4/CD8 in the associated matrix are good immune prognostic markers of colorectal cancer [42]. In another study, it was found that levels of peripheral blood (PB) DC1 and DC2 levels are negatively correlated with VEGF serum levels in patients with colorectal cancer. It has been suggested that the interval number and impaired function of PBDC may be related with neoplasm staging and VEGF level [43,44]. Additionally, the dosing of anti-VEGF therapy is critical, since studies have found that anti-VEGF therapy combined with tumor vaccination activates the immune system and inhibits tumor growth when anti-VEGF treatment is administered at 25% of the maximum dose. Compared with higher doses, lower doses lead to decreased MDSC infiltration. Lower doses of anti-VEGFR-2 also increase the distribution of the functional vascular system compared with higher doses. In conclusion, appropriate doses of anti-VEGF therapy normalize the vascular system of colorectal tumors and decrease the inhibition of the immune microenvironment [37,39,45,46].
Small molecule VEGF inhibitors: Sorafenib and regorafenib are two types of multi-kinase inhibitors that can also be used to inhibit VEGF and have been used for the treatment of colorectal cancer. They have been shown to affect immunogenicity, but their net effect is unknown [47-51]. First, sorafenib inhibits the function of DC, decreases the induction of antigen-specific T cells [52,53], and inhibits the function of NK cells [54,55]. Regorafenib is a third-line treatment for colorectal cancer but has a unique ability of reversing immune escape [56]. For example, regorafenib treatment enhances the responsiveness of adoptive chimeric antigen receptor modified T or NK cells (CAR-T or CAR-NK) in solid tumors. Regorafenib and CAR-NK-92 cells have been shown to exert synergistic effects on immune responses in human colorectal cancer xenografts in mouse models. Treatment with regorafenib enhances the ability of CAR-NK-92 cells to specifically recognize EpCAM-positive colorectal cancer cells and release cytokine killers, such as IFN-γ, perforin and granular enzyme B [57].
Anti-EGFR therapy: Panitumumab and cetuximab have been proven to enhance immune activity in colorectal cancer patients [58]. A large number of preclinical studies indicate that cetuximab treatment can induce antibody-dependent cell-mediated cytotoxicity (ADCC) [59,60]. Cetuximab is usually combined with chemotherapy but individualized gene expression is critical for its effect on immunity. Correale et al. found that EGFR expression is upregulated after chemotherapy, which subsequently leads to increased susceptibility of colorectal cancer cells to ADCC, independent of the k-ras pathway [61]. Cetuximab affects the proliferation and phagocytosis of colorectal cancer cells through DC. This suggests that CTL-dependent immunity is involved in the anti-tumor effects of cetuximab [62]. These studies also suggest that the order of administration is important for triggering an effective immune response. A critical finding presented in this study is that anti-EGFR therapy remains effective at suppressing immune escape even in cases of drastic mutations to the k-ras pathway. Compared with a standard FOLFOX regimen, the immune-activated chemotherapy regimen (with irinotecan and 5-FU administered on the first day and cetuximab administered on the third day) increases levels of CEA and thymidine synthase-specific CTL precursors in the blood of patients, as well as the number of killer T cells [63].
Potential immune costimulatory molecular drugs that reverse both immune escape and drug resistance
OX40 belongs to the tumor necrosis factor receptor superfamily and is expressed on the surface of antigen-presenting cells, natural killer cells and mast cells [64]. The sole ligand of OX40, OX40L (CD252), activates T cells by stimulating OX40 and initiating activation signals, such as NF-κB and nuclear factr of activated T cells (NFAT) [65]. These signals increase the expression of cyclin A, cyclin-dependent kinase, cytokines and their receptors [66]. OX40 is expressed on the surface of tumor infiltrating lymphocytes (TILs) of various tumor tissues [67]. In colorectal cancer, the high expression levels of OX40 on TILs of mesenteric lymph nodes and other sites have been found to be positively correlated with overall survival, particularly when the expression of OX40 is elevated on the surface of CD4+ T cells and on the surface of tumor-infiltrating Tregs [68,69]. Activation of the OX40 signaling pathway enhances the activity of CD4+/CD8+ T cells and inhibits the function of Tregs. However, T cell activation signals can be neutralized by inhibitory receptors, such as PD-1 or CTLA-4 [35,70]. Previous animal studies have shown that OX40 is expressed on the surface of T cells between 24 and 96 hours after antigen recognition and that the presence of the OX40 agonist antibody increases the survival of different subtypes of effector T cells [71-73]. As illustrated in Figure 2, OX40 affects the inhibitory function of Tregs because OX40 inhibits the expression of Foxp3 directly, which inhibits the antagonistic effects of TGF-β and is responsible for transforming T cells into Foxp3+ Tregs [74-76]. Additionally, Tr1 cells (inhibitory CD4+ T cells) induced in vitro can be blocked by the activation of OX40 [77]. However, the degree of OX40 activation depends on the immune microenvironment of T cells, since OX40 can only promote the proliferation of Treg in the absence of IFN-γ and IL-4 [78,79].
