Abstract
Random skin flaps have been widely applied in reconstructive and plastic surgery; however, necrosis usually happens due to insufficient blood supply in the ischemic area of flaps. Curcumin (CUR) is a primary bioactive compound of turmeric (Curcuma longa, L.), which has been proven to be effective on anticancer, decreasing oxidative stress and apoptosis through activating autophagy, and promoting angiogenesis in ischemic tissue. Therefore, the potential therapeutic effect of CUR on promoting survival of ischemic random skin flaps and its underlying mechanism associated with autophagy were investigated. After establishment of dorsal random skin flaps, sixty mice were randomly divided into three groups: Control, CUR or CUR+3-methyladenine (3-MA, an autophagy inhibitor). The results showed that CUR increased the viability area and blood flow as well as relieved the edema of skin flaps through promoting angiogenesis, decreasing oxidative stress, and inhibiting apoptosis of the ischemic area. Further study confirmed that CUR activated autophagy in the random skin flaps, and 3-MA effectively reversed the effect on viability, neovascularization, oxidative stress and apoptosis, suggesting autophagy played a vital role in these CUR’s protective effect on random skin flaps. Moreover, this CUR-induced autophagy should be mediated through downregulating the PI3K/AKT/mTOR signaling pathway. Together with secondary response of increased angiogenesis, reduced oxidative stress and apoptosis, CUR effectively improved survival of random skin flaps in vivo. To sum up, our research showed the great potential of CUR using as a promising flap protective therapy for random skin flap survival and regeneration.
Keywords: Curcumin, random skin flaps, angiogenesis, oxidative stress, autophagy
Introduction
Random skin flaps have been well applied in burn and plastic surgery for their flexibility and simplicity to heal the cutaneous defects [1,2]. Unlike perforator flaps, random skin flaps survive by their own blood supply through the subcutaneous plexus [3]. However, random flaps usually suffer ischemic and hypoxic injury because of the shortage of axial vessels at distal parts and their dependence on different microvascular plexuses to meet their metabolic needs [4]. Due to insufficient blood supply, necrosis usually happens, especially at the distal ends of the random skin flaps, resulting in secondary surgery and delayed healing [5,6]. Thus, it is necessary to promote angiogenesis for avoiding necrosis and enhancing the survival of the flaps. Besides, even after neovascularization, the restoration and reperfusion of blood supply may also cause ischemia-reperfusion injury (IRI) in ischemic part of skin flaps [7], resulting in the burst of reactive oxygen species (ROS), destruction of cellular redox homeostasis and severe cell apoptosis [8,9], ultimately the necrosis of random skin flaps. Consequently, a series of strategies including the inhibition of apoptosis, attenuation of oxidative stress and promotion of angiogenesis have been developed to improve the viability of random skin flaps [10,11].
Autophagy is a vital intracellular degradation process for regulating cell signaling, maintaining cellular homeostasis, and promoting cell survival [12]. The induction of autophagy can inhibit the ROS production via the PI3K/AKT/mTOR signaling pathway [13]. Autophagy also promoted angiogenesis in a rat model of deep partial-thickness burns via AMPK/AKT/mTOR signaling [14]. Besides, it has been proven that autophagy attenuated liver IRI [15] and lipopolysaccharide caused apoptosis in murine podocytes [16]. Therefore, these studies suggested that activating autophagy can promote angiogenesis, reduce apoptosis and oxidative stress, together may benefit the survival of skin flaps.
Curcumin (CUR), the major yellow pigment derived from turmeric, is a highly pleiotropic molecule with antioxidant and antiinflammatory biological effects [17,18]. Relevant clinical work has clarified the role of CUR in a variety of diseases, including cardiovascular disease, diabetes, and cancer [19,20]. It was reported that CUR can protect patients against diabetic cardiomyopathy through modulating interactions between autophagy and apoptosis [12]. Meanwhile, CUR can effectively reduce the myocardial IRI through activating the pro-survival kinases including PI3K/Akt pathway [21], and inhibit cell apoptosis and oxidative stress in ameliorating osteoarthritis progression [22]. Although CUR has been shown to inhibit angiogenesis in tumor, interestingly, recent study also reported that CUR promoted neo-angiogenesis in ischemic tissue such as the cutaneous wounds and gastric ulcers [23-25]. However, whether CUR can activate autophagy and promote the survival of the ischemic random skin flaps remains unknown.
