Abstract
Objective: To investigate the effects of vitamin D drops on immune function in children with recurrent respiratory tract infections (RRTI). Methods: The clinical data of 119 children with RRTI in our hospital were retrospectively retrieved, and they were divided into group A (n=59, receiving routine treatment) and group B (n=60, receiving vitamin D drops) based on their treatment modality. The clinical efficacy, symptom disappearance time, immune function index, insulin-like growth factor (IGF-1), 25-hydroxyvitamin D3 [25-(OH)D3], serum y-interferon (INF-y), and the number of episodes of respiratory tract infections were compared between the two groups. Results: The total effective rate of treatment in group B was 96.67%, which was significantly higher than 71.19% in group A (P<0.05). Children in group B had shorter time to disappearance of lung rales, cough, and fever than group A (P<0.05). Group B had higher IgA, IgG, and IgM levels, higher CD4+, CD3+ levels and lower CD8+ levels as well as higher IGF-1, 25-(OH)D3, INF-y levels, and fewer respiratory infections after treatment than group A (P<0.05). Conclusion: Vitamin D drops are effective in the treatment of children with RRTI, which is beneficial to the improvement of clinical symptoms and immune function.
Keywords: Recurrent, respiratory tract infections, children, vitamin D drops, immune function
Introduction
Clinically, recurrent respiratory tract infections (RRTI) are more common in children, and are characterized by recurring upper and lower respiratory tract infections, mainly in school-age and preschool children, and the incidence rate of RRTI accounts for about 20% of pediatric respiratory tract infections [1,2]. In recent years, the incidence of RRTI in children has increased significantly, and the common types include recurrent pneumonia, chronic bronchitis, recurrent bronchiolitis, recurrent pharyngeal- tonsillitis, and recurrent nasopharyngitis [3].
In most cases, respiratory tract infections develop rapidly, and children usually have typical symptoms such as sneezing and coughing, runny nose and congestion, and fever, which vary with age [4,5]. In addition to the above symptoms, RRTI are usually accompanied by symptoms such as yellow face and thinness, loss of appetite, etc. If effective measures are not taken in time, it may also lead to myocarditis, asthma and other diseases, seriously affecting the physical development and health of children [6,7]. Antibacterial drugs are often prescribed to control the infection, but the therapeutic effect of single-agent antimicrobial therapy needs to be further improved [8,9]. Studies in recent years have shown that the occurrence of RRTI is not only related to microbial infection and other factors, but also has a certain correlation with the nutritional status and immune function of children [10]. Vitamin D is one of the important micronutrients in the human body, and previous studies have shown that supplementation of this micronutrient is beneficial to improving immunity of the body [11].
Therefore, the present study explored the feasibility of the adjunctive use of vitamin D drops in the treatment of RRTI.
Materials and methods
Medical data
The clinical data of 119 children with RRTI were collected retrospectively and divided into two groups based on the treatment modality. Group A (n=59) received routine therapy while group B (n=60) received vitamin D drops. (1) Inclusion criteria: patients who were <6 years old; patients whose family members signed the informed consent; patients who were diagnosed with RRTI. This study was approved by the medical ethics committee of The First People’s Hospital of Fuyang Hangzhou. (2) Exclusion criteria: patients who requested withdrawal, those with poor treatment compliance, severe cardiac, hepatic and renal dysfunction, and treatment with vitamin D, hormonal drugs and immunostimulants within 2 months prior to enrollment; those with contraindications to the medication used in this study.
Methods
Group A: After admission, patients were given supportive therapy such as supplemental oxygen, cough suppressants, asthma and expectoration treatment according to their condition.
Group B: Vitamin D drops (H20113033, Qingdao Shuangjing Pharmaceutical Co., Ltd. 400 units * 10 tablets * 3 tablets) were taken orally at q.d 800 U. Twenty days of medication and 10 days without in every 30 consecutive days were a course of treatment, and a total of 3 courses were performed.
Outcome measurements
(1) Therapeutic efficacy criteria [12]: Cure: no recurrence of respiratory tract infection within 1 year after treatment. Significant improvement: reduction of >70% in the number of episodes of respiratory tract infections within 1 year after treatment. Effective: reduction of 40%-70%; Invalid: reduction of <40%. Total effective = Effective + Significant improvement + Cure.
(2) Time to the disappearance of symptoms in the two groups were compared.