Figure 2.
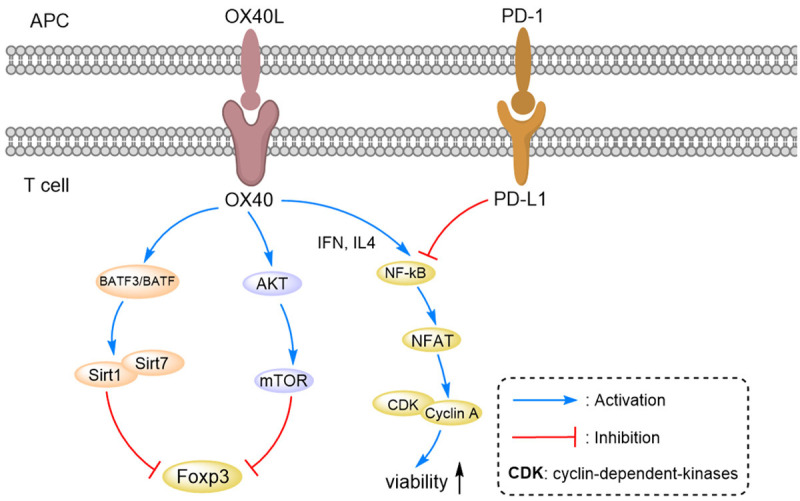
Pathway analysis of APC cells and T cells: the pathways that involve OX40/OX40L, PD-1/PD-L1, mTOR/Akt, NK-κB/NFAT and Cyclin A/CDK were analyzed.
Reversal of immune escape: The OX40 signaling pathway can be activated by OX40L-Fc fusion proteins, specific OX40 antibody agonists, and transfected tumors and dendritic cells (DCs). OX40 antibody agonists directly improve the effector function of T cells and neutralize invasive Tregs [80,81]. This is accomplished via antibody-dependent cell-mediated cytotoxicity (ADCC) or antibody-dependent cell-mediated phagocytosis (ADCP). Additionally, this process involves surface activated Fc γ receptors (human being: FcγR I and FcγR II a, mouse: FcγR I, FcγR III and FcγR IV) of NK cells that recognize antibodies bound to antigens on the surface of cell membranes and kill these specific cells. The antibody impacts the strength of the ADCC or ADCP response based on the following factors: (I) Antibody subtypes; for example, the ADCC effect of an IgG1 antibody is stronger than that of an IgG4 antibody [82-84]. (II) Glycosylation modification; for example, fucose can enhance the ADCC effect of natural killer cells. (III) Types of Fc receptors (and whether they are activated or inhibited) [85]. (IV) The density of macrophages in tumor lesions [86]. Additionally, the activation or depletion of OX40 antibodies depend on the expression of OX40 on the surface of different TIL subtypes. ADCC occurs only when NK cells are present and the activated Fc receptor is expressed [87]. Intravenous administration of OX40 antibody is more likely to activate peripheral lymphocytes, while intra-tumor administration can enhance the activation of tumor-specific immune responses and reduce systemic toxicity. ADCC can be further enhanced in combination with 4-1bb antibodies [88]. Decreasing the accumulation of bone marrow cells in tumors can weaken the tumor inhibition effect of the OX40 antibody [89]. However, OX40 antibody-mediated Tregs depletion does not account for all anti-tumor effects, since it has been observed that OX40 antibodies directly activate CD8+ T cells and CD4+ effector T cells to elicit anti-tumor effects [90,91]. In conclusion, Tregs depletion is a necessary but insufficient condition for OX40 antibody functioning, while the activation of effector T cells also play a critical role. This provides us with more useful details for future drug development efforts related to OX40 for the treatment of colorectal cancer.
