Abstract
The present study aimed to explore the role of kelch-like ECH-associated protein-1 (Keap1)/Nuclear factor erythroid 2-related factor 2 (Nrf-2) signaling pathway in regulating heme oxygenase-1 (HO-1) expression in adverse outcomes of preeclampsia (PE). Adult Wistar rats, HTR-8/SVneo and hESC cells were used for models in vitro and in vivo, respectively. Inhibition of Nrf-2 could slightly reduce the elevation of systolic blood pressure (SBP) and urinary protein in PE rats. The percentages of dead fetuses during pregnancy and within seven days of birth were decreased by Nrf-2 inhibitor. There was no significant effect on the pathology and HO-1 expression of Nrf-2 in placental tissue. Deficiency of Nrf-2 increased significantly the levels of chemokine 2 (CCL2), interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), angiotensin II receptor type 1 (AT1R) and reactive oxygen species (ROS) in the embryonic tissues. Knockdown of Nrf-2 suppressed cell proliferation, improved cell apoptosis and invasion with an increase of ROS and HO-1, but the effect on cells apoptosis was greater. Activation of Nrf-2 pathway could reduce oxidative stress in PE rats and trophoblast cells induced by Ang II, and enhance the adverse outcome of PE via increasing HO-1. Nrf-2 silence reshaped blood vessels and achieved the effect of treating PE. Our results might provide theoretical guidance for the application of Nrf-2 in the treatment of PE.
Keywords: Preeclampsia, nuclear factor erythroid 2-related factor 2, heme oxygenase-1, oxidative stress, apoptosis
Introduction
Preeclampsia (PE), a complex disorder of pregnancy, is the leading cause of rising incidence and mortality rates among pregnant women and perinatal infants in otherwise uncomplicated pregnancies in many developed countries [1,2]. The specific molecular mechanism of the occurrence and development of PE remained unclear, but several previous studies had shown that the pathogenesis of PE includes impaired infiltration of trophoblast cells, vascular endothelial deterioration and abnormal increase in the apoptosis of trophoblast cells, which were caused by oxidative damage and hypertension [3,4]. PE generally occurred in about 20 weeks of pregnancy and involved damage to several organs, including the heart, kidney, brain and liver, in late pregnancy [5]. In PE, the impairment of nitric oxide (NO) bioavailability resulted in endothelial dysfunction. NO deficiency was a multifactorial process involving the decrease of NO production and the increase of NO degradation by reactive oxygen species (ROS). It had been proven that ROS could be scavenged by antioxidant enzymes, which were downstream molecules of Nrf-2 [6].
Nuclear factor erythroid 2-related factor 2 (Nrf-2), a classical cytoprotective transcription factor, had been reported to exert protective effects against oxidative injury [7]. Nrf-2 was combined with its inhibitor kelch-like ECH-associated protein-1 (Keap1) as a heterodimer in the cytoplasm under normal conditions [8]. However, under conditions where oxidative stress was low, Nrf-2 moved into the nucleus after separating from Keap-1, and its interaction with antioxidant response elements induced the expression of different antioxidant enzymes [9]. Previous studies had shown that oxidative stress contribute to PE, and patients with severe PE might be protected against oxidative injury following an elevation in HO-1 and Nrf-2 levels [10]. Nrf-2 was likely to have a synergistic effect on HO-1 in PE. The present study was aimed at exploring the physiological roles of HO-1 and Nrf-2 in PE.
Nezu et al. [11] established a mouse model of pregnancy-associated hypertension (PAH) in which the antioxidant system Nrf-2 had been genetically or pharmacologically manipulated. Further stimulation of Keap-1/Nrf-2 signaling pathway (Keap-1 knock down) could indeed reduce ROS, but inhibit fetal development, reduce placental angiogenesis, and lead to deterioration of maternal health condition; however, Nrf-2 deficiency increased ROS levels, but promoted placental angiogenesis, therefore improved maternal and fetal survival rate, ameliorated intra-uterine growth retardation. Additionally, the activation of Keap-1/Nrf-2 in PAH pregnant mice could moderately induce the expression of HO-1 coding gene Hmox1, but it did not change the adverse outcomes. Therefore, the relationship between Nrf-2 and HO-1 in PE may not be a simple upstream and downstream relationship as it used to be.
However, no further studies have been conducted to explore the effects of Nrf-2 on trophoblast cells. Therefore, to evaluate the effects of Nrf-2 on trophoblast, we mimicked the pathological condition of oxidative stress in PE by inducing HTR-8/SVneo and hESC cells and employing angiotensin II (Ang-II) treatments in vitro. This study mainly observed the roles of Nrf-2 in placental tissues of PE rats and trophoblast cells belonging to the main components of placenta.
Materials and methods
Animals and experimental designs
Adult Wistar rats (200 ± 20 g) were procured from Jinan Jinfeng Experimental Animal Center (Shandong, China). Before experiments, rats were allowed to acclimatize for one week. Subsequently, rats were maintained at the conditions with 22-25°C temperature, 50-70% humidity and a 12/12 h light/dark cycle and they were freely allowed standard rodent chow and water. The male rats were raised in cages with female rats, and the female rats were naturally pregnant. On the morning after mating, the wet cotton swabs were gently inserted into the vagina of female rats to obtain the vaginal secretions, which were smeared on the slides and observed under an optical microscope. The presence of sperm in the vaginal smears was indicative of gestational day 0.
The female rats with a gestation of 1 week were divided into 2 groups using a random number table, including normal pregnancy group (control group) and PE model group. In PE model group, rats received 50 mg/kg L-NAME injection into the rats at multiple subcutaneous points at gestational day 7 to induce PE after minor adjustments according to previous studies [12,13]. In control group, equal dose of 0.9% normal saline (NS) was injected into the rats. All treatments were maintained until gestational day 11. After PE models were built, rats were divided into three subgroups, including PE group, PE+CDDO-Im group and PE+ML385 group. Rats were treated with Nrf-2 agonist, CDDO-Im (1.5 mg/kg, i.p.) or Nrf-2 inhibitor ML385 (30 mg/kg, i.p.; MCE Co. Ltd., Shanghai, China) and vehicle (0.5% DMSO, 5 mL/kg, i.p.) once daily for three days.
This study was approved by the Animal Ethics Committee of Dongying People’s Hospital. Housing and experimental treatment of the animals were in accordance with the Guide for the Care and Use of Laboratory Animals from the Institute for Laboratory Research [14].
Systolic blood pressure (SBP) measurement
On day 6, 12 and 21, the SBP was measured by the non-invasive tail cuff method when the rats were pre-warmed in a healing chamber at 37°C for 15 min. The SBP measurement was conducted five times, and the mean value of each rat was obtained.
Proteinuria measurement
On the day 6, 12 and 21 of pregnancy, 24 h urine of rats was collected for the determination of urinary protein. Then, the proteinuria was measured by a commercial kit (Sigma).