(3) Immune function indices [13]: Before and after treatment, 4 ml of fasting venous blood was drawn from both groups of children in the morning, and heparin was added for anticoagulation, followed by centrifugation at 8000 r/min for 10 min. Immunoglobulin IgA, IgG and IgM levels were detected by an automatic biochemical analyzer, and T lymphocyte subpopulations CD8+, CD4+ and CD3+ were detected by flow cytometry.
(4) Clinical parameters [14]: Before and after treatment, 2 ml of fasting venous blood was drawn from both groups of children in the morning, and the levels of insulin-like growth factor (IGF-1), 25-hydroxyvitamin D3 [25-(OH)D3], and serum y-interferon (INF-y) were measured by enzyme-linked immunosorbent assay after centrifugation (3000 r/min for 10 min).
(5) The number of episodes of respiratory infections before and after treatment were compared between the two groups of children.
Statistical methods
SPSS 22.0 was used for data analysis. Measurement data were indicated by mean ± standard deviation (mean ± SD). The t test was used for normally distributed data, while Mann-Whitney U test was used for non-normally distributed data. Count data expressed as [n (%)] was compared by chi-squared test. P<0.05 suggested statistical significance.
Results
Comparison of baseline data
There was no difference in terms of sex, age, disease duration and body mass in the two groups (P>0.05) (Table 1).
Table 1.
Comparison of baseline data [n (%)]/(x̅±s)
| Medical data | Group A (n=59) | Group B (n=60) | t/X2 | P | |
|---|---|---|---|---|---|
| Gender (cases) | Male | 41 (69.49) | 43 (71.67) | 0.068 | 0.795 |
| Female | 18 (30.51) | 17 (28.33) | |||
| Age (years) | 4.85±0.11 | 4.88±0.09 | 1.630 | 0.106 | |
| Duration of illness | 1.62±0.13 | 1.65±0.11 | 1.359 | 0.177 | |
| Weight (kg) | 21.35±2.28 | 21.38±2.25 | 0.072 | 0.943 | |
Comparison of the clinical efficacy
The total effective rate of group B was 96.67%, significantly higher than 71.19% in group A (P<0.05) (Table 2).
Table 2.
Comparison of the clinical efficacy [n (%)]
| Grouping | Cases | Cure | Significant improvement | Effective | Invalid | Total effective |
|---|---|---|---|---|---|---|
| Group A | 59 | 22 (37.29) | 11 (18.64) | 9 (15.25) | 17 (28.81) | 42 (71.19) |
| Group B | 60 | 37 (61.67) | 15 (25.00) | 6 (10.00) | 2 (3.33) | 58 (96.67)* |
| X2 | 14.395 | |||||
| P | 0.000 |
indicates comparison with group A, P<0.05.
Comparison of the time to disappearance of symptoms
Group B had a shorter time to disappearance of cough, lung rales, and fever than group A (P<0.05) (Figure 1).
Figure 1.
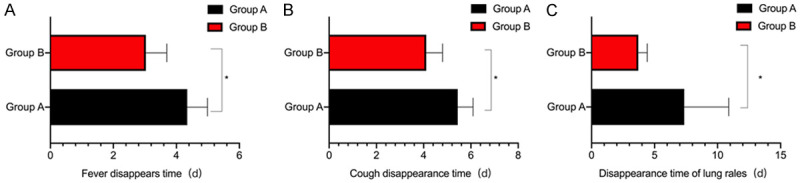
Comparison of the disappearance time of symptoms between the two groups. A. Disappearance time of fever; B. The time of disappearance of cough; C. The disappearance time of lung rales. *P<0.05, compared with group A.
Comparison of immunoglobulin levels
The levels of IgA, IgG, and IgM showed no significant difference between the two groups before treatment (P>0.05). After treatment, the levels of IgA, IgG, and IgM were increased in the two groups (P<0.05). The levels of IgA, IgG, and IgM in group B after treatment were higher than those in group A (P<0.05) (Figure 2).
Figure 2.