Reversing drug-resistance: OX40 antibodies and OX40 Fc fusion shows strong tumor suppressive effects on low tumor load in mice. However, OX40 antibody treatment has a weak therapeutic effects on larger or metastatic tumors, which can be improved when OX40 is used in a combined drug regimen [92]. The current strategy is to enhance antigen release for improved immune suppression and providing assistance to adoptive T cells, including using cyclophosphamide to kill tumor cells, release antigens and mediate Tregs inhibition [93]. Since the OX40 signaling pathway significantly prolongs the survival of antigen-activated CD4+ and CD8+ T cells, the combination of OX40 agonists with certain combination therapies increases antigen load [94,95]. For example, OX40L-Fc used in combination with chemotherapy and vaccines for the treatment of solid tumors [96]. Additionally, the survival of CD4+ and CD8+ effector T cells can be enhanced by inhibiting the function of Tregs (via depletion or attenuation of the inhibitory effect of Tregs). The most operable strategy is that OX40 agonists combined with immunosuppressants exert a synergistic effect for the treatment of metastatic colorectal cancer. PD-1 inhibitors combined with OX40 agonists significantly extend the survival time of 50% of experimental animals treated for colorectal cancer [97,98]. Similarly, OX40 antibody combined with arginase inhibitors significantly enhance the function of CD4+ T cells and CD8+ T cells, thereby inducing tumor shrinkage [99].
Summary and prospects
During recent years, many studies have explored potential treatment methods that modulate varying aspects of the immune response, in order to target cancers, such as colorectal cancer [100,101]. The advantages of immunotherapy are clear: a lasting response, a lack of drug-resistance, generation of immune memory and a decrease in non-specific toxicity [102,103]. However, immunotherapy is not suitable for every patient and may require a combination of multiple treatments to elicit an appropriate immune response and to address “immune-susceptible” tumors [104,105]. For example, metronomic chemotherapy (high frequency, low dose chemotherapy) has gained increasing attention during recent years, since its administration has been shown to exert positive effects on the immune response [106-109]. Metronomic chemotherapy can improve CTL activity and reduce the quantity of immunosuppressive cells (Tregs and MDSCs) in the tumor microenvironment by adequately stimulating cytotoxic immune cells without exhaustion. Checkpoint suppression can further enhance the immune response against tumors by keeping T cells in an activated state. Combining checkpoint suppression with metronomic chemotherapy drug delivery can produce synergistic effects that enhance immune responses against tumors and eliminate metabolic competition. This allows for the elimination of treatment-resistant cancer cells, an effect that cannot be achieved with either treatment alone [109,110]. The OX40 antibody has broad prospects in combination with other therapies, such as surgery, radiotherapy, vaccines and immunomodulators. At present, more studies are needed to find the most effective combination schedule and optimal dose to balance the direct anti-cancer effect of conventional therapy in synergy with immunotherapy to achieve maximum effectiveness using OX40 for the treatment of colorectal cancer.
Acknowledgements
This work was supported by the National Science Foundation for Young Scholars of China (No. 81502120) and the National Science Foundation (81973533) and Guangxi Medical University Training Program for Distinguished Young Scholars. China Postdoctoral Science Foundation (No. 2019M653812XB). 2019 Guangxi University High-level Innovation Team and the Project of Outstanding Scholars Program, and Guangxi Science and Technology Project (2019AC03004).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Duffy AG, Greten TF. Immunological off-target effects of standard treatments in gastrointestinal cancers. Ann Oncol. 2014;25:24–32. doi: 10.1093/annonc/mdt349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagnoli PA, Premenko-Lanier MF, Loria GD, Altman JD. CD8 T cell memory recall is enhanced by novel direct interactions with CD4 T cells enabled by MHC class II transferred from APCs. PLoS One. 2013;8:e56999. doi: 10.1371/journal.pone.0056999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engels B, Chervin AS, Sant AJ, Kranz DM, Schreiber H. Long-term persistence of CD4(+) but rapid disappearance of CD8(+) T cells expressing an MHC class I-restricted TCR of nanomolar affinity. Mol Ther. 2012;20:652–660. doi: 10.1038/mt.