Abstract
Objective: To investigate the effects of miR-24 and HMOX1 on the inflammatory response and neurological function in rats with cerebral vasospasm (CVS) after subarachnoid hemorrhage (SAH). Methods: Fifteen Sprague-Dawley rats were randomly assigned to the sham group (sham operation, treated with normal saline). Rat model of SAH-induced CVS was established in 90 rats, and these rats were randomly divided into the model, miR-24 NC (treated with miR-24-NC vector), miR-24 inhibitor (treated with miR-24 inhibitor vector), HMOX-NC (treated with HMOX1-NC vector), oe-HMOX1 (treated with HMOX1 overexpression vector), and miR-24 inhibitor + si-HMOX1 (treated with miR-24 inhibitor and si-HMOX1 vectors) groups. Adenoviral vectors containing the target sequences were injected into the hippocampus of the rats in the corresponding groups. Dual-luciferase reporter assay was conducted to verify the relationship between miR-24 and HMOX1. The learning and memory abilities, neurological function, cerebral edema, permeability of blood-brain barrier, myeloperoxidase activity, and levels of miR-24, HMOX1, interleukin-6, tumor necrosis factor-α, superoxide dismutase, and malondialdehyde in rats were examined. Results: miR-24 could negatively regulate HMOX1 expression. SAH-induced CVS was accompanied with increased miR-24 expression and decreased HMOX1 expression. Inhibiting miR-24 expression or enhancing the expression of its down streaming target, HMOX1, could partly reverse the increased oxidation and inflammation as well as functional deficits in the rats. Moreover, the effects of miR-24 inhibitor could be reversed by inhibiting HMOX1 expression. Conclusion: miR-24 downregulation can promote HMOX1 expression, thereby decreasing the inflammatory response and improving the neurological function of rats with CVS after SAH.
Keywords: miR-24, HMOX1, subarachnoid hemorrhage, cerebral vasospasm, inflammation, neurological function
Introduction
Subarachnoid hemorrhage (SAH) is a common type of hemorrhagic stroke with high mortality and morbidity rates which have been increasing in recent years [1]. In most cases, survivors of SAH cannot be fully recovered and show deficits in communication, memory, and executive functions [2]. Cerebral vasospasm (CVS), a key complication of SAH, has an occurrence rate of 30%-70% in SAH patients. CVS is the major cause of death and disability in patients with SAH and is one of the main causes of delayed cerebral ischemia (DCI) after aneurysmal SAH [3,4]. The total incidence rate of CVS, DCI, and infarction-induced neurological sequelae, such as neurological dysfunction and learning disability, is 10% to 20% [5]. Although it has been more than 60 years since the discovery of SAH-induced arteriostenosis, a better understanding of the pathophysiological mechanism of CVS is still a hotspot [6]. It is believed CVS may be induced by oxidative stress, inflammatory response, cellular apoptosis, and toxic substances stimulation following SAH, but the detailed mechanism remains unclear and no effective preventative measures currently exist [7].
MiRNAs, which are highly conservative in humans, can regulate about 30% of all human genes and serve critical roles in neurodevelopment, neuroplasticity, and other neurobiological processes and diseases [8]. Aberrant regulation of miRNA is associated with a series of nervous system disorders [9-12]. For example, miR-15a is found to be a miRNA related to SAH-induced CVS, and its expression is significantly increased in patients with SAH [13]. Some studies reported that the upregulation of miR-24 can lead to SAH-induced CVS by inhibiting the expression of endothelial nitric oxide synthase [14]. After SAH, the accumulation of heme-containing blood components, especially red blood cells saturated with hemoglobins, can cause brain inflammation, thereby resulting in neuronal damage and cognitive impairment. Heme oxygenase (HO), which is responsible for degrading heme, has two subtypes, HMOX1 and HMOX2. HMOX1 is an inducible enzyme expressed in most tissues, while HMOX2 is constitutively expressed in the brain, endothelium. and testis. HMOX1 is a cell-protection molecule, which contributes to the cellular homeostasis, and the absence of HMOX1 can lead to aggravation of inflammation [15]. In SAH, the heme in the blood overflowing into the subarachnoid cavity is metabolized by HO, leading to the generation of excessive free irons which can damage the cell membrane through free radicals. So far, the necessity of HMOX1 in neuroprotection has been evaluated in some studies [16].
In this study, we searched on a bioinformatics website and found the targeting relationship between miR-24 and HMOX1. We speculated that miR-24 may affect the inflammatory response and neurological function of rats with SAH-induced CVS through regulating the HMOX1 expression.
Materials and methods
Animal subjects and grouping
A total of 120 healthy male Sprague-Dawley rats (two weeks old, clean grade, bodyweight 35±5 g) from the laboratory animal center of Tongji University School of Medicine were used for the study. Of them, 30 rats were randomly assigned to the normal group (15 rats) or the sham group (sham operation, 15 rats), and the remaining 90 rats were used to construct the SAH-induced CVS model [17]. The animal experiments took place in the laboratory animal center of Tongji University School of Medicine. The study complied with national legislation and local Institutional Review Board requirements and was approved by the Ethics Committee of Brain Hospital Affiliated to Tongji University.
Modeling method: The rats were anesthetized by intraperitoneal injection of 40 mg/kg pentobarbital sodium, and the head of the rats was fixed in a stereotactic instrument. After hair removal in the occipital region, the muscles and periosteum of the rats were separated layer by layer. The tail was cut 5 cm away from the base of the tail, and 0.3 mL of autologous blood of the rats was drawn with a 1 mL syringe. Next, under the guidance of the stereotactic instrument, a puncture needle was vertically inserted 1 mm deep into the atlantooccipital joint membrane, and 0.1 mL of cerebrospinal fluid was extracted. The obtained autologous blood was then slowly injected into the cisterna magna of the rats at a speed of 0.1 mL/min using the puncture needle and micropump. The puncture hole was then sealed with bone wax, and the wound was sutured, followed by intramuscular injection of penicillin to prevent infection. Subsequently, the rats were kept in the prone position with the head lowered by 30° for at least 30 min. Afterward, the breathing pattern of the rats was carefully checked, and mechanical ventilation was provided if necessary. The rats were put back to the cage when they were fully awake. In the sham group, the autologous blood was not injected, whereas other steps of the procedure were the same as those described above. Injection of autologous blood into the subarachnoid cistern is a common and safe method to create an SAH-induced CVS model [17]. In this study, the disease model was created successfully in all the rats, and no rats died in the model creation.