Sample collection and measurement of fetal weight and dead fetuses
Animals were anesthetized with i.p. administration of 3% sodium pentobarbital (50 mg/kg) and sacrificed after administration (during early pregnancy), and placenta tissues were dissected immediately. Then, the placentas and fetal rats were taken out from the uterus, and the placentas in each group were washed with the normal saline to remove bloodiness. Fetal weight and the following parameters: litter size, number of stillbirths and neonatal survival were evaluated. A portion of the placental tissues was fixed for histological and immunohistochemistry analysis and the remaining tissue was stored at -80°C for further investigation. Homogenate preparation was used for ELISA assay, mRNA and protein detection.
Histopathology
Hematoxylin and eosin (H&E) staining (Boster, Wuhan, China) was used to detect the pathological changes of placental tissues of rats among each group. Placental tissues were fixed with paraformaldehyde and dehydrated with different concentrations of ethanol, followed by transparency with xylene. Then, the placental tissues were embedded with paraffin and sliced into approximately 5 μm-thick sections. After dewaxing and processing, the sections were stained with hematoxylin and eosin, and then analyzed under an optical microscope for pathological changes (Magnification, × 200).
Immunohistochemistry
The paraffin placental tissue sections were baked in an oven at 60°C for 30 min, followed by deparaffinization in xylene (5 min × 3 times) and incubated with citrate buffer for antigen retrieval. Sections were rinsed with phosphate-buffered saline (PBS) and the endogenous peroxidase was inhibited by 3% hydrogen peroxide-methanol. The anti-HO-1 antibody was diluted at 1:200 with PBS and incubated at 4°C overnight, followed by washing with PBS on a shaking table for 4 times. After the addition of secondary antibodies, the diaminobenzidine was adopted for color development. After that, 6 samples were randomly selected from each group, and five fields of vision were randomly selected from each sample for photography under a light microscope (Magnification, × 200).
Cell culture and transfection
Human trophoblast cell line (HTR-8/SVneo) and human normal endometrial stromal cell line (hESC) were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). HTR-8/SVneo cells were cultured in RPMI1640 and hESC cells were cultured in DMEM/F12 media, all containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2-humidified incubator. Cells were subcultured to 4-10 generations to obtain invasive phenotype for follow-up experiments. Subsequently, two cell lines were transfected with plasmids of siRNA-Nrf-2-1, siRNA-Nrf-2-2, siRNA-NC, siRNA-Keap1-1 and siRNA-Keap1-2 using Lipofectamine 2000 kits according to the manufacture’s protocol. qRT-PCR and western blot assays were used to measure Nrf-2 expression to verify transfection efficiency. Eventually, siRNA-Nrf-2-1 and siRNA-Keap1-1 plasmids were chosen as interfering plasmids for further experiments.
Cells treatment and grouping
HTR-8/SVneo and hESC cells (all 1 × 105 cells/ml) were treated with Angll (10 μM) for 48 h. Then, two cell lines were divided into four groups, respectively. Groups were as following: Normal-1 group (normal HTR-8/SVneo cells), Angll-1 group (HTR-8/SVneo cells were treated with Angll), Angll-1+siRNA-Nrf-2-1 group (HTR-8/SVneo cells were treated with Angll after transfected with siRNA-Nrf-2-1) and Angll-1+siRNA-Keap1-1 group (HTR-8/SVneo cells were treated with Angll after transfected with siRNA-Keap1-1); Normal-2 group (normal hESC cells), Angll-2 group (hESC cells were treated with Angll), Angll-2+siRNA-Nrf-2-1 group (hESC cells were treated with Angll after transfected with siRNA-Nrf-2-1) and Angll-2+siRNA-Keap1-1 group (hESC cells were treated with Angll after transfected with siRNA-Keap1-1).
MTT assay for cell proliferation
Cell proliferation among above groups was determined by the 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. Cells were seeded in 96-well plates at the density of 1 × 105 cells/well. Following 24 h incubation, the supernatant was removed and replaced with serum-free media. 20 μL of MTT (5 mg/mL) was then added and incubated for another 4 h. The absorbance at 490 nm was measured by a microplate reader (BIO-RAD). Values represent the mean ± SD of at least three independent experiments.
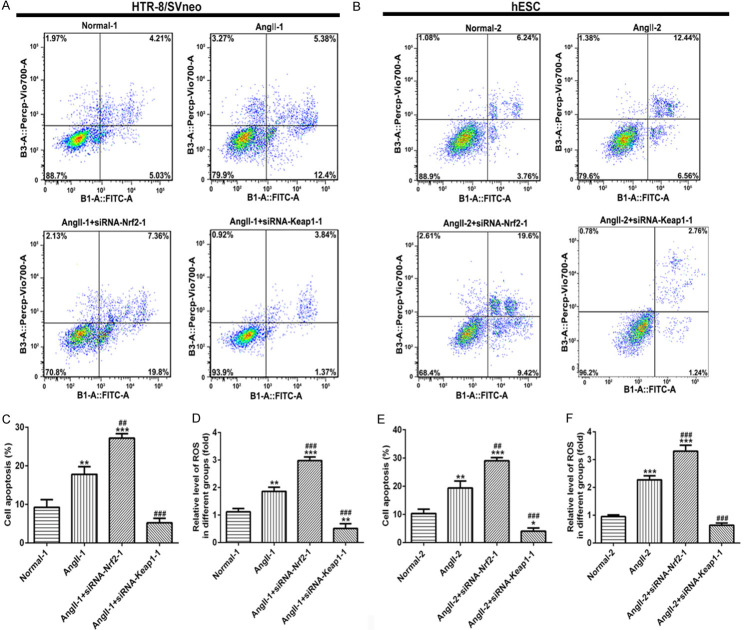
Flow cytometry for cell apoptosis
Cell apoptosis in two cell lines was detected by flow cytometry (FCM) using Annexin V-FITC/PI double staining. Cells in the logarithmic phase were fetched, digested and prepared into suspension with 0.25% trypsin-EDTA, and seeded into the medium with a 6-well plate. Then, cells were harvested and washed with PBS and incubated in binding buffer containing Annexin V-FITC and PI (Beyotime, Shanghai, China) for 15 min at 37°C in the dark. The apoptosis rate was calculated using FlowJo software (version 7.6.1).
Transwell assay for cell invasion
Cell invasion was measured using 24-transwell inserts with 8-µm microporous membranes. Cell suspensions (2 × 105 cells/ml) were added to the upper chamber of a Transwell migration system (BD Biosciences) and incubated for 12 h at 37°C. After starving for 8 h in FBS-free DMEM, the indicated treatments were added and the cells were allowed to migrate for 24 h. Non-migrating cells were removed from the upper surface of the insert with a cotton swab. Cells were fixed with 100% ethanol for 20 min, followed by staining with crystal violet solution. Images of migrating cells were captured using a microscope (Olympus).
Enzyme-linked immunosorbent assay (ELISA)
The placental tissues were homogenized with tissue lysis buffer for 10 min, followed by centrifugation. Then, the supernatant was collected from tissue homogenate and cells were collected and the ROS level were detected using the commercially-available kits according to the instructions.