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai YS, Wahyuningtyas R, Aui SP, Chang KT. Autocrine VEGF signalling on M2 macrophages regulates PD-L1 expression for immunomodulation of T cells. J Cell Mol Med. 2019;23:1257–1267. doi: 10.1111/jcmm.14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH, Go H. Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol. 2016;23:694–702. doi: 10.1245/s10434-015-4903-7. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki S, Schirripa M, Loupakis F, Cao S, Zhang W, Yang D, Ning Y, Berger MD, Miyamoto Y, Suenaga M. Tandem repeat variation near the HIC1 (hypermethylated in cancer 1) promoter predicts outcome of oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Cancer. 2017;123:4506–4514. doi: 10.1002/cncr.30880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satake H, Tsuji A, Nakamura M, Ogawa M, Kotake T, Hatachi Y, Yasui H, Takagane A, Okita Y, Nakamura K. Phase I study of primary treatment with 5-FU, oxaliplatin, irinotecan, levofolinate, and panitumumab combination chemotherapy in patients with advanced/recurrent colorectal cancer involving the wild-type RAS gene: the JACCRO CC-14 study. Int J Clin Oncol. 2018;23:490–496. doi: 10.1007/s10147-017-1228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115–123. doi: 10.1038/sj.bjc.6605465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 13.Stojanovska V, McQuade RM, Fraser S, Prakash M, Gondalia S, Stavely R, Palombo E, Apostolopoulos V, Sakkal S, Nurgali K. Oxaliplatin-induced changes in microbiota, TLR4+ cells and enhanced HMGB1 expression in the murine colon. PLoS One. 2018;13:e0198359. doi: 10.1371/journal.pone.0198359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 15.Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, Schreibelt G, de Boer A, Van Herpen CM, Kaanders JH. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121:3100–3108. doi: 10.1172/JCI43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Aparicio M, Alzuguren P, Mauleon I, Medina-Echeverz J, Hervas-Stubbs S, Mancheno U, Berraondo P, Crettaz J, Gonzalez-Aseguinolaza G, Prieto J. Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice. Gut. 2011;60:341–349. doi: 10.1136/gut.2010.211722. [DOI] [PubMed] [Google Scholar]
- 17.Du C, Huang D, Peng Y, Yao Y, Zhao Y, Yang Y, Wang H, Cao L, Zhu WG, Gu J. 5-fluorouracil targets histone acetyltransferases p300/CBP in the treatment of colorectal cancer. Cancer Lett. 2017;400:183–193. doi: 10.1016/j.canlet.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 19.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2:628–639. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Shigeta K, Hasegawa H, Okabayashi K, Tsuruta M, Ishii Y, Endo T, Ochiai H, Kondo T, Kitagawa Y. Randomized phase II trial of TEGAFIRI (tegafur/uracil, oral leucovorin, irinotecan) compared with FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) in patients with unresectable/recurrent colorectal cancer. Int J Cancer. 2016;139:946–954. doi: 10.1002/ijc.30127. [DOI] [PubMed] [Google Scholar]
- 21.Melichar B, Touskova M, Vesely P. Effect of irinotecan on the phenotype of peripheral blood leukocyte populations in patients with metastatic colorectal cancer. Hepatogastroenterology. 2002;49:967–970. [PubMed] [Google Scholar]
- 22.Kim HS, Park HM, Park JS, Sohn HJ, Kim SG, Kim HJ, Oh ST, Kim TG. Dendritic cell vaccine in addition to FOLFIRI regimen improve antitumor effects through the inhibition of immunosuppressive cells in murine colorectal cancer model. Vaccine. 2010;28:7787–7796. doi: 10.1016/j.vaccine.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Luan L, Wang X. A randomized Phase II clinical study of combining panitumumab and bevacizumab, plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for patients with metastatic colorectal cancer and KRAS mutation. Onco Targets Ther. 2015;8:1061–1068. doi: 10.2147/OTT.S81442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasa S, Nagashima K, Yamaguchi T, Matsumoto H, Ichikawa Y, Goto A, Yasui H, Kato K, Okita NT, Shimada Y. S-1 and irinotecan with or without bevacizumab versus 5-fluorouracil and leucovorin plus oxaliplatin with or without bevacizumab in metastatic colorectal cancer: a pooled analysis of four phase II studies. Cancer Chemother Pharmacol. 2015;76:605–614. doi: 10.1007/s00280-015-2834-4. [DOI] [PubMed] [Google Scholar]