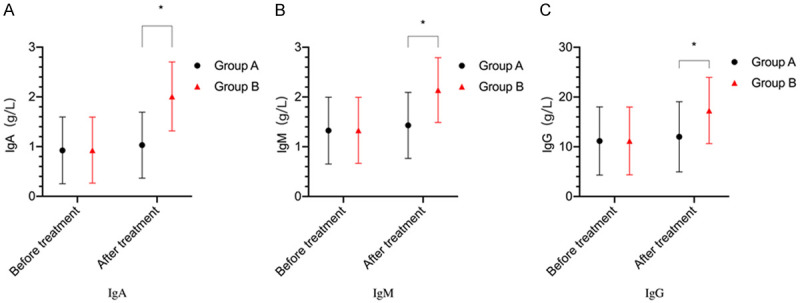
Comparison of immunoglobulin levels between the two groups. A: IgA levels; B: IgM levels; C: IgG levels. *P<0.05, compared with group A.
Comparison of two subpopulations of T lymphocytes
The levels of CD8+, CD4+ and CD3+ revealed no significant difference in the two groups before treatment (P>0.05). After treatment, CD4+ and CD3+ levels were increased and CD8+ levels were decreased in both groups (P<0.05). Group B showed higher levels of CD4+ and CD3+ and lower CD8+ levels than group A (P<0.05) (Figure 3).
Figure 3.
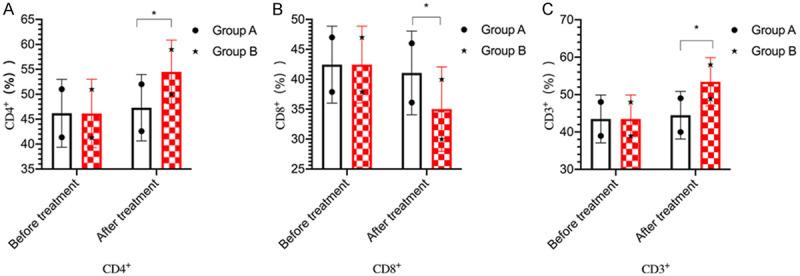
Comparison of T-lymphocyte subsets between the two groups. A: CD4+ levels; B: CD8+ levels; C: CD3+ levels. *P<0.05, compared with group A.
Comparison of clinical indicators
There was no difference in the levels of IGF-1, 25-(OH)D3, and INF-y between the two groups before treatment (P>0.05). After treatment, the levels of IGF-1,25-(OH)D3, and INF-y were increased in both groups (P<0.05). Group B had higher levels of IGF-1, 25-(OH)D3, and INF-y than group A after treatment (P<0.05) (Figure 4).
Figure 4.
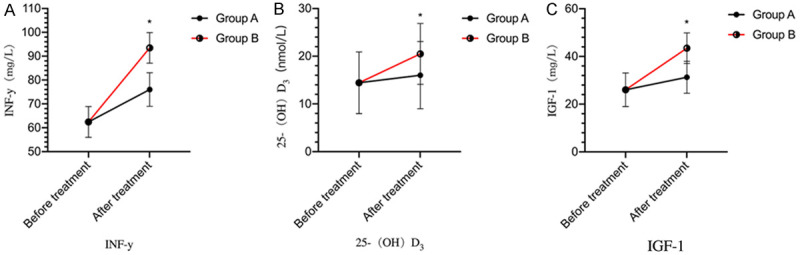
Comparison of clinical indices between the two groups. A: INF-y levels; B: 25-(OH)D3 levels; C: IGF-1 levels. *P<0.05, compared with group A.
Comparison of the number of episodes of respiratory infection
There was no difference in the number of respiratory tract infections between the two groups before treatment (P>0.05). The number was reduced in both groups after treatment (P<0.05) and was less in group B in contrast to group A (P<0.05) (Table 3).
Table 3.
Comparison of the number of episodes of respiratory infection (x̅±s, min)
| Grouping | Pre-treatment | Post-treatment |
|---|---|---|
| Group A (n=59) | 6.52±0.19 | 4.12±0.13# |
| Group B (n=60) | 6.58±0.12 | 2.25±0.11#,* |
| t | 2.063 | 84.760 |
| P | 0.041 | 0.000 |
indicates a comparison with pre-treatment, P<0.05;
indicates a comparison with group A, P<0.05.
Discussion
RRTI in children is a common respiratory disease with a complex pathology and etiological mechanisms [15]. It is believed clinically that the disease onset is associated with immune dysfunction, vitamin deficiency, malnutrition, microbial infections, and improper care [16]. At present, symptomatic therapy is usually used for RRTI, but the therapeutic effect needs to be further improved [17,18]. The specific pathogenesis of the disease and novel treatments should be explored [19].