Quantitative real-time PCR (qRT-PCR) analysis for Nrf-2 expression
Total RNA was isolated from cells among different groups using Trizol reagent (Invitrogen; Thermo Fisher Scientific, Irvine, CA, USA). The complementary DNA (cDNA) was synthesized using a cDNA reverse transcription kit (Invitrogen; Thermo Fisher Scientific, Irvine, CA, USA). The mRNA level of Nrf-2 was detected by qRT-PCR using the SYBR® Green Master Mix (Invitrogen; Thermo Fisher Scientific, Irvine, CA, USA) according to the manufacturer’s instructions. The primer of Nrf-2 was following, forward, 5’-TCCAGTCAGAAACCAGTGGAT-3’ and reverse, 5’-GAATGTCTGCGCCAAAAGCTG-3’. GAPDH was used to as control and the relative expression of mRNA was calculated using 2ΔΔCt method. Three repeated experiments were performed for each qPCR reaction.
Western blot analysis for proteins expression
The placental tissues and cells were homogenized to obtain total protein using protein isolation kits (Beyotime Institute of Biotechnology). Protein concentrations were detected using a bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology). The protein samples were separated on 8-12% SDS-PAGE gels and were transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked in 5% TBST for 2 h at room temperature and incubated overnight at 4°C with primary antibodies including HO-1, CCL2, IL-1β, TNF-α, AT1R, Nrf-2, VEGFR, Bcl-2, Bax, Cleaved-Caspase-3 and Caspase3. After washing, membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit antibody for 1 h at room temperature. Membranes were then washed three times in TBST and the protein signals were detected using electrochemiluminescence (ECL) detection system. Signals were quantified by Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Significant differences between groups were assessed using GraphPad Prism 5.0 software (Graphpad Software, San Diego, CA, USA). Differences between the means of groups were calculated by one-way ANOVA followed by the Tukey’s post-hoc test and the quantitative variables were compared using the paired two-tailed Student’s-t test. Differences of P<0.05 were considered statistically significant.
Results
Effects of Nrf-2 on systolic blood pressure, proteinuria and fetal weight in PE rats
The levels of systolic blood pressure (SBP) and 24 h proteinuria in rats were detected on the day 6, 12 and 21 of pregnancy, as well as the fetal weight and the rate of dead fetuses were assessed after rats were sacrificed. As shown in Figure 1, compared with the control group (normal pregnant rats), the levels of SBP and proteinuria in PE rats were significantly elevated, which continued to rise after Nrf-2 activation (CDDO-Im treatment); however the levels of SBP and proteinuria were decreased after Nrf-2 inhibition (ML385 treatment) when compared with PE+CDDO-Im group, but there were all no significant difference with PE group on day 12 and 21 (Figure 1A and 1B). Meanwhile, the fetal weight was inversely correlated with the Nrf-2 expression. The fetal weight was markedly decreased in PE rats and CDDO-Im (Nrf-2 agonist) could expand the downward trend comparing to control, but ML385 (Nrf-2 inhibitor) could raise the fetal weight when compared with PE group (Figure 1C). The mortality of rats during pregnancy and after birth were not entirely consistent. When compared with control group, the rate of dead fetuses increased significantly in PE rats both during pregnancy and within 7 days after birth, CDDO-Im administration accelerated mortality of fetal rat in PE group, which was suppressed greatly with ML385 treatment during pregnancy (Figure 1D). Inversely, CDDO-Im could decrease the rate of dead fetuses of PE rats and ML385 could continue to enhance this trend within 7 days after birth (Figure 1E).
Figure 1.
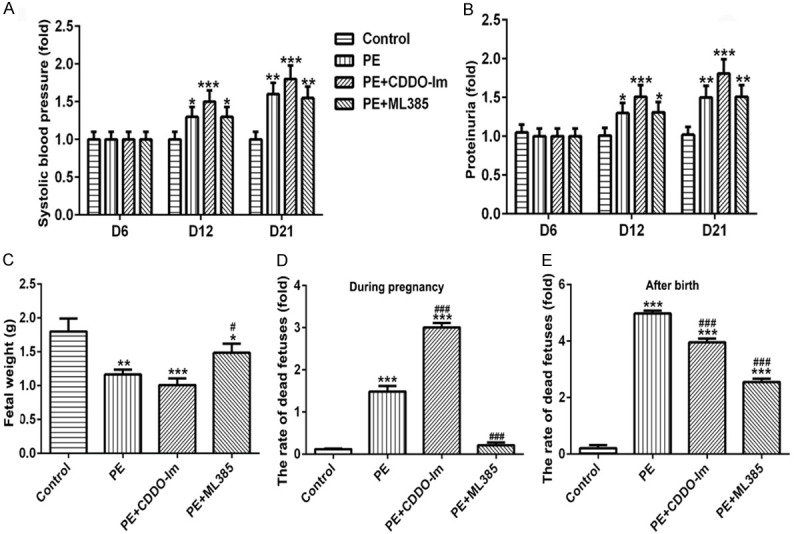
The effects of Nrf-2 on SBP, proteinuria and fetal weight. The level of SBP (A) and 24 h proteinuria (B) in rats of different groups on the day 6, 12 and 21 of pregnancy. The fetal weight (C) of rats in different groups. The rate of dead fetuses of rats during pregnancy (D) and within 7 days after birth (E) in different groups. n=8, *P<0.05, **P<0.01 and ***P<0.001 vs. Control; #P<0.05 and ###P<0.001 vs. PE. SBP, systolic blood pressure; CDDO-Im, Nrf-2 agonist; ML385, Nrf-2 inhibitor.
Effects of Nrf-2 on the changes in placental pathology and HO-1 expression
As shown in Figure 2, HE staining showed that the villous basement membrane was thickened, thrombosis was formed, and hemorrhagic infarction of placenta was observed in the placenta tissue of PE rats. Placental villus cells in the pregnant rats in the control group were evenly distributed with normal cell morphology and smooth tube wall. Additionally, the pathological changes were not obvious with only slight improvement in lumen stenosis after PE rats were treated with CDDO-Im and ML385 (Figure 2A). HO-1 was expressed in the placental tissue and the brown granules presented positive expression. As shown in Figure 2B, in PE group, the expression of HO-1 decreased compared with the control group and increased weakly after CDDO-Im intervention compared with PE group; after treatment with ML385, there was no significant effect on the expression of HO-1. These results from immunohistochemical staining were consistent with the results of Western blot showing in Figure 2C.
Figure 2.