- 25.Xu RH, Muro K, Morita S, Iwasa S, Han SW, Wang W, Kotaka M, Nakamura M, Ahn JB, Deng YH. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018;19:660–671. doi: 10.1016/S1470-2045(18)30140-2. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz HI, Tan BR, Reeves JA, Xiong H, Somer B, Lenz HJ, Hochster HS, Scappaticci F, Palma JF, Price R. Phase II randomized trial of sequential or concurrent FOLFOXIRI-bevacizumab versus FOLFOX-bevacizumab for metastatic colorectal cancer (STEAM) Oncologist. 2019;24:921–932. doi: 10.1634/theoncologist.2018-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landre T, Maillard E, Taleb C, Ghebriou D, Guetz GD, Zelek L, Aparicio T. Impact of the addition of bevacizumab, oxaliplatin, or irinotecan to fluoropyrimidin in the first-line treatment of metastatic colorectal cancer in elderly patients. Int J Colorectal Dis. 2018;33:1125–1130. doi: 10.1007/s00384-018-3053-3. [DOI] [PubMed] [Google Scholar]
- 28.Hanna DL, Loupakis F, Yang D, Cremolini C, Schirripa M, Li M, Matsusaka S, Berger MD, Miyamoto Y, Zhang W. Prognostic value of ACVRL1 expression in metastatic colorectal cancer patients receiving first-line chemotherapy with bevacizumab: results from the triplet plus bevacizumab (TRIBE) study. Clin Colorectal Cancer. 2018;17:e471–e488. doi: 10.1016/j.clcc.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aparicio T, Ghiringhelli F, Boige V, Le Malicot K, Taieb J, Bouche O, Phelip JM, Francois E, Borel C, Faroux R. Bevacizumab maintenance versus no maintenance during chemotherapy-free intervals in metastatic colorectal cancer: a randomized phase III trial (PRODIGE 9) J. Clin. Oncol. 2018;36:674–681. doi: 10.1200/JCO.2017.75.2931. [DOI] [PubMed] [Google Scholar]
- 30.Naito H, Wakabayashi T, Kidoya H, Muramatsu F, Takara K, Eino D, Yamane K, Iba T, Takakura N. Endothelial side population cells contribute to tumor angiogenesis and antiangiogenic drug resistance. Cancer Res. 2016;76:3200–3210. doi: 10.1158/0008-5472.CAN-15-2998. [DOI] [PubMed] [Google Scholar]
- 31.Lin CY, Siow TY, Lin MH, Hsu YH, Tung YY, Jang T, Recht L, Chang C. Visualization of rodent brain tumor angiogenesis and effects of antiangiogenic treatment using 3D DeltaR2-muMRA. Angiogenesis. 2013;16:785–793. doi: 10.1007/s10456-013-9355-8. [DOI] [PubMed] [Google Scholar]
- 32.Farsaci B, Donahue RN, Coplin MA, Grenga I, Lepone LM, Molinolo AA, Hodge JW. Immune consequences of decreasing tumor vasculature with antiangiogenic tyrosine kinase inhibitors in combination with therapeutic vaccines. Cancer Immunol Res. 2014;2:1090–1102. doi: 10.1158/2326-6066.CIR-14-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J. Clin. Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correale P, Cusi MG, Tagliaferri P. Immunomodulatory properties of anticancer monoclonal antibodies: is the ‘magic bullet’ still a reliable paradigm? Immunotherapy. 2011;3:1–4. doi: 10.2217/imt.10.92. [DOI] [PubMed] [Google Scholar]
- 35.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, Yanai K, Koga K, Nakamura M, Tanaka M, Morisaki T. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 2009;29:881–888. [PubMed] [Google Scholar]
- 37.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dings RP, Vang KB, Castermans K, Popescu F, Zhang Y, Oude Egbrink MG, Mescher MF, Farrar MA, Griffioen AW, Mayo KH. Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res. 2011;17:3134–3145. doi: 10.1158/1078-0432.CCR-10-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Zhang J, Yao X, Jiang C, Ni P, Cheng L, Liu J, Ni S, Chen Q, Li Q. Bevacizumab-enhanced antitumor effect of 5-fluorouracil via upregulation of thymidine phosphorylase through vascular endothelial growth factor A/vascular endothelial growth factor receptor 2-specificity protein 1 pathway. Cancer Sci. 2018;109:3294–3304. doi: 10.1111/cas.13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huh JE, Kang JW, Nam D, Baek YH, Choi DY, Park DS, Lee JD. Melittin suppresses VEGF-A-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. J Nat Prod. 2012;75:1922–1929. doi: 10.1021/np300446c. [DOI] [PubMed] [Google Scholar]
- 42.Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M, Filho AL. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genomics Proteomics. 2013;10:55–67. [PubMed] [Google Scholar]
- 43.Huang A, Gilmour JW, Imami N, Amjadi P, Henderson DC, Allen-Mersh TG. Increased serum transforming growth factor-beta1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin Exp Immunol. 2003;134:270–278. doi: 10.1046/j.1365-2249.2003.02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Della Porta M, Danova M, Rigolin GM, Brugnatelli S, Rovati B, Tronconi C, Fraulini C, Russo Rossi A, Riccardi A, Castoldi G. Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology. 2005;68:276–284. doi: 10.1159/000086784. [DOI] [PubMed] [Google Scholar]