Clinical studies have confirmed that there is a strong correlation between reduced body resistance, immune dysfunction and recurrent respiratory infections in children [20-22]. Improving immune function is one of the important ways to improve the therapeutic effect of RRTI in children. Vitamin D drops belong to steroid derivatives, are fat-soluble, and play an important role in promoting human growth and development [23]. It was found that vitamin D drops 11.85A after treatment, suggesting that vitamin D drops improved the nutritional status as an adjunct to treatment of RRTI. Vitamin D exists in various forms in the human body, among which vitamin D3 is a crucial one. 1,25-(OH)2D3 is a bioactive metabolite of vitamin D3. Due to its relatively poor stability in the human body, 25-(OH)D3 is often used because it is stable and can be used to effectively reflect the body’s nutritional status and vitamin D levels. In addition, this study also showed that the IGF-1 and INF-y levels of children in group B were higher than those in group A after treatment, suggesting that vitamin D drops can improve the body’s immunity. We speculated that IGF-1 is one of the regulatory factors mediating growth hormone, which can not only promote the secretion and synthesis of immunoglobulin, but also promote the differentiation and proliferation of B and T lymphocytes. A decrease in IGF-1 levels indicates a decrease in body immunity. IFN-γ is a cytokine that is critical to innate and adaptive immunity against viral, some bacterial and protozoal infections.
Studies have shown that vitamin D deficiency also affects the levels of calcium ions involved in lymphocyte activation, which in turn affects T-cell proliferation and immune function, so it is necessary to observe the changes in T-cells [28]. In this study, group B showed higher levels of CD4+ and CD3+ and lower levels of CD8+, suggesting that children with RRTI exhibited immune dysregulation, but their immune function was significantly improved by vitamin D therapy. Secondly, IgA, IgG and IgM levels of group B were higher than those of group A after treatment, suggesting that vitamin D therapy can play a key role in immune regulation. In the study of Ouyang [29], it was also found that the immunoglobulin IgA, IgG and IgM levels of the children in group A (vitamin D administration) after treatment were all higher than those in group B (routine treatment), which was highly consistent with the results of this study. This may be attributed to the fact that IgA is a mucosal local immune factor, while IgM is a crucial marker of recent infection, which kills microbial phagocytosis by activating complement and thus provides immune defense against early infection. IgG, on the other hand, regulates phagocytosis and protects the immune function. Vitamin D drops can improve the humoral immune function and thus reduce the incidence of respiratory infections.
In conclusion, vitamin D drop treatment has shown significant efficacy in improving various clinical symptoms and immune function.
Although this study has achieved some results, it also has the limitation of small sample size, which needs to be further expanded in the future for more-in-depth research and analysis.
Acknowledgements
This research received no specific funding from any agency in the public, commercial, or not-for-profit sectors.
Disclosure of conflict of interest
None.
References
- 1.Okamoto M, Dapat CP, Sandagon AMD, Batangan-Nacion LP, Lirio IC, Tamaki R, Saito M, Saito-Obata M, Lupisan SP, Oshitani H. Molecular characterization of respiratory syncytial virus in children with repeated infections with subgroup B in the philippines. J Infect Dis. 2018;218:1045–1053. doi: 10.1093/infdis/jiy256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breuer O, Schultz A, Turkovic L, de Klerk N, Keil AD, Brennan S, Harrison J, Robertson C, Robinson PJ, Sly PD, Ranganathan S, Stick SM, Caudri D. Changing prevalence of lower airway infections in young children with cystic fibrosis. Am J Respir Crit Care Med. 2019;200:590–599. doi: 10.1164/rccm.201810-1919OC. [DOI] [PubMed] [Google Scholar]