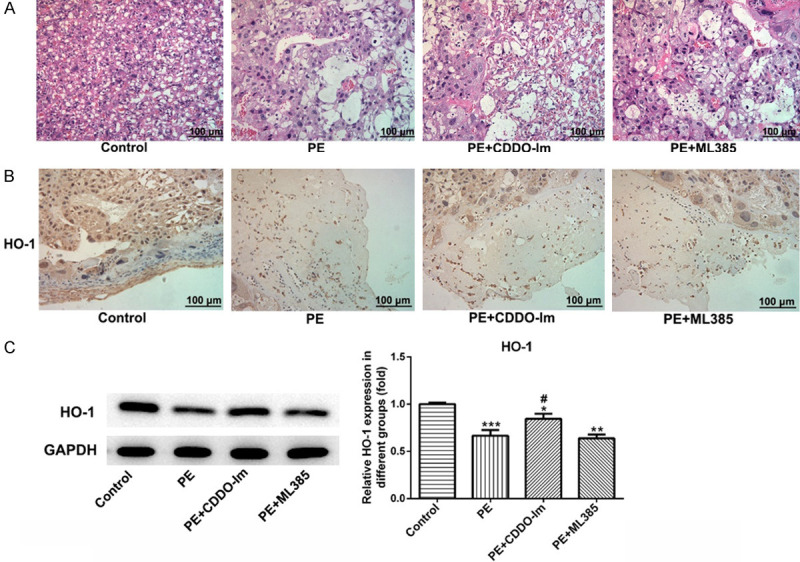
The effect of Nrf-2 on the changes in placental pathology and HO-1 expression. A. Representative photographs of HE-stained placental sections (200 ×). B. Immunohistochemical detection of HO-1 (brow) in the placental tissue in different groups (200 ×). C. The expression of HO-1 in the placental tissue in different groups were examined by western blot. n=8. Scale bar =100 μm. CDDO-Im, Nrf-2 agonist; ML385, Nrf-2 inhibitor.
Levels of CCL2, IL-1β, TNF-α, AT1R and ROS in placental tissue
Western blot results showed that protein levels of CCL2 and TNF-α were decreased with no difference, but IL-1β and AT1R increased markedly in PE rats (Figure 3A). However, CDDO-Im could suppress the expression of CCL2, IL-1β and TNF-α, but ML385 could elevate their expression when compared with PE group (Figure 3A). In addition, treatment with CDDO-Im or ML385 had no effect on AT1R expression in placental tissue of PE rats (Figure 3A). The level of ROS raised significantly in PE rats, and CDDO-Im could reduce the ROS while ML385 administration could upregulate ROS level sharply when compared with PE group (Figure 3B).
Figure 3.
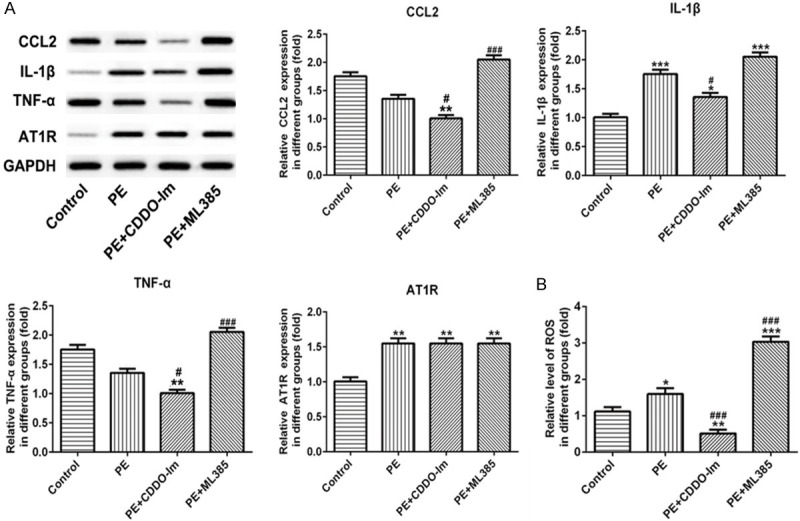
The effect of Nrf-2 on the level of CCL2, IL-1β, TNF-α, AT1R and ROS in placental tissue. A. The expression of CCL2, IL-1β, TNF-α, and AT1R of placental tissue in different groups were examined by western blot. B. The level of ROS in placental tissue was detected using ELISA kits. n=8, *P<0.05, **P<0.01 and ***P<0.001 vs. Control; #P<0.05 and ###p<0.001 vs. PE. ROS, Reactive oxygen species; CDDO-Im, Nrf-2 agonist; ML385, Nrf-2 inhibitor.
Expression of Nrf-2 in human trophoblast cells (HTR-8/SVneo) and human normal endometrial stromal cells (hESC) after transfection
After cells were transfected with siRNA-Nrf-2-1 and siRNA-Nrf-2-2, the mRNA level of Nrf-2 was decreased significantly in both HTR-8/SVneo and hESC cells (Figure 4A and 4B). The results of protein expression from western blot (Figure 4C and 4D) were consistent with the results of RT-PCR analysis. Both at the protein and mRNA levels, the siRNA-Nrf-2-1 plasmid displayed the best transfection effect, which was therefore selected for subsequent experiments.
Figure 4.
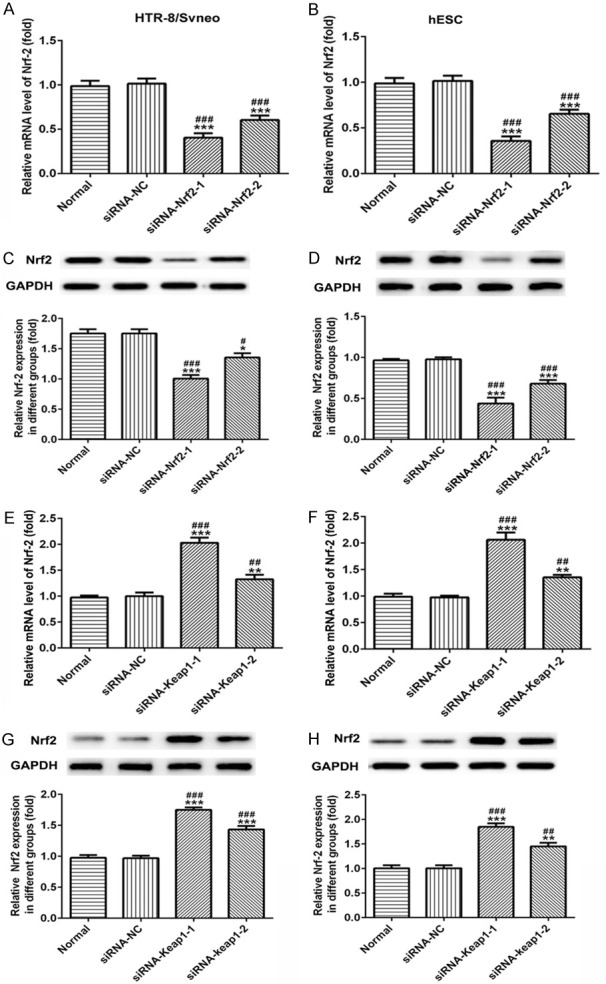
Validation of transfection efficiency of Nrf-2 overexpressed or low-expressed plasmids in human trophoblast cells (HTR-8/SVneo) and human normal endometrial stromal cells (hESC). A, B. The analysis results of RT-qPCR for Nrf-2 in HTR-8/SVneo and hESC cells after transfected with siRNA-Nrf-2-1 and siRNA-Nrf-2-2 plasmids. C, D. Western blot images and quantitative analyses of Nrf-2 in HTR-8/SVneo and hESC cells. E, F. The analysis results of RT-qPCR for Nrf-2 in HTR-8/SVneo and hESC cells after transfected with siRNA-Keap-1 and siRNA-Keap-2 plasmids. G, H. Western blot images and quantitative analyses of Nrf-2 in HTR-8/SVneo and hESC cells. *P<0.05, **P<0.01 and ***P<0.001 vs. Normal; #P<0.05, ##P<0.01 and ###P<0.001 vs. siRNA-NC.