- 45.Lacal PM, Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res. 2018;136:97–107. doi: 10.1016/j.phrs.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Do K, Cao L, Kang Z, Turkbey B, Lindenberg ML, Larkins E, Holkova B, Steinberg SM, Raffeld M, Peer CJ. A phase II study of sorafenib combined with cetuximab in EGFR-expressing, KRAS-mutated metastatic colorectal cancer. Clin Colorectal Cancer. 2015;14:154–161. doi: 10.1016/j.clcc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pehserl AM, Ress AL, Stanzer S, Resel M, Karbiener M, Stadelmeyer E, Stiegelbauer V, Gerger A, Mayr C, Scheideler M. Comprehensive analysis of miRNome alterations in response to sorafenib treatment in colorectal cancer cells. Int J Mol Sci. 2016;17:2011. doi: 10.3390/ijms17122011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks EI, Tan C, Zhang J, Zhou L, Yang Z, Scicchitano A, El-Deiry WS. Regorafenib with a fluoropyrimidine for metastatic colorectal cancer after progression on multiple 5-FU-containing combination therapies and regorafenib monotherapy. Cancer Biol Ther. 2015;16:1710–1719. doi: 10.1080/15384047.2015.1113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuoka K, Nakagawa F, Tanaka N, Okabe H, Matsuo K, Takechi T. Effective sequential combined chemotherapy with trifluridine/tipiracil and regorafenib in human colorectal cancer cells. Int J Mol Sci. 2018;19:2915. doi: 10.3390/ijms19102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roed Skarderud M, Polk A, Kjeldgaard Vistisen K, Larsen FO, Nielsen DL. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: a systematic review. Cancer Treat Rev. 2018;62:61–73. doi: 10.1016/j.ctrv.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Mulder SF, Jacobs JF, Olde Nordkamp MA, Galama JM, Desar IM, Torensma R, Teerenstra S, Mulders PF, Vissers KC, Punt CJ. Cancer patients treated with sunitinib or sorafenib have sufficient antibody and cellular immune responses to warrant influenza vaccination. Clin Cancer Res. 2011;17:4541–4549. doi: 10.1158/1078-0432.CCR-11-0253. [DOI] [PubMed] [Google Scholar]
- 53.Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 54.Lohmeyer J, Nerreter T, Dotterweich J, Einsele H, Seggewiss-Bernhardt R. Sorafenib paradoxically activates the RAS/RAF/ERK pathway in polyclonal human NK cells during expansion and thereby enhances effector functions in a dose- and time-dependent manner. Clin Exp Immunol. 2018;193:64–72. doi: 10.1111/cei.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi L, Lin H, Li G, Jin RA, Xu J, Sun Y, Ma WL, Yeh S, Cai X, Chang C. Targeting androgen receptor (AR)→IL12A signal enhances efficacy of sorafenib plus NK cells immunotherapy to better suppress HCC progression. Mol Cancer Ther. 2016;15:731–742. doi: 10.1158/1535-7163.MCT-15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grothey A, Marshall JL, Seery TE. Current options for third-line treatment of metastatic colorectal cancer. Clin Adv Hematol Oncol. 2016;14:1–15. [PubMed] [Google Scholar]
- 57.Zhang Q, Zhang H, Ding J, Liu H, Li H, Li H, Lu M, Miao Y, Li L, Zheng J. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J Immunol Res. 2018;2018:4263520. doi: 10.1155/2018/4263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 59.Kawaguchi Y, Kono K, Mimura K, Sugai H, Akaike H, Fujii H. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer. 2007;120:781–787. doi: 10.1002/ijc.22370. [DOI] [PubMed] [Google Scholar]
- 60.Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, Touge H, Makino H, Takata M, Miyata M. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 61.Correale P, Marra M, Remondo C, Migali C, Misso G, Arcuri FP, Del Vecchio MT, Carducci A, Loiacono L, Tassone P. Cytotoxic drugs up-regulate epidermal growth factor receptor (EGFR) expression in colon cancer cells and enhance their susceptibility to EGFR-targeted antibody-dependent cell-mediated-cytotoxicity (ADCC) Eur J Cancer. 2010;46:1703–1711. doi: 10.1016/j.ejca.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi MM, Gori Savellini G, Bestoso E, Apollinari S, Mannucci S, Marra M. Cetuximab +/- chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. 2012;130:1577–1589. doi: 10.1002/ijc.26181. [DOI] [PubMed] [Google Scholar]
- 63.Botta C, Bestoso E, Apollinari S, Cusi MG, Pastina P, Abbruzzese A, Sperlongano P, Misso G, Caraglia M, Tassone P. Immune-modulating effects of the newest cetuximab-based chemoimmunotherapy regimen in advanced colorectal cancer patients. J Immunother. 2012;35:440–447. doi: 10.1097/CJI.0b013e31825943aa. [DOI] [PubMed] [Google Scholar]