Gene interferences of miR-24 and HMOX1 were performed in rats 24 hours after modeling. The rats were anesthetized by intraperitoneal injection of 40 mg/kg pentobarbital sodium and the rat’s head was fixed in a stereotaxic instrument, and then the scalp was cut along the sagittal direction. With the anterior fontanelle as the base point, two small holes were drilled 3 mm backward from the base point and 2 mm to the left and 2 mm to the right of the median sagittal suture, respectively. The microsampling device was then inserted 3.5 mm deep, and the following drugs (1 μL) were injected correspondingly: sham group (normal saline), model group (normal saline), miR-24-NC group (miR-24-NC vector), miR-24 inhibitor group (miR-24 inhibitor vector), HMOX1-NC group (HMOX1-NC vector), oe-HMOX1 group (HMOX1 overexpression vector), miR-24 inhibitor + si HMOX1 group (miR-24 inhibitor and si-HMOX1 vectors). Each group had 15 rats. Subsequently, the skull was sealed with bone wax, and the scalp was sutured. After wound disinfection, the rats were injected with penicillin to prevent infection. The adenoviral vectors for miR-24 NC, miR-24 inhibitor, HMOX1 NC, oe-HMOX1, and si-HMOX1 were designed, synthesized, and provided by OBiO Technology (Shanghai, China). The dosage administered was 1012 vg/kg, and the injection on each side lasted for 5 min with needles also retained for 5 min.
One week after gene interferences, the Morris water maze (MWM) test was conducted, and neurological functions were assessed in all rats. After intraperitoneal injection of 40 mg/kg pentobarbital sodium for anesthesia, three rats were randomly selected from each group and they were euthanatized by fast cervical dislocation. Their brains were obtained to measure the permeability of blood-brain barrier (BBB). Remaining rats were sacrificed for blood collection from the apex of the heart for ELISA test followed by craniotomy immediately. The fresh brain tissues of three rats were randomly selected from each group for assessing cerebral edema, whereas the hippocampal tissues of the remaining rats were isolated and kept in liquid nitrogen for later use.
Dual-luciferase reporter (DLR) assay
The miR-24 binding site on HMOX1 was first analyzed through a bioinformatics website (www.targetscan.org) and the targeting relationship between miR-24 and HMOX1 was verified by DLR assay. The HMOX1 DLR gene vectors with or without a mutant miR-24 binding site were constructed and named as PGL3-HMOX1 mut and PGL3-HMOX1 wt, respectively. Rellina plasmids and the two reporter plasmids were cotransfected into HEK293T cells with miR-24 or NC plasmids. After 24 hours of transfection, DLR assay was performed and the luciferase activity was measured according to the manufacturer’s instructions of the DLR assay kit (Promega, USA). Relative luciferase activity = firefly luciferase activity/renilla luciferase activity.
MWM test
The water maze was a round pool (diameter 150 cm, height 60 cm, temperature 20°C-25°C) and was divided into four quadrants: right-lower quadrant, right upper quadrant, left lower quadrant, and left upper quadrant. A platform was installed in the right lower quadrant. After 7 days of training, the rats were placed in the water with face towards the water, and the escape latency, the time they spent finding the platform within 2 minutes, was recorded.
Assessment of neurological function
The neurological function of the rats with cerebral hemorrhage was assessed using Weaver’s 15-point scale. On the 7th day after operation, the forelimb movement after tail fixation, autonomic movement of the four limbs, spontaneous activity, climbing movement, and response to whisker stimulation in rats were scored. A higher score represented a better neurological function [18].
Permeability of BBB
The permeability of BBB in rats was determined by Evans Blue (EB) fluorescence spectrophotometry. After anesthesia, 2% EB saline solution (4 mL/kg) was injected into the femoral vein of the rats. One hour later, thoracotomy was performed, and the normal saline solution was perfused into the left ventricle (perfusion pressure 110 mmHg) of the rats until the fluid flowing out of the right ventricle was colorless. The rat brain was taken out, and the right dorsolateral cortex was isolated, weighed, and homogenized in 50% trichloroacetic acid (TCA). Afterward, the samples were centrifuged at 10,000 rpm for 25 min. The supernatant was collected and diluted with anhydrous ethanol at the ratio of 1:3. After mixing, the fluorescence value at the wavelength of 610 nm was measured using a fluorescence spectrophotometer. The calibration standard was 50% TCA added with anhydrous ethanol in a 1:3 ratio. EB content (μg/g brain tissue) was calculated according to the standard curve.
ELISA
Blood was collected at the apex of the rat heart using a 1 mL syringe wetted with heparin and then transferred to a 1.5 mL Eppendorf tube. The blood samples were placed at room temperature for 2 h followed by centrifugation at 8,000 rpm for 10 min. The supernatant was collected and centrifuged again at 3,000 rpm for 10 min to obtain the supernatant. The levels of the pro-inflammatory factors IL-6 and TNF-α in the serum were measured using ELISA kit (69-25328, 69-40133, MSKBio, Wuhan, China).
Assessment of cerebral edema
The whole brain of the rats was taken out and weighed immediately. The weight of the whole brain minus the weight of the slide was defined as the wet weight. Afterward, the brains were heated in the oven at 105°C for 24 hours and weighed again. The weight of the heated brain minus the weight of the slide was defined as the dry weight. The cerebral edema index was calculated using the following formula: cerebral edema index (%) = (wet weight - dry weight)/wet weight * 100.