Therefore, in this study, it is hypothesized that CUR can promote the survival of random skin flaps through activating autophagy and the following secondary response including angiogenesis, anti-oxidative stress, and anti-apoptosis. The therapeutic effect of CUR was evaluated by investigating its function on the flap survival associated with autophagy and the following response on angiogenesis, oxidative stress, and apoptosis in the random skin flaps.
Materials and methods
Materials and chemicals
CUR (C21H20O6, purity ≥99%) was provided by MedChemExpress (Shanghai, China). 3-methyladenine (3-MA), dimethylsulphoxide (DMSO) and pentobarbital sodium were acquired from Sigma-Aldrich (St. Louis, USA). Primary antibodies against AKT, p-AKT, PI3K, p-PI3K, mTOR, p-mTOR were obtained from Cell Signaling Technologies (product numbers 4691, 4060, 4228, 4257, 2983, 5536; Beverly, MA, USA). The antibodies specific for GAPDH, VEGF, SOD1, VPS34, MMP9, CTSD, HO1, CAPS3 were purchased from Proteintech Group (product numbers 60004-1, 66828-1, 10269-1, 12452-1, 10375-2, 21327-1, 10701-1 and 19677-1; Chicago, IL, USA). The antibodies specific for LC-3, SQSTM1/p62 were purchased from Abcam (ab48394, ab56416; Cambridge, UK). Goat anti-Mouse IgG Secondary Antibody [HRP (Horseradish Peroxidase)] was acquired from Santa Cruz Biotechnology (Dallas, USA). Antifading Mounting Medium with DAPI solution, H&E Staining Kit and DAB developer were provided by Solarbio life Science (Beijing, China). Fluorescein isothiocyanate (FITC)-conjugated IgG secondary antibody and the BCA Kit were purchased from Beyotime Biotechnology (Jiangsu, China). The Electrochemiluminescence (ECL) Plus Reagent Kit was obtained from PerkinElmer Life Sciences (Waltham, USA).
Random skin flap model and CUR administration
Sixty healthy C57BL/6 mice (male, 20-25 g) were obtained from Experimental Animal Facility of Wenzhou Medical University and the animal experiments including operation, treatment, and postoperative care were approved by the Animal Care and Use Committee. All mice were individually kept in a standard experimental cage with free access to food and water at 25°C. Sixty mice were randomly divided into the Control, CUR, and CUR+3-MA groups (n = 20 per group). Before operation, 1% pentobarbital sodium (50 mg·kg-1) was injected into mice for anesthetization. The back of each mouse was shaved and sterilized with 75% ethanol and iodophor solution, and a full-thickness random skin flap (1.5 cm × 4.5 cm) was created. Then, the bilateral sacral arteries providing blood supply of the flap were excised. The flap area was equally divided into the proximal (area I), intermediate (area II), and distal (area III) parts.
CUR was dissolved in a 2% DMSO/corn oil solution and 3-MA was dissolved in phosphate buffered saline (PBS) to achieve a concentration of 5 mg/ml and 3 mg/ml before use, respectively. After surgery, each mouse in CUR group got intraperitoneal injection of CUR (20 mg/kg per day) until the mice were sacrificed. The dosage was determined by a preliminary experiment based on the dosage of curcumin in different animal models in the literature [26,27]. Every time before CUR administration, the CUR+3-MA group received 3-MA (15 mg·kg-1 per day) i.p. injection 30 min before CUR [28]. Control mice were injected with an equivalent dose of DMSO/corn oil solution (vehicle control). All mice were sacrificed with pentobarbital sodium injection after 7 days and flap tissues were harvested.