- 3.Ramette A, Spycher BD, Wang J, Goutaki M, Beardsmore CS, Kuehni CE. Longitudinal associations between respiratory infections and asthma in young children. Am J Epidemiol. 2018;187:1714–1720. doi: 10.1093/aje/kwy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarantino V, Savaia V, D’Agostino R, Silvestri M, Passali FM, Di Girolamo S, Ciprandi G. Bacteriotherapy in children with recurrent upper respiratory tract infections. Eur Rev Med Pharmacol Sci. 2019;23:39–43. doi: 10.26355/eurrev_201903_17347. [DOI] [PubMed] [Google Scholar]
- 5.Munteanu AN, Surcel M, Huică RI, Isvoranu G, Constantin C, Pîrvu IR, Chifiriuc C, Ulmeanu C, Ursaciuc C, Neagu M. Peripheral immune cell markers in children with recurrent respiratory infections in the absence of primary immunodeficiency. Exp Ther Med. 2019;18:1693–1700. doi: 10.3892/etm.2019.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamoto M, Sakamoto M, Dapat C, Saito M, Saito-Obata M, Tamaki R, Lupisan SP, Oshitani H. Complete genome sequences of 12 human respiratory syncytial virus (human orthopneumovirus) strains detected in children with repeated subgroup b infections in the philippines. Microbiol Resour Announc. 2018;7:e01017–e01018. doi: 10.1128/MRA.01017-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Hoog MLA, Venekamp RP, Damoiseaux R, Schilder AGM, Sanders EAM, Smit HA, Bruijning-Verhagen P. Impact of repeated influenza immunization on respiratory illness in children with preexisting medical conditions. Ann Fam Med. 2019;17:7–13. doi: 10.1370/afm.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YF, Shen FC, Wang SL, Kuo PH, Tsai HP, Liu CC, Wang JR, Chi CY. Molecular epidemiology and clinical manifestations of adenovirus respiratory infections in taiwanese children. Medicine (Baltimore) 2016;95:e3577. doi: 10.1097/MD.0000000000003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner JC, Pyles RB, Miller AL, Nokso-Koivisto J, Loeffelholz MJ, Chonmaitree T. Determining persistence of bocavirus DNA in the respiratory tract of children by pyrosequencing. Pediatr Infect Dis J. 2016;35:471–476. doi: 10.1097/INF.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breuer O, Schultz A, Garratt LW, Turkovic L, Rosenow T, Murray CP, Karpievitch YV, Akesson L, Dalton S, Sly PD, Ranganathan S, Stick SM, Caudri D. Aspergillus infections and progression of structural lung disease in children with cystic fibrosis. Am J Respir Crit Care Med. 2020;201:688–696. doi: 10.1164/rccm.201908-1585OC. [DOI] [PubMed] [Google Scholar]
- 11.Teo SM, Tang HHF, Mok D, Judd LM, Watts SC, Pham K, Holt BJ, Kusel M, Serralha M, Troy N, Bochkov YA, Grindle K, Lemanske RF Jr, Johnston SL, Gern JE, Sly PD, Holt PG, Holt KE, Inouye M. Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe. 2018;24:341–352. e345. doi: 10.1016/j.chom.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xepapadaki P, Bachert C, Finotto S, Jartti T, Konstantinou GN, Kiefer A, Kowalski M, Lewandowska-Polak A, Lukkarinen H, Roumpedaki E, Sobanska A, Sintobin I, Vuorinen T, Zhang N, Zimmermann T, Papadopoulos NG. Contribution of repeated infections in asthma persistence from preschool to school age: design and characteristics of the PreDicta cohort. Pediatr Allergy Immunol. 2018;29:383–393. doi: 10.1111/pai.12881. [DOI] [PubMed] [Google Scholar]
- 13.Hansen TE, Evjenth B, Holt J. Lower respiratory tract infections appear to be the most important risk factor for current asthma in subarctic schoolchildren. Acta Paediatr. 2019;108:911–919. doi: 10.1111/apa.14603. [DOI] [PubMed] [Google Scholar]
- 14.McLean HQ, Caspard H, Griffin MR, Gaglani M, Peters TR, Poehling KA, Ambrose CS, Belongia EA. Association of prior vaccination with influenza vaccine effectiveness in children receiving live attenuated or inactivated vaccine. JAMA Netw Open. 2018;1:e183742. doi: 10.1001/jamanetworkopen.2018.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortaz E, Azempour E, Mansouri D, Tabarsi P, Ghazi M, Koenderman L, Roos D, Adcock IM. Common infections and target organs associated with chronic granulomatous disease in Iran. Int Arch Allergy Immunol. 2019;179:62–73. doi: 10.1159/000496181. [DOI] [PubMed] [Google Scholar]