Determination of superoxide dismutase (SOD) and malondialdehyde (MDA) levels and myeloperoxidase (MPO) activity in the hippocampus of the rats
Three hippocampal tissues from each group were randomly selected for this test. The SOD level in the hippocampal tissue was measured by WST-1 colorimetry (xanthine oxidase method, test kit: A001-3-2, Nanjing Jiancheng Bioengineering Institute, China), the MDA level was measured by thiobarbituric acid colorimetry (test kit: A003-1-2, Nanjing Jiancheng Bioengineering Institute, China), and the MPO activity was measured by spectrophotometry (test kit: A044, Nanjing Jiancheng Bioengineering Institute, China). The experiments were carried out according to the manufacturer’s instructions of the kits. SOD, MDA, and MPO results are expressed in U/mg brain tissue, mmol/mg brain tissue, and U/mg brain tissue, respectively.
qRT-PCR
Total RNAs were extracted from the hippocampus using TRIzol (Thermo Fisher Scientific, USA). The cDNAs were reversely transcribed using SuperScriptTM One-Step RT-PCR System with PlatinumTM Taq DNA Polymerase (Thermo Fisher Scientific, USA), and the fluorescence quantitative PCR was conducted with SYBR® PremixExTaqTMII kit (Xingzhi Biotechnology, China). The reaction system consisted of 3.2 μL of deionized-distilled water, 0.4 μL of PCR forward primer, 0.4 μL of PCR reverse primer, 5 μL of SYBR® PremixExTaqTMII (2×), 1 μL of ROX Reference Dye (50×), and 1 μL of DNA template. The running parameters (PCR system: ABI 7500, USA) were as follows: predenaturation at 95°C for 5 min, denaturation at 95°C for 15 s, and annealing at 60°C for 1 min; the cycle was repeated 40 cycles followed by extension at 60°C for 1 min. U6 was used as the internal control for miR-24, and GAPDH was used as the internal control for HMOX1. The relative expression level of each gene was calculated using the 2-ΔΔCt method. Primers are listed in Table 1.
Table 1.
qRT-PCR primer sequences
| Gene | Sequence |
|---|---|
| miR-24 | Forward: 5’-GCGGCGGTGGCTCAGTACAGC-3’ |
| Reverse: 5’-GTGCAGGGTCCGAGGT-3’ | |
| HMOX1 | Forward: 5’-CTGGAGGAGGAGATrGAGCG-3’ |
| Reverse: 5’-TGGCACTGGCAATGTTGG-3’ | |
| U6 | Forward: 5’-CTCGCTTCGGCAGCACATATACT-3’ |
| Reverse: 5’-ACGCTTCACGAATTTGCGTGTC-3’ | |
| GAPDH | Forward: 5’-CCAATGTGTCCGTCGTGGATCT-3’ |
| Reverse: 5’-GTTGAAGTCGCAGGAGACAACC-3’ |
Western blot
The total proteins of the hippocampal tissues were extracted using RIPA lysis buffer containing PMSF (R0010, Solarbio, Beijing, China). The protein concentration was measured with the BCA Kit (Thermo Fisher Scientific, USA.) and adjusted with the deionized water. The samples were mixed with the sample buffer and boiled for 10 min. Each lane of the PAGE was loaded with 30 µg protein samples for electrophoresis at 80 V for 2 h. The proteins were then transferred to a polyvinylidene fluoride membrane (ISEQ00010, Millipore, MA, USA) at 110 V for 2 h. Next, the membrane was blocked with 5% skimmed milk at 4°C for 2 h. The blocking solution was then discarded, and the membrane was washed in TBST for 3 times. The membrane was incubated with rabbit anti-mouse HMOX1 (ab13248, 1:1,000, Abcam, UK) and rabbit anti-GAPDH (ab70699, 1:2,000, Abcam, UK) antibodies at 4°C overnight, followed by washing in TBST for 3 times (10 min per wash). Afterward, the samples were treated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies (1:5,000, ZSBio, Beijing, China). Subsequently, the samples were treated with an ECL detection kit (BB-3501, Amersham, UK) and visualized in a gel imaging system. The results were photographed using Bio-Rad image analysis system (Bio-Rad, USA) and analyzed with ImageJ software. The relative protein content was calculated as the grayscale value of the protein band/the grayscale value of GAPDH band.
Statistical analysis
SPSS 21.0 software was applied for statistical analysis. Measurement data are presented as mean ± standard deviation (x̅ ± sd). Comparisons of means between multiple groups were compared by Tukey post-hoc test. Comparisons among multiple groups were conducted by one-way analysis of variance. P<0.05 indicated a statistically significant difference.
Results
miR-24 can target HMOX1 and negatively regulate its expression
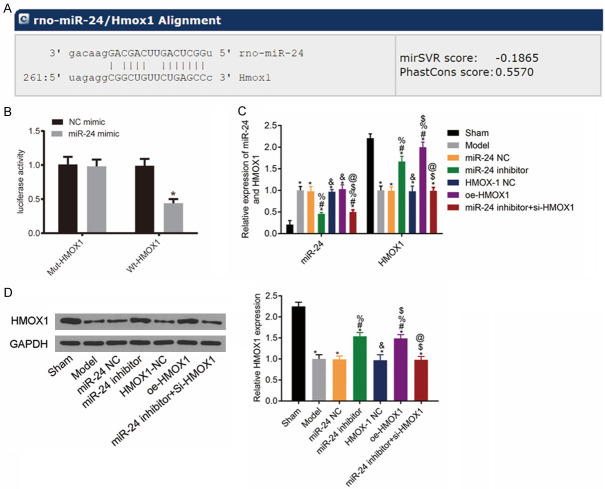
The bioinformatics website (www.targetscan.org) predicted the existence of the miR-24 binding site on HMOX1 gene (Figure 1A). The results of DLR assay revealed that compared with the NC mimic group, the luciferase activity of the cells co-transfected with wt-HMOX1 and miR-24 mimic was much lower (P<0.05), whereas the luciferase activity of the cells co-transfected with mut-HMOX1 and miR-24 mimic remained unchanged (P>0.05). This result indicated that miR-24 can target HMOX1 gene and negatively regulate its expression (Figure 1B).