Macroscopic evaluation of flap survival
The gross healing progress of the skin flaps were evaluated after the surgery. At day 7 post-operation, images of skin flaps were taken to evaluate their macroscopic development and viability by Image-Pro Plus 6.0 software. The percentage of survival area was determined by the following calculation: the survival area × 100%/total area.
Tissue edema measurement
After 7 days of operation, flap edema was measured and calculated by the water content of random flap according to previous studies [29]. Briefly, after mice were euthanized, six samples were collected from each group, followed by weight measurement, and dehydration. All specimens were weighed daily until no weight change can be found in more than two days. The percentage of water content can be calculated as follows: (W0-Wf)/W0 × 100%, whereas W0 and Wf represent for the final and initial weight, respectively.
Laser doppler blood flow (LDBF) analysis
Microvascular network reconstruction in the random skin flap was assessed by LDBF imaging. At day 7 post-operation, all treated mice were anesthetized, and skin flaps were scanned under a laser doppler instrument (MoorLDI-2, Moor Instruments Limited, UK) with the following parameters: 633 nm wavelength, 29 cm distance and scan time of 8 min at (25±5)°C. MoorLDI Review software was used to quantify the results and blood flow intensity was recorded as flux with perfusion unit (PU).
H&E staining
At day 7, six samples (1.5 × 1.5 cm) from flap area II were acquired in each group for histopathologic analysis. Samples were embedded in paraffin for section after fixing with 4% paraformaldehyde for at least 1 day. The 5-μm tissue sections were then obtained and used to perform H&E staining according to the protocol. Images were acquired using a microscope (Olympus Corp., Japan). Six randomly selected fields (200 ×) from each slide were used to assess the microvessels regeneration that can be evaluated by the mean vessel density. Other histological changes including granulation formation and tissue swelling were also analyzed from the images.
Immunohistochemistry
Before performing immunohistochemistry (IHC), tissue sections were deparaffinized in xylene, followed by rehydration treatment in graded ethanol solutions. Then the endogenous peroxidase activity was inhibited by 3% H2O2. After washing, sections underwent antigen retrieval in sodium citrate buffer (10.2 mM, 20 min, 95°C), and were blocked with 10% bovine serum albumin (BSA) solution for 10 min. The primary antibody of CD34 (1:100), VEGF (1:200), cadherin5 (1:200), SOD1 (1:150), or CTSD (1:150) was incubated with slides at 4°C overnight, further treated with HRP-conjugated secondary antibody (1:1000) at room temperature (RT) for 2 h. Immunoreactivity was visualized by staining with a DAB detection kit and counterstained with hematoxylin. Images from at least six random fields were taken using a microscope (Olympus Corp., Japan) for further analysis.
Immunofluorescence staining
The embedded tissue samples were deparaffinized, rehydrated and heat-induced antigen retrieval as above described. After washing, samples were permeabilized by 0.1% Triton X-100 solution for 30 min and blocked in 10% BSA for 1 h. Specimens were incubated with primary antibody against LC3II (1:200) for 2 h at RT, then treated with the anti-rabbit secondary antibody and DAPI staining. Slides were observed and images were obtained under an Olympus microscope for further analysis.
Western blotting
At day 7, flap samples from the intermediate (area II) were harvested and kept at -80°C for Western blotting. Briefly, tissue sample was extracted by lysis buffer, then protein concentration was detected by a BCA Kit. The extracted protein (60 μg) was separated by SDS/PAGE using 12% or 8% polyacrylamide gel. After being elctrotransferred to a PVDF membrane, proteins were blocked by 5% nonfat powdered milk, incubated with primary antibody overnight at 4°C, and then treated with the HRP-conjugated IgG secondary antibody for 2 h at RT. For detection, the membranes were treated with an electrochemiluminescence reagent and bands were then visualized using a ChemiDoc XRS System. Finally, the density of protein band was quantified by an Image Lab 3.0 software (Bio-Rad, USA).
Statistical analysis
The data of at least three individual experiments were expressed as mean ± standard deviation (SD). Comparison between two groups was analyzed by the independent-sample t-test using GraphPad Prism 7.0. Statistical significance was set at *P < 0.05, **P < 0.01 and ***P < 0.001 versus the indicated group.