Meanwhile, siRNA-Keap1-1 and siRNA-Keap1-2 (Nrf-2 activator) were transfected into both HTR-8/SVneo and hESC cells, the results from RT-PCR (Figure 4E and 4F) and western blot (Figure 4G and 4H) assays indicated that the mRNA and protein levels of Nrf-2 were significantly elevated when compared with normal cells. The Nrf-2 expression increased most significantly after transfection with siRNA-Keap1-1 plasmid, therefore, it was selected for follow-up experiments.
Effects of Nrf-2 on cell proliferation, apoptosis, and ROS level in HTR-8/SVneo and hESC cells
After cells were predisposed with Angll, siRNA-Nrf-2-1 and siRNA-Keap1-1 were transfected into both HTR-8/SVneo and hESC cells, respectively. MTT assay showed that cell proliferation was decreased significantly in Angll-induced group both in HTR-8/SVneo (Figure 5A) and hESC (Figure 5B) cells. Nrf-2 overexpression could raise cell proliferation, which was inhibited following the knockdown of Nrf-2, when compared with Angll group at 48 h and 72 h (Figure 5A and 5B). Similarly, Nrf-2 overexpression upregulated the expressions of PCNA and Ki67, but Nrf-2 silence suppressed their expression when compared with Angll group both in HTR-8/SVneo (Figure 5C) and hESC (Figure 5D) cells. Treatment with Angll could induce cell apoptosis in HTR-8/SVneo (Figure 6A) cells and hESC cells (Figure 6B). After interfering with siRNA-Nrf-2-1, cell apoptosis was significantly elevated, while overexpression of Nrf-2 significantly reduced cell apoptosis in both HTR-8/SVneo (Figure 6A and 6C) and hESC (Figure 6B and 6E) cells. Additionally, the ROS level was higher in AngII-induced cells than that in normal cells, as well as ROS raised continuously after Nrf-2 was suppressed, while declined when Nrf-2 was overexpressed, both in HTR-8/SVneo (Figure 6D) and hESC cells (Figure 6F).
Figure 5.
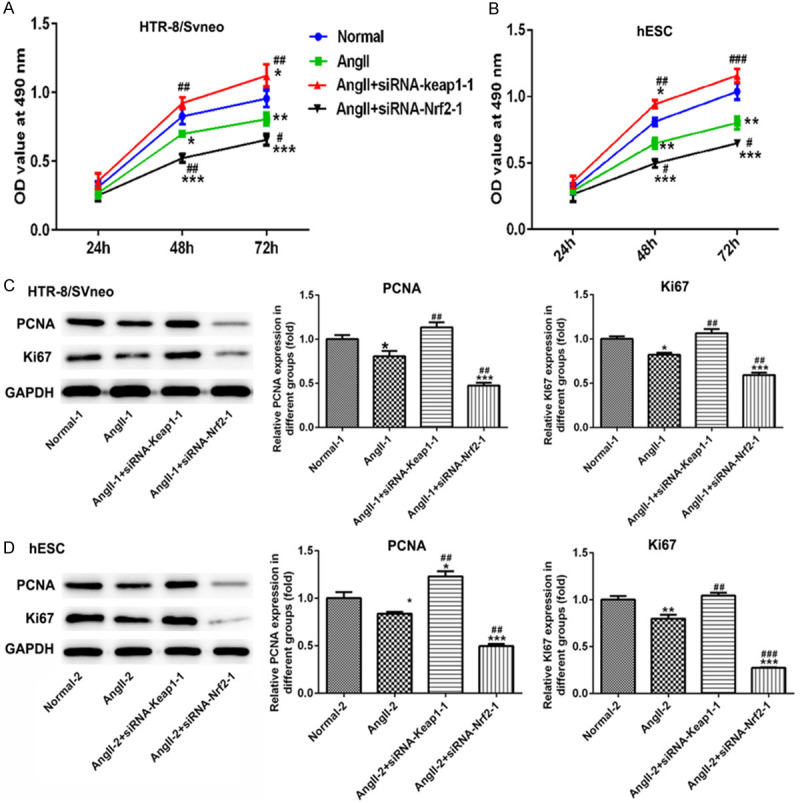
The effect of Nrf-2 on cell proliferation of HTR-8/SVneo and hESC cells. In HTR-8/SVneo cells (A) and hESC cells (B) transfected with siRNA-Nrf-2-1 and siRNA-Keap1-1, a three-day MTT assay was carried out to detect the proliferation rates. (C, D) Western blot images and quantitative analyses of PCNA and Ki67 expressions in HTR-8/SVneo and hESC cells. *P<0.05, **P<0.01 and ***P<0.001 vs. Normal; #P<0.05, ##P<0.01 and ###P<0.001 vs. Angll.
Figure 6.
The effect of Nrf-2 on cell apoptosis and ROS level in HTR-8/SVneo and hESC cells. After staining with Annexin V-FITC and PI, apoptotic cells in HTR-8/SVneo cells (A) and hESC cells (B) transfected with siRNA-Nrf-2-1 and siRNA-Keap1-1 were analyzed using a flow cytometer. Histograms of data statistics of A and B are showed in (C) and (E). The level of ROS in HTR-8/SVneo cells (D) and hESC cells (F) transfected with siRNA-Nrf-2-1 and siRNA-Keap1-1 was detected using ELISA kits. *P<0.05, **P<0.01 and ***P<0.001 vs. Normal; #P<0.05, ##P<0.01 and ###P<0.001 vs. Angll. ROS, Reactive oxygen species.