Figure 1.
miR-24 can inhibit Notch signaling pathway in rat hippocampus. A: 3’-UTR region of miR-24 that binds to HMOX1; B: Luciferase activity results; compared with NC mimic group, *P<0.05; C: Histogram of miR-24 and HMOX1 mRNA levels in rat hippocampus; D: HMOX1 protein band image and protein levels; compared with the sham group, *P<0.05; compared with the model group, #P<0.05; compared with the miR-24 NC group, %P<0.05; compared with the miR-24 inhibitor group, &P<0.05; compared with the HMOX-1 NC group, $P<0.05; compared with the oe-HMOX1 group, @P<0.05, n=3.
To further clarify the relationship between miR-24 and Notch signaling pathway, we measured the expression levels of related markers in the hippocampus of rats in each group. The results showed that compared with the sham group, the model rats had higher expression levels of miR-24 and lower expression levels of HMOX1 mRNA and protein (all P<0.05). Compared with the model group, the NC group had similar expression levels of each gene (all P>0.05), whereas the miR-24 inhibitor and the oe-HMOX1 groups had higher HMOX1 mRNA expression levels (all P<0.05). The HMOX1 mRNA expression level was lower in the miR-24 inhibitor + si-HMOX1 group than that in the miR-24 inhibitor group (P<0.05). See Figure 1C, 1D.
Scores for neurological function in each group
Compared with the sham group, the model rats had lower scores for neurological function (all P<0.05). Compared with the model group, the NC group had a similar score (P>0.05), whereas the miR-24 inhibitor and the oe-HMOX1 groups had higher scores for neurological function (both P<0.05). The miR-24 inhibitor + si-HMOX1 group had lower score for neurological function than the miR-24 inhibitor group (P<0.05). See Figure 2.
Figure 2.
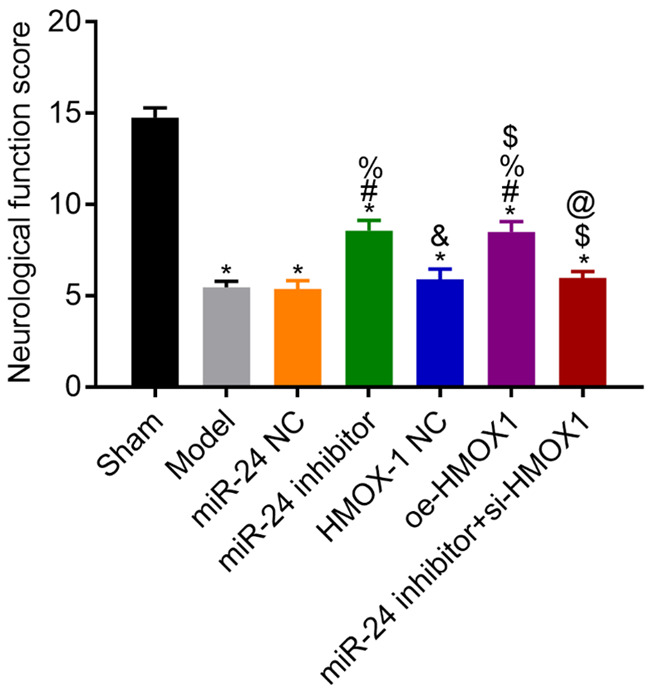
Scores for the neurological function of rats in each group. Compared with the sham group, *P<0.05; compared with the model group, #P<0.05; compared with the miR-24 NC group, %P<0.05; compared with the miR-24 inhibitor group, &P<0.05; compared with the HMOX-1 NC group, $P<0.05; compared with the oe-HMOX1 group, @P<0.05, n=3.
Cerebral edema in each group
Compared with the sham group, the brain edema indexes of model rats were higher (all P<0.05). Compared with the model group, the NC group had a similar result (P>0.05), whereas the brain edema index in the miR-24 inhibitor and the oe-HMOX1 groups were lower (both P<0.05). The brain edema index in the miR-24 inhibitor + si-HMOX1 group was higher than that in the miR-24 inhibitor group (P<0.05). See Figure 3.
Figure 3.
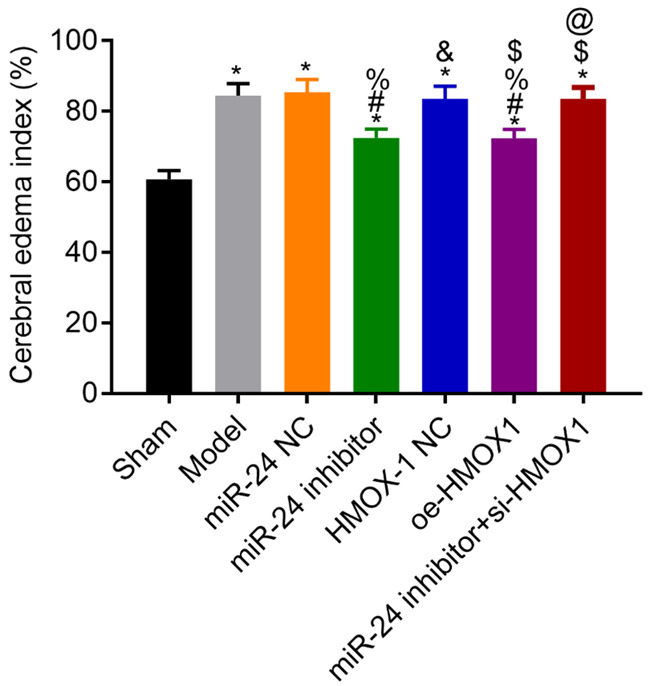
Cerebral edema indexes in each group. Compared with the sham group, *P<0.05; compared with the model group, #P<0.05; compared with the miR-24 NC group, %P<0.05; compared with the miR-24 inhibitor group, &P<0.05; compared with the HMOX-1 NC group, $P<0.05; compared with the oe-HMOX1 group, @P<0.05, n=3.