- 64.Willoughby J, Griffiths J, Tews I, Cragg MS. OX40: structure and function - what questions remain? Mol Immunol. 2017;83:13–22. doi: 10.1016/j.molimm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Lan P, Fan Y, Zhao Y, Lou X, Monsour HP, Zhang X, Choi Y, Dou Y, Ishii N, Ghobrial RM, Xiao X, Li XC. TNF superfamily receptor OX40 triggers invariant NKT cell pyroptosis and liver injury. J Clin Invest. 2017;127:2222–2234. doi: 10.1172/JCI91075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 68.Petty JK, He K, Corless CL, Vetto JT, Weinberg AD. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134) Am J Surg. 2002;183:512–518. doi: 10.1016/s0002-9610(02)00831-0. [DOI] [PubMed] [Google Scholar]
- 69.Cepowicz D, Gryko M, Zareba K, Stasiak-Bermuta A, Kedra B. Assessment of activity of an adhesion molecule CD134 and CD137 in colorectal cancer patients. Pol Przegl Chir. 2011;83:641–645. doi: 10.2478/v10035-011-0102-9. [DOI] [PubMed] [Google Scholar]
- 70.Foote JB, Kok M, Leatherman JM, Armstrong TD, Marcinkowski BC, Ojalvo LS, Kanne DB, Jaffee EM, Dubensky TW, Emens LA. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol Res. 2017;5:468–479. doi: 10.1158/2326-6066.CIR-16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guttman-Yassky E, Pavel AB, Zhou L, Estrada YD, Zhang N, Xu H, Peng X, Wen HC, Govas P, Gudi G. GBR 830, an anti-OX40, improves skin gene-signatures and clinical scores in atopic dermatitis. J Allergy Clin Immunol. 2019;144:482–493. doi: 10.1016/j.jaci.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X, Xiao X, Lan P, Li J, Dou Y, Chen W, Ishii N, Chen S, Xia B, Chen K. OX40 costimulation inhibits Foxp3 expression and Treg induction via BATF3-dependent and independent mechanisms. Cell Rep. 2018;24:607–618. doi: 10.1016/j.celrep.2018.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang CY, Chiang SF, Ke TW, Chen TW, You YS, Chen WT, Chao KSC. Clinical significance of programmed death 1 ligand-1 (CD274/PD-L1) and intra-tumoral CD8+ T-cell infiltration in stage II-III colorectal cancer. Sci Rep. 2018;8:15658. doi: 10.1038/s41598-018-33927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim EJ, Lee JG, Kim JY, Song SH, Joo DJ, Huh KH, Kim MS, Kim BS, Kim YS. Enhanced immune-modulatory effects of thalidomide and dexamethasone co-treatment on T cell subsets. Immunology. 2017;152:628–637. doi: 10.1111/imm.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamaki S, Ine S, Kawabe T, Okuyama Y, Suzuki N, Soroosh P, Mousavi SF, Nagashima H, Sun SL, So T. OX40 and IL-7 play synergistic roles in the homeostatic proliferation of effector memory CD4(+) T cells. Eur J Immunol. 2014;44:3015–3025. doi: 10.1002/eji.201444701. [DOI] [PubMed] [Google Scholar]
- 76.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, Bonneau C, Kondratova M, Kuperstein I, Zinovyev A, Givel AM, Parrini MC, Soumelis V, Vincent-Salomon A, Mechta-Grigoriou F. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–479. e410. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 77.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baeyens A, Saadoun D, Billiard F, Rouers A, Gregoire S, Zaragoza B, Grinberg-Bleyer Y, Marodon G, Piaggio E, Salomon BL. Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context. J Immunol. 2015;194:999–1010. doi: 10.4049/jimmunol.1400504. [DOI] [PubMed] [Google Scholar]
- 79.Ruby CE, Yates MA, Hirschhorn-Cymerman D, Chlebeck P, Wolchok JD, Houghton AN, Offner H, Weinberg AD. Cutting edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–480. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 82.White AL, Chan HT, French RR, Willoughby J, Mockridge CI, Roghanian A, Penfold CA, Booth SG, Dodhy A, Polak ME. Conformation of the human immunoglobulin G2 hinge imparts superagonistic properties to immunostimulatory anticancer antibodies. Cancer Cell. 2015;27:138–148. doi: 10.1016/j.ccell.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li F, Ravetch JV. A general requirement for FcgammaRIIB co-engagement of agonistic anti-TNFR antibodies. Cell Cycle. 2012;11:3343–3344. doi: 10.4161/cc.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DiLillo DJ, Ravetch JV. Fc-receptor interactions regulate both cytotoxic and immunomodulatory therapeutic antibody effector functions. Cancer Immunol Res. 2015;3:704–713. doi: 10.1158/2326-6066.CIR-15-0120. [DOI] [PubMed] [Google Scholar]