Effects of Nrf-2 on cell invasion and the expression of related molecules in HTR-8/SVneo and hESC cells
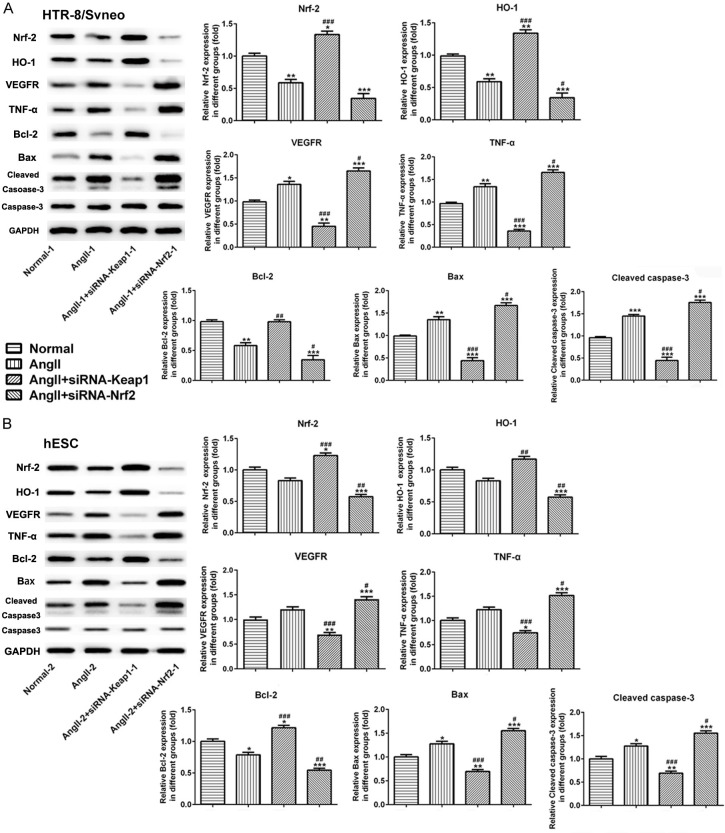
The results from Transwell assay demonstrated that cell migration was increased following Angll treatment both in HTR-8/SVneo and hESC cells. Subsequently, Nrf-2 overexpression suppressed cell migration, which was promoted due to Nrf-2 knockdown (Figure 7A and 7B). We then examined the expression of proteins associated with migration, oxidative stress, angiogenesis, inflammation and apoptosis. As shown in Figure 7C and 7D, data from western blot demonstrated that Angll caused an elevation on the expression of MMP2 and MMP9 both in HTR-8/SVneo and hESC cells. However, MMP2 and MMP9 were reduced significantly in siRNA-Keap1-1-induced groups and were increased markedly in siRNA-Nrf-2-induced group. Meanwhile, in the Figure 8A and 8B, Nrf-2 expression was reduced rapidly in the Angll-induced group comparing to normal cells. Then, siRNA-Keap1-1 (Nrf-2 overexpression) and siRNA-Nrf-2-1 (Nrf-2 interference) plasmids were transfected into the Angll-induced cells, and the results of western blot showed that the transfection effect of these two plasmids was excellent. The expression of HO-1 was lower in the Angll group than that in normal group, which trend was similar to its expression in PE rats. Overexpression of Nrf-2 could reverse the effect of Angll on HO-1 expression to upregulate HO-1 expression significantly, and inhibition of Nrf-2 could enhance the effect of Angll on HO-1 expression to down-regulate HO-1 expression.
Figure 7.
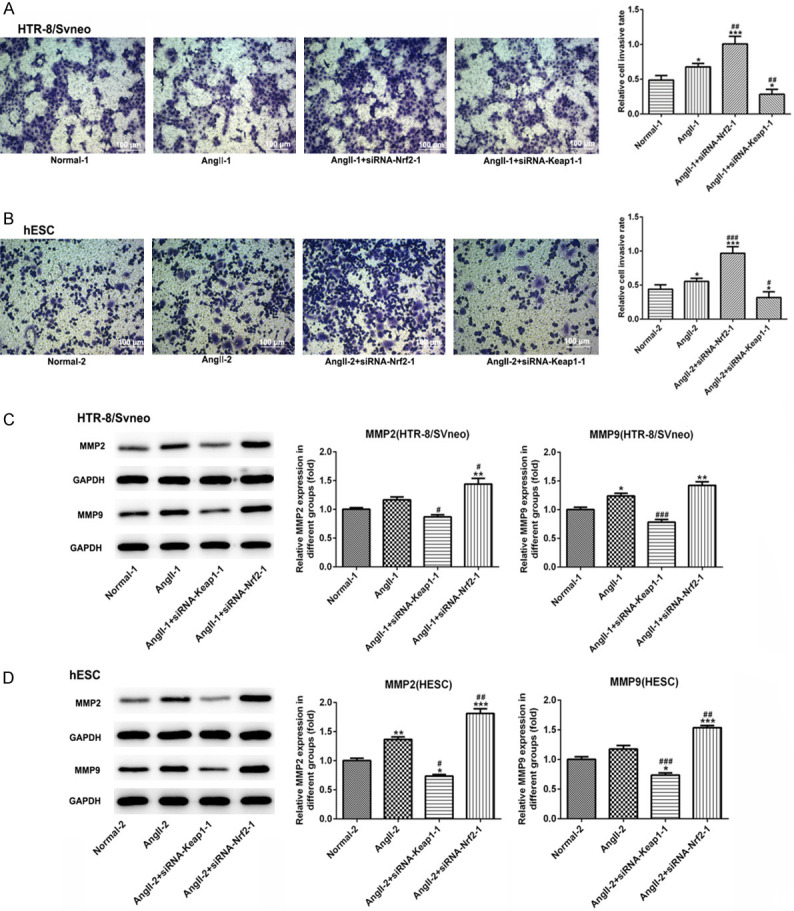
The effect of Nrf-2 on cell invasion and the related proteins in HTR-8/SVneo and hESC cells. Representative images of Transwell membranes (magnification, × 200; stain 0.05% crystal violet) and quantification of migrated cells of HTR-8/SVneo (A) and hESC cells (B) in each group. Scale bar =100 μm. Western blot analysis was performed to detect the expression levels of MMP2 and MMP9, and statistical analysis of relative expression in HTR-8/SVneo (C) and hESC (D) cells in different groups. *P<0.05, **P<0.01 and ***P<0.001 vs. Normal; #P<0.05, ##P<0.01 and ###P<0.001 vs. Angll.
Figure 8.
The effect of Nrf-2 on the expression of related molecules in HTR-8/SVneo and hESC cells. Western blot analysis was performed to detect the expression levels of Nrf-2, HO-1, VEGFR, TNF-α, Bax, Bcl-2 and Cleaved caspase-3 proteins and statistical analysis of relative these proteins expression in HTR-8/SVneo (A) and hESC (B) cells in different groups. *P<0.05, **P<0.01 and ***P<0.001 vs. Normal; #P<0.05, ##P<0.01 and ###P<0.001 vs. Angll.
Meanwhile, Angll treatment induced the upregulation of VEGFR, TNF-α, Bax and Cleaved caspase-3, and suppressed Bcl-2 expression in both HTR-8/SVneo and hESC cells. Overexpression of Nrf-2 inhibited the expression levels of VEGFR, TNF-α, Bax and Cleaved caspase-3, and upregulated Bcl-2 expression significantly. On the contrary, knockdown of Nrf-2 had the opposite effect from overexpression of Nrf-2 (Figure 8A and 8B). These results suggested that Nrf-2 was associated with migration, oxidative stress, angiogenesis, inflammation and apoptosis in cells on both sides of the maternal-fetal interface.