Permeability of BBB in each group
Compared with the sham group, the model rats had higher permeability of BBB (all P<0.05). Compared with the model group, the NC group had a similar result (P>0.05), whereas the miR-24 inhibitor and the oe-HMOX1 groups had lower BBB permeability (both P<0.05). The BBB permeability in the miR-24 inhibitor + si-HMOX1 group was higher than that in the miR-24 inhibitor group (P<0.05). See Figure 4.
Figure 4.
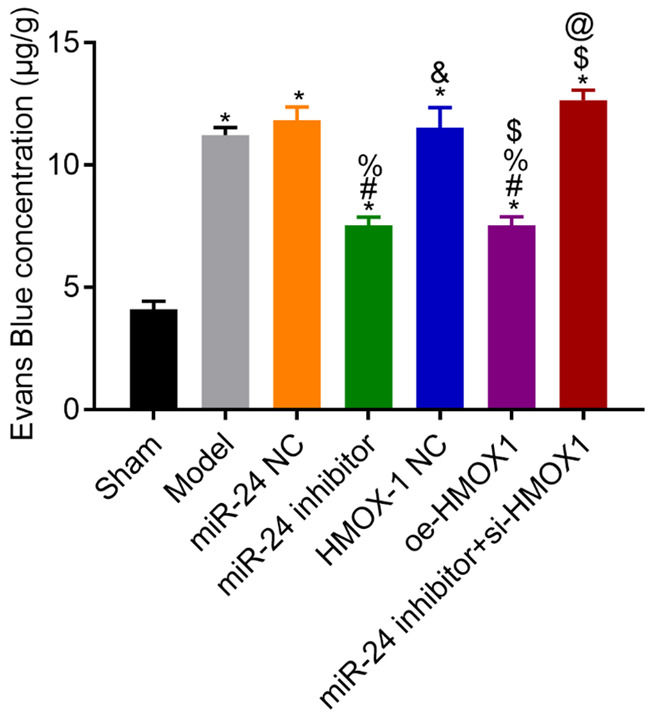
Permeability of the blood-brain barrier in each group. Compared with the sham group, *P<0.05; compared with the model group, #P<0.05; compared with the miR-24 NC group, %P<0.05; compared with the miR-24 inhibitor group, &P<0.05; compared with the HMOX-1 NC group, $P<0.05; compared with the oe-HMOX1 group, @P<0.05, n=3.
Learning and memory abilities in each group
The results of the MWM test showed that compared with the sham group, the model rats had longer escape latency (all P<0.05). Compared with the model group, the NC group had a similar result in escape latency (P>0.05), whereas the miR-24 inhibitor and the oe-HMOX1 groups had shorter escape latency (both P<0.05). Compared with the miR-24 inhibitor group, the latency in the miR-24 inhibitor + si-HMOX1 group was longer (P<0.05). See Figure 5.
Figure 5.
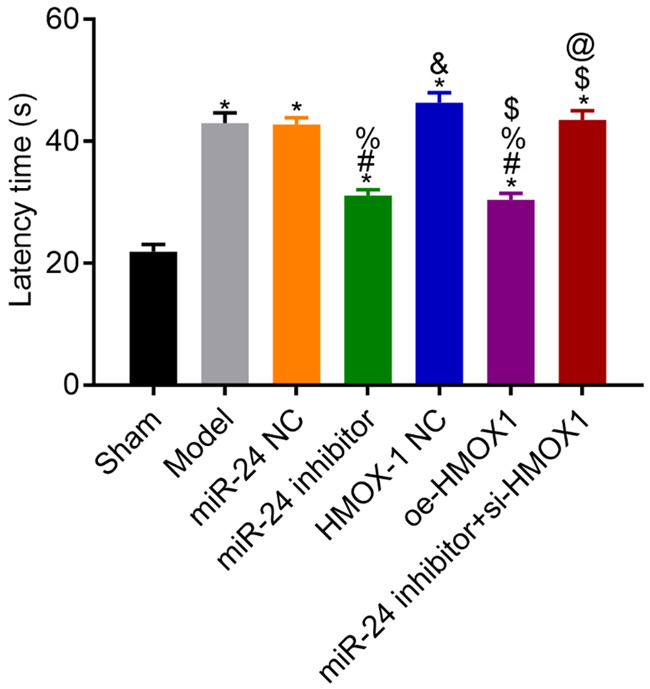
Escape latency in each group. Compared with the sham group, *P<0.05; compared with the model group, #P<0.05; compared with the miR-24 NC group, %P<0.05; compared with the miR-24 inhibitor group, &P<0.05; compared with the HMOX-1 NC group, $P<0.05; compared with the oe-HMOX1 group, @P<0.05, n=3.
Levels of SOD and MDA and activity of MPO in each group
Compared with the sham group, the model rats had lower SOD levels, higher MDA levels, and higher MPO activities (all P<0.05). Compared with the model group, the NC group had similar results in these markers (all P>0.05), whereas the miR-24 inhibitor and the oe-HMOX1 groups had higher SOD levels, lower MDA levels, and lower MPO activities (all P<0.05). Compared with the miR-24 inhibitor group, the miR-24 inhibitor + si-HMOX1 group had lower SOD level, higher MDA level, and higher MPO activity (all P<0.05). See Figure 6.
Figure 6.
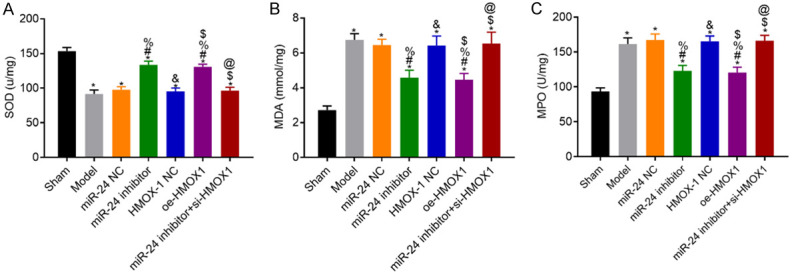
Levels of SOD and MDA and activities of MPO in rat hippocampus in each group. A: SOD levels in each group; B: MDA levels in each group; C: MPO activities in each group; compared with the sham group, *P<0.05; compared with the model group, #P<0.05; compared with the miR-24 NC group, %P<0.05; compared with the miR-24 inhibitor group, &P<0.05; compared with the HMOX-1 NC group, $P<0.05; compared with the oe-HMOX1 group, @P<0.05, n=3. SOD: superoxide dismutase; MDA: malondialdehyde; MPO: myeloperoxidase.