- 16.Muro F, Mosha N, Hildenwall H, Mtei F, Harrison N, Schellenberg D, Olomi R, Reyburn H, Todd J. Variability of respiratory rate measurements in children suspected with non-severe pneumonia in North-east Tanzania. Trop Med Int Health. 2017;22:139–147. doi: 10.1111/tmi.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visseaux B, Collin G, Ichou H, Charpentier C, Bendhafer S, Dumitrescu M, Allal L, Cojocaru B, Desfrère L, Descamps D, Mandelbrot L, Houhou-Fidouh N. Usefulness of multiplex PCR methods and respiratory viruses’ distribution in children below 15 years old according to age, seasons and clinical units in France: a 3 years retrospective study. PLoS One. 2017;12:e0172809. doi: 10.1371/journal.pone.0172809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarantino V, Savaia V, D’Agostino R, Damiani V, Ciprandi G. Oral bacteriotherapy in children with recurrent respiratory infections: a real-life study. Acta Biomed. 2020;91:73–76. doi: 10.23750/abm.v91i1-S.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harun SN, Holford NHG, Grimwood K, Wainwright CE, Hennig S. Pseudomonas aeruginosa eradication therapy and risk of acquiring Aspergillus in young children with cystic fibrosis. Thorax. 2019;74:740–748. doi: 10.1136/thoraxjnl-2018-211548. [DOI] [PubMed] [Google Scholar]
- 20.Kartal Öztürk G, Eşki A, Çelebi Çelik F, Conkar S, Gülen F, Demir E, Keskinoğlu A. Prospective evaluation of vascular changes in acute respiratory infections in children with cystic fibrosis. Turk J Med Sci. 2020;50:1007–1014. doi: 10.3906/sag-2002-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao L, Xing C, Yang Z, Xu S, Wang M, Du H, Liu K, Huang Z. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. Br J Nutr. 2015;114:1026–1034. doi: 10.1017/S000711451500207X. [DOI] [PubMed] [Google Scholar]
- 22.Do NT, Ta NT, Tran NT, Than HM, Vu BT, Hoang LB, van Doorn HR, Vu DT, Cals JW, Chandna A, Lubell Y, Nadjm B, Thwaites G, Wolbers M, Nguyen KV, Wertheim HF. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Glob Health. 2016;4:e633–641. doi: 10.1016/S2214-109X(16)30142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andaloro C, Santagati M, Stefani S, La Mantia I. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a oral spray for children with recurrent streptococcal pharyngotonsillitis: a randomized placebo-controlled clinical study. Eur Arch Otorhinolaryngol. 2019;276:879–887. doi: 10.1007/s00405-019-05346-3. [DOI] [PubMed] [Google Scholar]
- 24.Segerer FJ, Seeger K, Maier A, Hagemann C, Schoen C, van der Linden M, Streng A, Rose MA, Liese JG. Therapy of 645 children with parapneumonic effusion and empyema-A German nationwide surveillance study. Pediatr Pulmonol. 2017;52:540–547. doi: 10.1002/ppul.23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, Montin D. SARS-CoV-2-induced kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146:e20201711. doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 26.Shinjoh M, Sugaya N, Yamaguchi Y, Iibuchi N, Kamimaki I, Goto A, Kobayashi H, Kobayashi Y, Shibata M, Tamaoka S, Nakata Y, Narabayashi A, Nishida M, Hirano Y, Munenaga T, Morita K, Mitamura K, Takahashi T. Inactivated influenza vaccine effectiveness and an analysis of repeated vaccination for children during the 2016/17 season. Vaccine. 2018;36:5510–5518. doi: 10.1016/j.vaccine.2018.07.065. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto N, Takeishi K. Change in the efficacy of influenza vaccination after repeated inoculation under antigenic mismatch: a systematic review and meta-analysis. Vaccine. 2018;36:949–957. doi: 10.1016/j.vaccine.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Moore DP, Higdon MM, Hammitt LL, Prosperi C, DeLuca AN, Da Silva P, Baillie VL, Adrian PV, Mudau A, Deloria Knoll M, Feikin DR, Murdoch DR, O’Brien KL, Madhi SA. The incremental value of repeated induced sputum and gastric aspirate samples for the diagnosis of pulmonary tuberculosis in young children with acute community-acquired pneumonia. Clin Infect Dis. 2017;64:S309–S316. doi: 10.1093/cid/cix099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang K. Analysis of the immunomodulatory effects of vitamin D on children with recurrent respiratory infections. J Gannan Med Coll. 2014;34:607–608. [Google Scholar]