Discussion
Trophoblast function is programmed by elaborate and complicated genomic mechanism and signal pathway. As a placenta-derived disorder, the pathogenesis of PE is considered closely related to the dysfunction of trophoblast, which triggers placental angiogenesis disorder. Several studies had supported an increase in angiotensin II (Angll) receptor type 1 (AT1R) signaling in preeclampsia patients [15,16]. The deficiency of HO-1 results in impaired placental angiogenesis [17]. In the present study, our finding revealed a decrease in HO-1 in PE rats, and in HTR-8/SVneo and hESC cells induced by Angll, which was a vasoactive octapeptide generated by enzymatic reaction cascades [18]. HO-1 is an antioxidant enzyme that protects cells from oxidative stress and may have protective roles against PE [19]. In the vasculature of PE patients, augmented AT1R signaling induced the production of reactive oxygen species (ROS) [20,21], accompanied by an increase in the tumor necrosis factor-α (TNF-α) and proinflammatory cytokines IL-1β in PE [22]. Activated inflammatory cells produced large amounts of chemokines and cytokines, such as CCL-2, which further potentiated inflammation and regulated placental vascularization [23,24]. In PE rats, we found that except the inherent features of PE, the levels of IL-1β, ROS and AT1R in the placental tissues were highly expressed, and there was a slight decrease but no significant difference in the CCL-2 and TNF-α level (Figure 3). Interestingly, the levels of VEGFR, TNF-α, Bax/Bcl-2, Cleaved caspase-3 and ROS were higher in the in HTR-8/SVneo and hESC cells induced by AngII, with a decline in Nrf-2 expression (Figure 8). Previous studies had shown that appropriate amounts of ROS were proangiogenic factors that act through various mechanisms, including the enhancement of VEGF and cell apoptosis [25,26], which facilitated the process of vascular remodeling in PE. However, excess ROS accumulation induced oxidative stress which modified and damaged proteins, lipids, and nucleic acids [27], thereby decreased proliferation and increased apoptosis of other normal trophoblast cells or intimal cells in the placental tissue through other signaling pathways [11].
The activation of Nrf-2 had been reported in preeclampsia [28]. Others had found inactivation of Nrf-2 induced ROS accumulation and angiogenic chemokine expression in PAH placentas, whereas Nrf-2 over-activation impaired angiogenesis and enhanced preeclampsia-mediated adverse outcomes [11]. Nrf-2 had been proven to be beneficial in the angiogenic potential of brain tissues by improving vascular endothelial growth factor (VEGF) [29]. VEGF was one of the most important angiogenesis regulating factors leading to angiogenesis to ensure survival of the embryo and the VEGF receptor (VEGFR) was crucial in the process of angiogenesis and known to regulate endometrial angiogenesis [30,31]. Over the past decade, the Nrf-2-Keap1 pathway had been characterized as an important endogenous mechanism for combating oxidative stress [32]. From above, Nrf-2 could inhibit the production of ROS, reduce the level of oxidative stress, and inhibit apoptosis and chemotaxis in vivo. However, it had been shown that normal vascular recasting required oxidative stress, apoptosis, and chemotaxis during eclampsia recovery. The 2-cyano-3, 12 dioxooleana-1, 9 diene-28-imidazolide (CDDO-Im) also activated the Nrf-2-Keap1 pathway in both in vitro and in vivo models [33] and the Nrf-2 inhibitor (ML385) was used to inhibit the Nrf-2 antioxidant pathway in vivo. The present study demonstrated that overexpression of Nrf-2 induced by CDDO-Im slightly upregulated the level of SBP and urinary protein, indicating that Nrf-2 enrichment was not beneficial to PE rats (Figure 1A and 1B). Unexpectedly, the inhibitor of Nrf-2 induced by ML385 had no effects on SBP level and urinary protein in PE rats (Figure 1). We assumed that the reasons for this phenomenon was due to the Nrf-2 originally expressed little in PE rats, accompanied by a high level of SBP and urine protein at the same time. When the inhibitors of Nrf-2 (ML385) was used to suppress the Nrf-2 expression, the inhibitory effect of ML385 was very small because of Nrf-2 itself expression was low in PE rats. Therefore, Nrf-2 inhibitor had no a significant inhibitory effect to SBP and the level of urine protein in PE rats. The specific molecular mechanism by which Nrf-2 inhibitor had no significant effect on SBP and urinary proteins in PE rats need to be further validated by further experiments.
Interestingly, fetal weight was decreased, while the rate of dead fetuses during pregnancy and 7 days after birth was increased significantly in PE rats. Inhibiting Nrf-2 levels significantly restored fetal weight and reduced the rate of dead fetuses. Astonishingly, overexpression of Nrf-2 enhanced the rate of dead fetuses during pregnancy but reduced the rate of dead fetuses during 7 days after birth (Figure 1), which was not consistent with previous perceptions. These results suggested that the mechanism of Nrf-2’s influence on the rate of dead fetus of pregnant rats with PE during pregnancy and within 7 days after birth was different. There might be a time critical point, and the Nrf-2 might have opposite effects on both sides of this time critical point. Although the inhibitory effect of overexpression of nrf2 on fetal mortality is inconsistent with previous studies, the conclusion that knocking down nrf2 can inhibit fetal mortality remains unchanged, which is consistent with the conclusion of this study. In the other hand, whether Nrf-2 was activated or not had a weak effect on the pathology of placental tissue and the expression of HO-1, just HO-1 was slightly up-regulated after the activation of Nrf-2 (Figure 2). HO-1 and Nrf-2 belonged to the protective proteins, which were involved in combating oxidative stress. Nrf-2 was also an essential upstream transcription factor regulating HO-1. These evidences indicated that the role of Nrf-2 in PE rats during pregnancy was complex.
Based on these observations, we found that HO-1 was decreased, whereas CCL-2, IL-1β, TNF-α and ROS were increased when lacking Nrf-2 (Figure 3). All these tests were performed during placenta formation in the early stage of pregnancy. Additionally, it had been shown that normal vascular recasting required oxidative stress, apoptosis, and chemotaxis during eclampsia recovery. Therefore, deficiency of Nrf-2 could increase oxidative stress, apoptosis and inflammation in the placental tissue by increasing the levels of CCL2, IL-1β, TNF-α, AT1R, ROS, Bax/Bcl-2, Cleaved caspase-3, to support the restoration of the damaged angiogenesis. These results suggested that Nrf-2 knockdown was beneficial to the vascular remodeling process in PE rats in vivo, which could be developed as a key target for the treatment of PE. Subsequently, we continued to culture the human trophoblast cell line (HTR-8/SVneo) and human normal endometrial stromal cell line (hESC) in vitro and transfected different plasmids to knockdown or overexpress Nrf-2 expression. Like experiments in vivo, after the Nrf-2 was interfered by siRNA, cell proliferation was decreased, and cell apoptosis and ROS level in both two cell lines were increased (Figures 5 and 6). The invasion of trophoblast cells were essential steps of normal placentation and successful pregnancy. Inadequate invasion by trophoblast cells might lead to poor perfusion of the placenta or complications such as PE [34]. Additionally, knockdown of Nrf-2 induced cell invasion, apoptosis, inflammation and angiogenesis via upregulating VEGFR, TNF-α, Bax/Bcl-2 and Cleaved caspase-3 expressions both in HTR-8/SVneo and hESC cells (Figure 8). These evidences also confirmed that deficiency of Nrf-2 could promote vascular remodeling by up-regulating appropriate oxidative stress, apoptosis and inflammation in cells on both side of the matinal-fetal interface, thereby alleviating preeclampsia. Subsequent experiments in the future may require intermolecular interactions or reverse suppression of inflammatory, oxidative stress, and apoptosis processes to further find the possible molecular mechanism of Nrf-2.