Levels of IL-6 and TNF-α in venous blood of rats in each group
Compared with the sham group, the model rats had higher IL-6 and TNF-α levels (all P<0.05). Compared with the model group, the NC group had similar results in these markers (both P>0.05), whereas the miR-24 inhibitor and the oe-HMOX1 groups had lower levels of IL-6 and TNF-α (P<0.05). Compared with the miR-24 inhibitor group, the IL-6 and TNF-α levels in the miR-24 inhibitor + si-HMOX1 group were higher (both P<0.05). See Figure 7.
Figure 7.
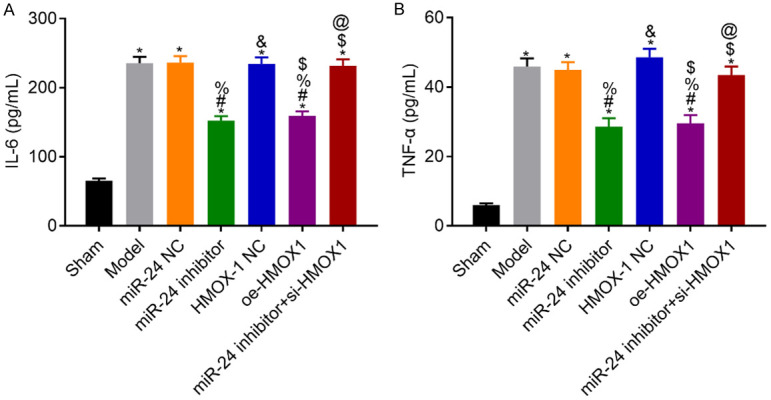
Levels of IL-6 and TNF-α in venous blood of rats in each group. A: IL-6 levels in each group; B: TNF-α levels in each group; compared with the sham group, *P<0.05; compared with the model group, #P<0.05; compared with the miR-24 NC group, %P<0.05; compared with the miR-24 inhibitor group, &P<0.05; compared with the HMOX-1 NC group, $P<0.05; compared with the oe-HMOX1 group, @P<0.05, n=10. IL, interleukin; TNF: tumor necrosis factor.
Discussion
CVS usually occurs between 3 and 9 days after aneurysmal SAH [19]. Although the ruptured aneurysm can be successfully treated by surgery and endovascular therapy, the incidence rates of CVS and permanent ischemic neurologic deficit in these patients are 48.3% and 16.1%, respectively, with the total mortality rate of 6.5% [19-22]. Notwithstanding extensive research has been conducted to explain the potential mechanism of CVS and many advanced therapies have been tried clinically, there is still no method that can treat this disease effectively [23,24].
At present, many studies have demonstrated that HMOX1 expression decreases in experimental CVS [25-28]. Ogawa et al. found that the injection of HMOX1 protein can attenuate SAH-induced CVS in rats [25]. Shimada et al. also reported that enhancing the expression of HO-1 may be an effective way to improve the prognosis of delayed CVS and other diseases associated with abnormal accumulation of heme and oxidative stress [26]. In this study, we found that after overexpression of HMOX1, the rats exhibited increased SOD level, decreased MDA, IL-6, and TNF-α levels, better neurological function and the integrity of BBB, and decreased activity of MPO. It has been reported that inhibiting HO-1 expression in the basilar artery can slow down the clearance of oxyhemoglobin and deoxyhemoglobin from the subarachnoid space and aggravate vasospasm [27]. The results of our study are consistent with the previous research, suggesting that overexpression of HMOX1 may reduce the brain injury of rats with autoimmune inflammation in the central nervous system associated with SAH-induced CVS.
Previous studies have reported that miR-24 play an important role in inflammatory reaction [29,30]. For example, Lin et al. reported that miR-24 could inhibit inflammatory responses in rats with LPS-induced acute lung injury [31]. Maegdefessel et al. performed in vivo and in vitro studies and found that miR-24 mediated chitinase 3-like 1 (Chi3l1) to regulate cytokine synthesis in macrophages as well as their survival, promote aortic smooth muscle cell migration and cytokine production, and stimulate adhesion molecule expression in vascular endothelial cells [32]. In our study, the miR-24 expression level was much higher and HMOX1 expression level was much lower in the hippocampus of the model rats. In addition, we also found that there was a binding site between miR-24 with HMOX1 on a bioinformatics website, and the DLR assay verified that miR-24 can negatively regulate HMOX1 expression. We speculated that miR-24 is an upstream molecule of HMOX1, which can regulate the expression of HMOX1.
Therefore, the model rats were injected with miR-24 inhibitor or miR-24 inhibitor combined with si-HMOX1 to further explore the effect of miR-24 and HMOX1 in vivo. The results showed that miR-24 downregulation could upregulate HMOX1 expression, thereby improving the learning and memory abilities, increasing the level of SOD, decreasing the levels of MDA, MPO, IL-6, and TNF-α, and improving the BBB integrity; moreover, the effects of silencing miR-24 could be reversed by silencing HMOX1. These results indicated that the interaction between miR-24 and HMOX1 can serve a critical role in brain injury. Since previous studies have not investigated miR-24 in central nervous system diseases, miR-24 may be a new treatment target for CVS (Figure 8).
Figure 8.
The model figure of this study.