Results
CUR ameliorated the survival of random skin flaps
After establishment of random skin flaps in mice, all the distal parts of flaps became pale and swollen. The necrosis expanded to the pedicle of the flaps at the early days and then gradually subsided until the postoperative day 7. The area I of both groups survived, while tissue of area III became dark, dry, stiff and spread to area II (Figure 1A). Compared with Control, the flap survival rate in CUR group was significantly improved ((44.36±4.50)% vs (73.17±7.78)%, respectively; Figure 1B). To evaluate the tissue edema, the inner part of the flap was collected. It can be clearly seen that the distal part in Control group showed significantly higher extent of edema with more apparent venous blood stasis than CUR group ((56.38±4.24)% vs (38.89±3.42)%, respectively; Figure 1C and 1D). The microvascular network reconstruction in random skin flaps was then visualized by LDBF. As described in Figure 1E, 1F, the blood flow intensity in CUR group ((209.40±38.41) PU) was significantly stronger than Control group ((340.3±45.64)PU). The blood vessels reconstruction was also evaluated by H&E staining. Compared with control ((19.98±3.62)/mm2), the vessel density of area II exhibited in Figure 1G, 1H was significantly enhanced by the CUR treatment ((61.00±5.20)/mm2). Furthermore, CD34 immunostaining results showed that more positive-stained microvessels can be found in CUR group than Control, with (30.58±4.16)/mm2 and (86.84±4.67)/mm2, respectively (Figure 1I, 1J).
Figure 1.
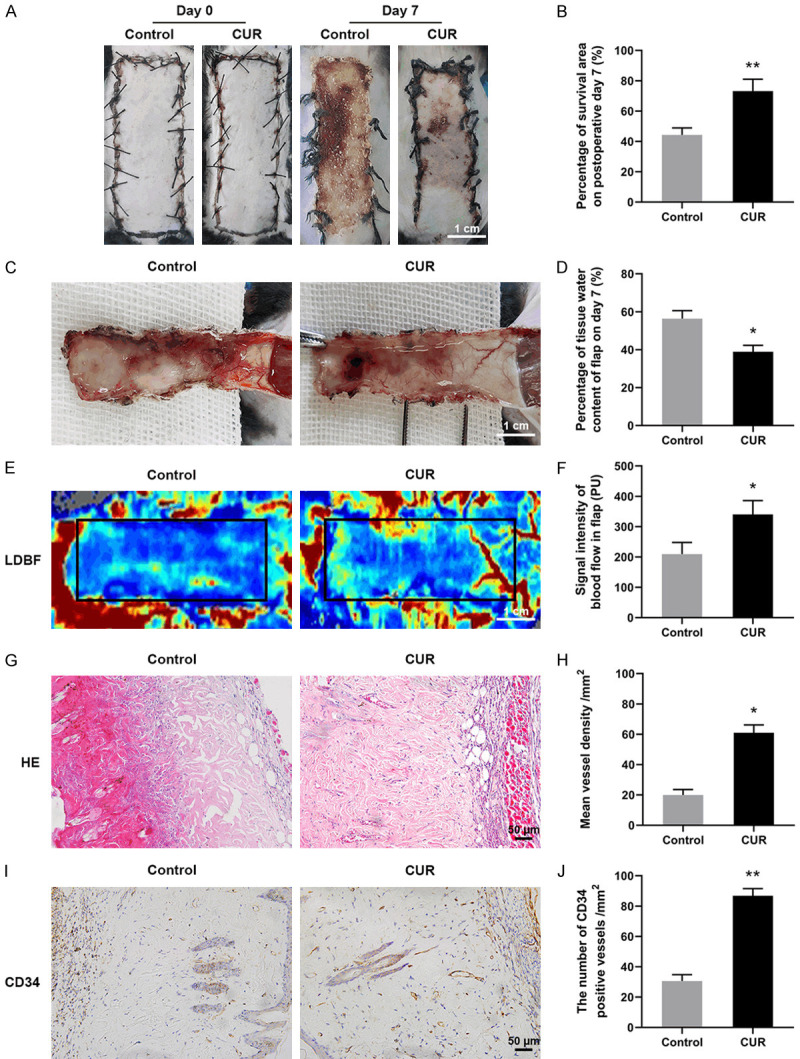
CUR promoted the survival and reduced edema of random skin flaps. A. Representative images of flaps treated with corn oil (Control) and CUR at day 7 (scale bar: 1.0 cm); B. The percentage of survival area at day 7; C. Representative images of the inner surface showing necrosis, edema and vascular network of the flaps (scale bar: 1.0 cm); D. The percentage of tissue water content in each group; E. LDBF photographs of subcutaneous vascular flow and blood supply in Control and CUR groups (scale bar: 1.0 cm); F. Quantitative data of blood flow signal intensity; G. Representative H&E staining images in each group (× 200, scale bar: 50 µm); H. Quantitative data of mean vessel density in the flaps (/mm2); I. Representative CD34 immunostaining images in each group (× 200, scale bar: 50 µm); J. Quantitative data of the density of CD34 positive stained blood vessels (/mm2). Data are mean ± SD, *P < 0.05 and **P < 0.01 vs Control (n = 6 per group).