Although the present study illustrates a series of scientific conclusions, it also had some limitations. One major viewpoint of our study was that inhibition of Nrf-2 might alleviate or prevent PE by upregulating ROS to promote placenta angiogenesis. However, how Nrf-2 in trophoblast affected placenta angiogenesis function and the relevant underlying signaling mechanisms were not sufficiently studied and discussed. Only VEGFR was assessed but there was no evaluation of other molecules and further angiogenesis-supporting functional assay. Importantly, it will be very interesting to further find the possible molecular mechanism via deficiency of Nrf-2 significantly increased the levels of CCL2, IL-1β, TNF-α, AT1R and ROS in the embryonic tissues, as well as the target molecule by knockdown of Nrf-2 induced cell invasion, apoptosis, inflammation and angiogenesis via upregulating VEGFR, TNF-α, Bax/Bcl-2 and Cleaved caspase-3 both in HTR-8/SVneo and hESC cells. These shortcomings also indicate our future research direction.
Conclusions
The main fertilized egg is a hapantigen, and many links in the first trimester require certain immune activity and certain immune tolerance. These two contradictory processes should be kept in a dynamic balance. Furthermore, the present study was presented for the first time that the relationship between Nrf-2 and HO-1 in PE might not be a simple upstream and downstream relationship as often, and their role in different stages of PE might vary significantly, which also provided a theoretical basis for the dialectical treatment of eclampsia. Although excessive oxidative stress and apoptosis could suppress the growth of normal cells in the placenta; herein, we found that inhibition of Nrf-2 could increase oxidative stress, apoptosis and inflammation in the placental tissue, to support the restoration of the damaged angiogenesis, ultimately achieve process for the treatment of PE. In summary, data strongly suggested that deficiency of Nrf-2 increased placental angiogenesis and improved fetal and maternal outcomes. These findings would provide theoretical basis for developing new approaches to treat preeclampsia.
Disclosure of conflict of interest
None.
References
- 1.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012;2:72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia: association with posterior reversible encephalopathy syndrome and stroke. Stroke. 2018;49:524–530. doi: 10.1161/STROKEAHA.117.018416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Rao H, Bai Y, Zhang F, Li Q, Zhuang B, Luo X, Qi H. The role of SATB1 in HTR8/SVneo cells and pathological mechanism of preeclampsia. J Matern Fetal Neonatal Med. 2019;32:2069–2078. doi: 10.1080/14767058.2018.1425387. [DOI] [PubMed] [Google Scholar]
- 6.Wang RY, Liu LH, Liu H, Wu KF, An J, Wang Q, Liu Y, Bai LJ, Qi BM, Qi BL, Zhang L. Nrf2 protects against diabetic dysfunction of endothelial progenitor cells via regulating cell senescence. Int J Mol Med. 2018;42:1327–1340. doi: 10.3892/ijmm.2018.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandran R, Kim T, Mehta SL, Udho E, Chanana V, Cengiz P, Kim H, Kim C, Vemuganti R. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J Cereb Blood Flow Metab. 2018;38:1818–1827. doi: 10.1177/0271678X17738701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Biswas C, Shah N, Muthu M, La P, Fernando AP, Sengupta S, Yang G, Dennery PA. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J Biol Chem. 2014;289:26882–26894. doi: 10.1074/jbc.M114.567685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Zhou J, Ye Y, Liu Q, Wang X, Zhang N, Wang X. Increased heme oxygenase-1 and nuclear factor erythroid 2-related factor-2 in the Placenta have a cooperative action on preeclampsia. Gynecol Obstet Invest. 2016;81:543–551. doi: 10.1159/000451025. [DOI] [PubMed] [Google Scholar]
- 11.Nezu M, Souma T, Yu L, Sekine H, Takahashi N, Wei AZ, Ito S, Fukamizu A, Zsengeller ZK, Nakamura T, Hozawa A, Karumanchi SA, Suzuki N, Yamamoto M. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci Signal. 2017;10:eaam5711. doi: 10.1126/scisignal.aam5711. [DOI] [PubMed] [Google Scholar]
- 12.Shu W, Li H, Gong H, Zhang M, Niu X, Ma Y, Zhang X, Cai W, Yang G, Wei M, Yang N, Li Y. Evaluation of blood vessel injury, oxidative stress and circulating inflammatory factors in an L-NAME-induced preeclampsia-like rat model. Exp Ther Med. 2018;16:585–594. doi: 10.3892/etm.2018.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ijomone OK, Shallie PD, Naicker T. Oligodendrocytes death induced sensorimotor and cognitive deficit in N-nitro-L-arginine methyl rat model of pre-eclampsia. Neurochem Res. 2020;45:902–914. doi: 10.1007/s11064-020-02969-5. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council. Guide for the care and use of laboratory animals. eighth edition. Washington DC: The National Academies Press; 2011. [Google Scholar]
- 15.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, Ozen M, Wong RJ, Stevenson DK. Heme oxygenase-1 in pregnancy and cancer: similarities in cellular invasion, cytoprotection, angiogenesis, and immunomodulation. Front Pharmacol. 2014;5:295. doi: 10.3389/fphar.2014.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall JE. Historical perspective of the renin-angiotensin system. Mol Biotechnol. 2003;24:27–39. doi: 10.1385/MB:24:1:27. [DOI] [PubMed] [Google Scholar]
- 19.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 22.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep. 2007;9:480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 23.Red-Horse K, Drake PM, Fisher SJ. Human pregnancy: the role of chemokine networks at the fetal-maternal interface. Expert Rev Mol Med. 2004;6:1–14. doi: 10.1017/S1462399404007720. [DOI] [PubMed] [Google Scholar]
- 24.Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl) 2013;91:323–328. doi: 10.1007/s00109-013-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 27.Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014;20:709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- 28.Acar N, Soylu H, Edizer I, Ozbey O, Er H, Akkoyunlu G, Gemici B, Ustunel I. Expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and peroxiredoxin 6 (Prdx6) proteins in healthy and pathologic placentas of human and rat. Acta Histochem. 2014;116:1289–1300. doi: 10.1016/j.acthis.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Guo H, Adah D, James PB, Liu Q, Li G, Ahmadu P, Chai L, Wang S, Liu Y, Hu L. Xueshuantong injection (lyophilized) attenuates cerebral ischemia/reperfusion injury by the activation of Nrf2-VEGF pathway. Neurochem Res. 2018;43:1096–1103. doi: 10.1007/s11064-018-2523-x. [DOI] [PubMed] [Google Scholar]
- 30.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 31.Li L, Jiang H, Wei X, Geng D, He M, Du H. Bu Shen Zhu Yun decoction improves endometrial receptivity via VEGFR-2-mediated angiogenesis. Evid Based Complement Alternat Med. 2019;2019:3949824. doi: 10.1155/2019/3949824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reisman SA, Buckley DB, Tanaka Y, Klaassen CD. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol. 2009;236:109–114. doi: 10.1016/j.taap.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 34.Deng Q, Chen Y, Yin N, Shan N, Luo X, Tong C, Zhang H, Baker PN, Liu X, Qi H. N-acetylglucosaminyltransferase V inhibits the invasion of trophoblast cells by attenuating MMP2/9 activity in early human pregnancy. Placenta. 2015;36:1291–1299. doi: 10.1016/j.placenta.2015.08.014. [DOI] [PubMed] [Google Scholar]