The present study preliminarily proved that miR-24 mediated upregulation of HMOX1 expression can exert protective effects on rat model of SAH-induced CVS. However, whether other signal molecules participate in the interaction between miRNA and HMOX1 and how HMOX1 regulates the levels of SOD, MDA, and inflammatory factors require further investigation. Meanwhile, there may other mechanisms for SAH-induced CVS as since the effect of normalizing miR-24 or HMOX only partially rescued the phenotype. For example, Yang et al. found that Cx43 and the PKC pathway were novel targets for developing treatments for SAH-induced CVS [33]. Liu et al. reported that cystatin C could induce autophagy to prevent SAH-induced CVS [34]. The results of Chen et al. showed that the administration of specific NLK agonist could prevent or reduce CVS or neuronal apoptosis caused by SAH [35]. In addition, more preclinical researches such as RNA sequence, and better targets screening and the safety test of RNA therapy should be performed by using pharmacological and genetic approaches. Some brain tissues could also be collected from patients to further verify our results in human.
In conclusion, miR-24 is highly expressed in the hippocampus of rats with CVS after SAH, and miR-24 downregulation can improve the recovery from brain injury in these rats by upregulating the expression of HMOX1 gene.
Disclosure of conflict of interest
None.
References
- 1.Huang YH, Chung CL, Tsai HP, Wu SC, Chang CZ, Chai CY, Lee TC, Kwan AL. Hyperglycemia aggravates cerebral vasospasm after subarachnoid hemorrhage in a rat model. Neurosurg. 2017;80:809–815. doi: 10.1093/neuros/nyx016. [DOI] [PubMed] [Google Scholar]
- 2.Malinova V, Psychogios MN, Tsogkas I, Koennecke B, Bleuel K, Iliev B, Rohde V, Mielke D. MR-angiography allows defining severity grades of cerebral vasospasm in an experimental double blood injection subarachnoid hemorrhage model in rats. PLoS One. 2017;12:e0171121. doi: 10.1371/journal.pone.0171121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darkwah Oppong M, Iannaccone A, Gembruch O, Pierscianek D, Chihi M, Dammann P, Köninger A, Müller O, Forsting M, Sure U, Jabbarli R. Vasospasm-related complications after subarachnoid hemorrhage: the role of patients’ age and sex. Acta Neurochir (Wien) 2018;160:1393–1400. doi: 10.1007/s00701-018-3549-1. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Chen Y, Zhang X, Zuo S, Ge H, Chen Y, Liu X, Zhang JH, Ruan H, Feng H. Scutellarin attenuates vasospasm through the Erk5-KLF2-enos pathway after subarachnoid hemorrhage in rats. J Clin Neurosci. 2016;34:264–270. doi: 10.1016/j.jocn.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Xu D, Bai X, Pan R, Wang B, Sun S, Chen R, Sun J, Huang Y. Curcumin mitigates cerebral vasospasm and early brain injury following subarachnoid hemorrhage via inhibiting cerebral inflammation. Brain Behav. 2017;7:e00790. doi: 10.1002/brb3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aydogmus E, Gul S, Bahadir B. Neuroprotective effects of hesperidin on cerebral vasospasm after experimental subarachnoid hemorrhage in rats: biochemical, pathologic, and histomorphometric analysis. World Neurosurg. 2019;122:e1332–e1337. doi: 10.1016/j.wneu.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Haruma J, Teshigawara K, Hishikawa T, Wang D, Liu K, Wake H, Mori S, Takahashi HK, Sugiu K, Date I, Nishibori M. Anti-high mobility group Box-1 (HMGB1) antibody attenuates delayed cerebral vasospasm and brain injury after subarachnoid hemorrhage in rats. Sci Rep. 2016;6:37755. doi: 10.1038/srep37755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stylli SS, Adamides AA, Koldej RM, Luwor RB, Ritchie DS, Ziogas J, Kaye AH. miRNA expression profiling of cerebrospinal fluid in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126:1131–1139. doi: 10.3171/2016.1.JNS151454. [DOI] [PubMed] [Google Scholar]
- 9.Pulcrano-Nicolas AS, Proust C, Clarençon F, Jacquens A, Perret C, Roux M, Shotar E, Thibord F, Puybasset L, Garnier S, Degos V, Trégouët DA. Whole-blood miRNA sequencing profiling for vasospasm in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2018;49:2220–2223. doi: 10.1161/STROKEAHA.118.021101. [DOI] [PubMed] [Google Scholar]
- 10.Huang F, Yi J, Zhou T, Gong X, Jiang H, Yao X. Toward understanding non-coding RNA roles in intracranial aneurysms and subarachnoid hemorrhage. Transl Neurosci. 2017;8:54–64. doi: 10.1515/tnsci-2017-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan MTH, Wong JYY, Leung AKT, Lu G, Poon WS, Lau AY, Chan WY, Wong GKC. Plasma and CSF miRNA dysregulations in subarachnoid hemorrhage reveal clinical courses and underlying pathways. J Clin Neurosci. 2019;62:155–161. doi: 10.1016/j.jocn.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Lopes KP, Vinasco-Sandoval T, Vialle RA, Paschoal FM, Bastos V, Bor-Seng-Shu E, Teixeira MJ, Yamada ES, Pinto P, Vidal AF, Ribeiro-Dos-Santos A, Moreira F, Santos S, Paschoal EHA, Ribeiro-Dos-Santos Â. Global miRNA expression profile reveals novel molecular players in aneurysmal subarachnoid haemorrhage. Sci Rep. 2018;8:8786. doi: 10.1038/s41598-018-27078-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikkawa Y, Ogura T, Nakajima H, Ikeda T, Takeda R, Neki H, Kohyama S, Yamane F, Kurogi R, Amano T, Nakamizo A, Mizoguchi M, Kurita H. Altered expression of MicroRNA-15a and Kruppel-like factor 4 in cerebrospinal fluid and plasma after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2017;108:909–916. e3. doi: 10.1016/j.wneu.