CUR promoted neovascularization in ischemic area of flaps
Angiogenesis is a critical factor for determining the random skin flaps’ survival. As described above, CUR promoted the microvascular network reconstruction in random skin flaps. Here, the angiogenic markers’ level of the flaps at day 7 were assessed by IHC and western blotting. As exhibited in Figure 2A, 2B, positive VEGF expression was higher in CUR group than Control. Similarly, the expression of Cadherin5 was also increased by CUR treatment (Figure 2C, 2D). Furthermore, the levels of neovascularization related proteins of the ischemic flaps, including MMP9, VEGF and Cadherin5, were also detected (Figure 2E-H). Compared with Control, all the three proteins were significantly increased by CUR treatment. These results suggested that CUR benefited angiogenesis and promoted neovascularization in the random skin flaps.
Figure 2.
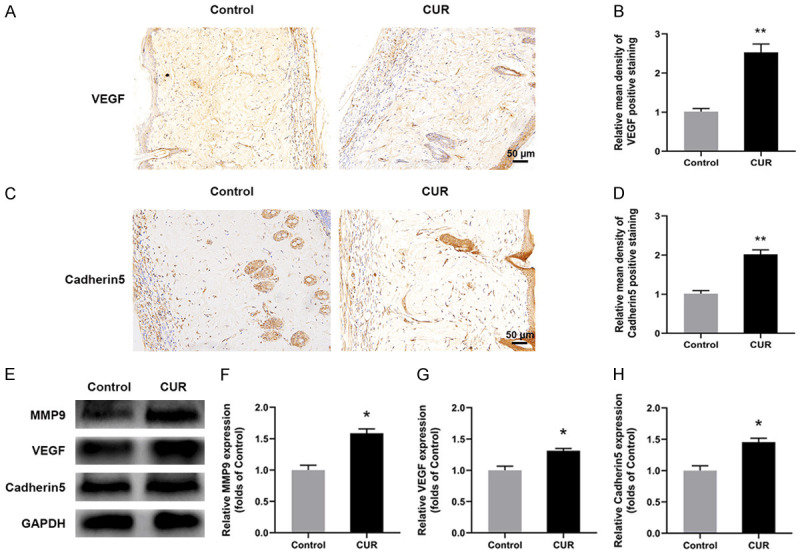
CUR benefited neovascularization of the flaps. A, C. Representative IHC images for VEGF and Cadherin5 expressions in Control and CUR groups at day 7 (× 200, scale bar, 50 µm); B, D. Relative mean density of VEGF and Cadherin5 positive stained tissue; E-H. Western blotting results of MMP9, VEGF and Cadherin5 in Control and CUR groups. Data were expressed as means ± SD, *P < 0.05 and **P < 0.01 vs Control (n = 6 per group).