- 85.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 86.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, Levy R, Brody J. Combination strategies to enhance antitumor ADCC. Immunotherapy. 2012;4:511–527. doi: 10.2217/imt.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 91.Kitamura N, Murata S, Ueki T, Mekata E, Reilly RT, Jaffee EM, Tani T. OX40 costimulation can abrogate Foxp3+ regulatory T cell-mediated suppression of antitumor immunity. Int J Cancer. 2009;125:630–638. doi: 10.1002/ijc.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y, Lizee G, Hwu P. Strong emerging rationale for combining oncogene-targeted agents with immunotherapy. Oncoimmunology. 2013;2:e22730. doi: 10.4161/onci.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2:142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu B, Yu H, Sun G, Sun X, Jin H, Zhang C, Shi W, Tian D, Liu K, Xu H, Li X, Yin J, Hong X, Zhang D. OX40 promotes obesity-induced adipose inflammation and insulin resistance. Cell Mol Life Sci. 2017;74:3827–3840. doi: 10.1007/s00018-017-2552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian J, Zheng Y, Zheng C, Wang L, Qin H, Hong S, Li H, Lu Y, He J, Yang J. Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood. 2012;119:161–169. doi: 10.1182/blood-2011-07-368472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shrimali RK, Ahmad S, Verma V, Zeng P, Ananth S, Gaur P, Gittelman RM, Yusko E, Sanders C, Robins H. Concurrent PD-1 blockade negates the effects of OX40 agonist antibody in combination immunotherapy through inducing T-cell apoptosis. Cancer Immunol Res. 2017;5:755–766. doi: 10.1158/2326-6066.CIR-17-0292. [DOI] [PubMed] [Google Scholar]
- 98.Leyland R, Watkins A, Mulgrew KA, Holoweckyj N, Bamber L, Tigue NJ, Offer E, Andrews J, Yan L, Mullins S. A novel murine GITR ligand fusion protein induces antitumor activity as a monotherapy that is further enhanced in combination with an OX40 agonist. Clin Cancer Res. 2017;23:3416–3427. doi: 10.1158/1078-0432.CCR-16-2000. [DOI] [PubMed] [Google Scholar]
- 99.Gough MJ, Killeen N, Weinberg AD. Targeting macrophages in the tumour environment to enhance the efficacy of alphaOX40 therapy. Immunology. 2012;136:437–447. doi: 10.1111/j.1365-2567.2012.03600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang C, Cai X, Zhang H, Xia X, Zhang B, Xia L. Activity and immune correlates of a programmed death-1 blockade antibody in the treatment of refractory solid tumors. J Cancer. 2018;9:205–212. doi: 10.7150/jca.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. 2017;9:347–360. doi: 10.2217/imt-2016-0141. [DOI] [PubMed] [Google Scholar]
- 103.Kather JN, Poleszczuk J, Suarez-Carmona M, Krisam J, Charoentong P, Valous NA, Weis CA, Tavernar L, Leiss F, Herpel E. In silico modeling of immunotherapy and stroma-targeting therapies in human colorectal cancer. Cancer Res. 2017;77:6442–6452. doi: 10.1158/0008-5472.CAN-17-2006. [DOI] [PubMed] [Google Scholar]
- 104.Kitahara M, Hazama S, Tsunedomi R, Takenouchi H, Kanekiyo S, Inoue Y, Nakajima M, Tomochika S, Tokuhisa Y, Iida M. Prediction of the efficacy of immunotherapy by measuring the integrity of cell-free DNA in plasma in colorectal cancer. Cancer Sci. 2016;107:1825–1829. doi: 10.1111/cas.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Correale P, Botta C, Ciliberto D, Pastina P, Ingargiola R, Zappavigna S, Tassone P, Pirtoli L, Caraglia M, Tagliaferri P. Immunotherapy of colorectal cancer: new perspectives after a long path. Immunotherapy. 2016;8:1281–1292. doi: 10.2217/imt-2016-0089. [DOI] [PubMed] [Google Scholar]
- 106.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 107.Biziota E, Mavroeidis L, Hatzimichael E, Pappas P. Metronomic chemotherapy A potent macerator of cancer by inducing angiogenesis suppression and antitumor immune activation. Cancer Lett. 2017;400:243–251. doi: 10.1016/j.canlet.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 108.Chen YL, Chang MC, Cheng WF. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett. 2017;400:282–292. doi: 10.1016/j.canlet.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 109.Mpekris F, Baish JW, Stylianopoulos T, Jain RK. Role of vascular normalization in benefit from metronomic chemotherapy. Proc Natl Acad Sci U S A. 2017;114:1994–1999. doi: 10.1073/pnas.1700340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kareva I. A combination of immune checkpoint inhibition with metronomic chemotherapy as a way of targeting therapy-resistant cancer cells. Int J Mol Sci. 2017;13:18. doi: 10.3390/ijms18102134. [DOI] [PMC free article] [PubMed] [Google Scholar]