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Li HT, Wang J, Li SF, Cheng L, Tang WZ, Feng YG. Upregulation of microRNA-24 causes vasospasm following subarachnoid hemorrhage by suppressing the expression of endothelial nitric oxide synthase. Mol Med Rep. 2018;18:1181–1187. doi: 10.3892/mmr.2018.9050. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc RH 3rd, Chen R, Selim MH, Hanafy KA. Heme oxygenase-1-mediated neuroprotection in subarachnoid hemorrhage via intracerebroventricular deferoxamine. J Neuroinflammation. 2016;13:244. doi: 10.1186/s12974-016-0709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schallner N, Pandit R, LeBlanc R 3rd, Thomas AJ, Ogilvy CS, Zuckerbraun BS, Gallo D, Otterbein LE, Hanafy KA. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–2625. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Xia X, Li X. Plasmid pLXSN-mediated adrenomedullin gene therapy for cerebral vasospasm following subarachnoid hemorrhage in rats. Med Sci Monit. 2017;23:3293–3302. doi: 10.12659/MSM.901914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver A, Goncalves da Silva A, Nuttall RK, Edwards DR, Shapiro SD, Rivest S, Yong VW. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB J. 2005;19:1668–1670. doi: 10.1096/fj.04-2030fje. [DOI] [PubMed] [Google Scholar]
- 19.Konczalla J, Wanderer S, Mrosek J, Gueresir E, Schuss P, Platz J, Seifert V, Vatter H. Levosimendan, a new therapeutic approach to prevent delayed cerebral vasospasm after subarachnoid hemorrhage? Acta Neurochir (Wien) 2016;158:2075–2083. doi: 10.1007/s00701-016-2939-5. [DOI] [PubMed] [Google Scholar]
- 20.Guvenc Tuna B, Lachkar N, de Vos J, Bakker EN, VanBavel E. Cerebral artery remodeling in rodent models of subarachnoid hemorrhage. J Vasc Res. 2015;52:103–115. doi: 10.1159/000431366. [DOI] [PubMed] [Google Scholar]
- 21.Aladag MA, Turkoz Y, Parlakpinar H, Gul M. Nebivolol attenuates cerebral vasospasm both by increasing endothelial nitric oxide and by decreasing oxidative stress in an experimental subarachnoid haemorrhage. Br J Neurosurg. 2017;31:439–445. doi: 10.1080/02688697.2017.1297367. [DOI] [PubMed] [Google Scholar]
- 22.Huang CY, Wang LC, Shan YS, Pan CH, Tsai KJ. Memantine attenuates delayed vasospasm after experimental subarachnoid hemorrhage via modulating endothelial nitric oxide synthase. Int J Mol Sci. 2015;16:14171–14180. doi: 10.3390/ijms160614171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aydin HE, Bektur NE, Ozbek Z, Oner S, Baycu C, Kilic FS. Comparison of the effects and mechanism of the curcumin with different drugs in experimental vasospasm after subarachnoid hemorrhage. Turk Neurosurg. 2017;27:884–893. doi: 10.5137/1019-5149.JTN.17432-16.2. [DOI] [PubMed] [Google Scholar]
- 24.Huang Q, Wang G, Hu YL, Liu JX, Yang J, Wang S, Zhang HB. Study on the expression and mechanism of inflammatory factors in the brain of rats with cerebral vasospasm. Eur Rev Med Pharmacol Sci. 2017;21:2887–2894. [PubMed] [Google Scholar]
- 25.Ogawa T, Hänggi D, Wu Y, Michiue H, Tomizawa K, Ono S, Matsui H, Date I, Steiger HJ. Protein therapy using heme-oxygenase-1 fused to a polyarginine transduction domain attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31:2231–2242. doi: 10.1038/jcbfm.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada Y, Tsunoda H, Zang L, Hirano M, Oka T, Tanaka T. Synergistic induction of heme oxygenase-1 by nicaraven after subarachnoid hemorrhage to prevent delayed cerebral vasospasm. Eur J Pharmacol. 2009;620:16–20. doi: 10.1016/j.ejphar.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Kanamaru K, Tsunoda H, Inada H, Kuroki M, Sun H, Waga S, Tanaka T. Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. J Clin Invest. 1999;104:59–66. doi: 10.1172/JCI5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono S, Komuro T, Macdonald RL. Heme oxygenase-1 gene therapy for prevention of vasospasm in rats. J Neurosurg. 2002;96:1094–1102. doi: 10.3171/jns.2002.96.6.1094. [DOI] [PubMed] [Google Scholar]
- 29.Dai B, Yang Y, Li H, Chen B, He J, Xiang D, Qiu J. MiR-24 can promote the effects of induction of pluripotent stem cells on myocardial infarction fibrosis. Int J Clin Exp Pathol. 2016;9:12315–12322. [Google Scholar]
- 30.Zhao Y, Yue CH, Cao HL, Liu RM. Effect and mechanism of upregulating mir-24 on the inflammation of acute lung injury in infant rats. J Xi’an Jiaotong Univ (Medical ences) 2019;40:684–689. 715. [Google Scholar]
- 31.Lin Y, Yang Y. MiR-24 inhibits inflammatory responses in LPS-induced acute lung injury of neonatal rats through targeting NLRP3. Pathol Res Pract. 2019;215:683–688. doi: 10.1016/j.prp.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, Jin H, Roy J, Hultgren R, Caidahl K, Schrepfer S, Hamsten A, Eriksson P, McConnell MV, Dalman RL, Tsao PS. Mir-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014;5:5214. doi: 10.1038/ncomms6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Yan J, Zhang JA, Zhou XH, Fang C, Zeng EM, Tang B, Duan J, Lu GH, Hong T. The important role of connexin 43 in subarachnoid hemorrhage-induced cerebral vasospasm. J Transl Med. 2019;17:433. doi: 10.1186/s12967-019-02190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Cai H, Wang Z, Li J, Wang K, Yu Z, Chen G. Induction of autophagy by cystatin c: a potential mechanism for prevention of cerebral vasospasm after experimental subarachnoid hemorrhage. Eur J Med Res. 2013;18:21. doi: 10.1186/2047-783X-18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, Feng D, Zhang L, Dang B, Liu H, Wang Z. Expression of nemo-like kinase (NLK) in the brain in a rat experimental subarachnoid hemorrhage model. Cell Biochem Biophys. 2013;66:671–680. doi: 10.1007/s12013-012-9511-6. [DOI] [PubMed] [Google Scholar]