CUR attenuated oxidative stress in ischemic area of flaps
To confirm whether CUR affected oxidative stress level in flaps, the SOD1 level was evaluate by immunochemical staining. The results in Figure 3A, 3B showed that CUR treatment significantly upregulated SOD1 expression, which can defense against the oxidative stress in area II and help the survival of the flaps. Additionally, compared with control, remarkably higher levels of SOD1, eNOS and HO1 in CUR group were observed through western blotting, indicating that oxidative stress was inhibited in the flaps by the CUR treatment (Figure 3C-F).
Figure 3.
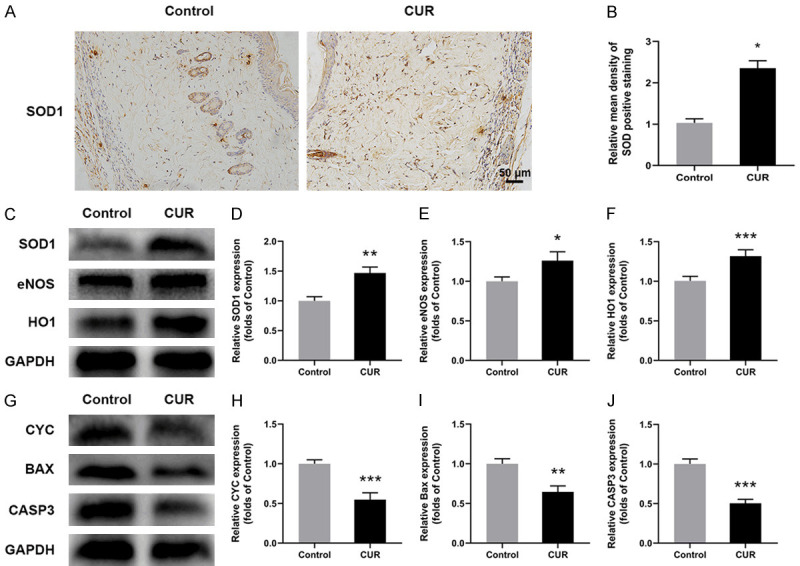
CUR attenuated oxidative stress and apoptosis of the flaps. A. Representative IHC images for SOD expression in the ischemic flaps of Control and CUR groups at day 7 (× 200, scale bar, 50 µm); B. Relative mean density of SOD positive stained tissue; C-F. Western blotting results of SOD1, eNOS and HO in the flaps; G-J. Western blotting results of CYC, Bax and CASP3 expression in Control and CUR groups. Data were expressed as means ± SD, *P < 0.05, **P < 0.01 and ***P < 0.001 vs Control (n = 6 per group).
CUR inhibited apoptosis in ischemic area of flaps
The expression of apoptosis-related proteins of flaps, including CYC, Bax, and CASP3, were evaluated by western blotting. In comparation with Control, CUR group showed significantly lower levels of CYC, Bax, and CASP3 in the skin flaps (Figure 3G-J), which indicated that CUR treatment can inhibit the apoptosis process in vivo and further promote the survival of random skin flaps.
CUR facilitated autophagy through PI3K/AKT/mTOR pathway in random skin flaps
Autophagy indicators including LC3, CTSD, Beclin1, VPS34 and p62 in the skin flaps were also detected by immunostaining or western blotting. The positive staining of autophagosomes’ marker of LC3 in flap area II from CUR group was remarkably higher than Control (Figure 4A, 4B). Compared with control, CTSD level was also effectively elevated by the CUR treatment (Figure 4C, 4D). Furthermore, western blotting analysis confirmed that significantly increased protein expression of Beclin1, LC3II, VPS34 and CTSD and lower expression of p62 were found in CUR group when compared with Control (Figure 4E, 4F, 4H), suggesting autophagy was activated by CUR treatment. Moreover, the role of PI3K/AKT/mTOR signaling in CUR associated autophagy was investigated. Compared with Control, the results in Figure 4G, 4I showed that CUR significantly down-regulated the levels of p-mTOR, p-PI3K, p-AKT. These results indicated that CUR activated autophagy in random skin flaps, which could be modulated by the PI3K/AKT/mTOR signaling pathway.
Figure 4.
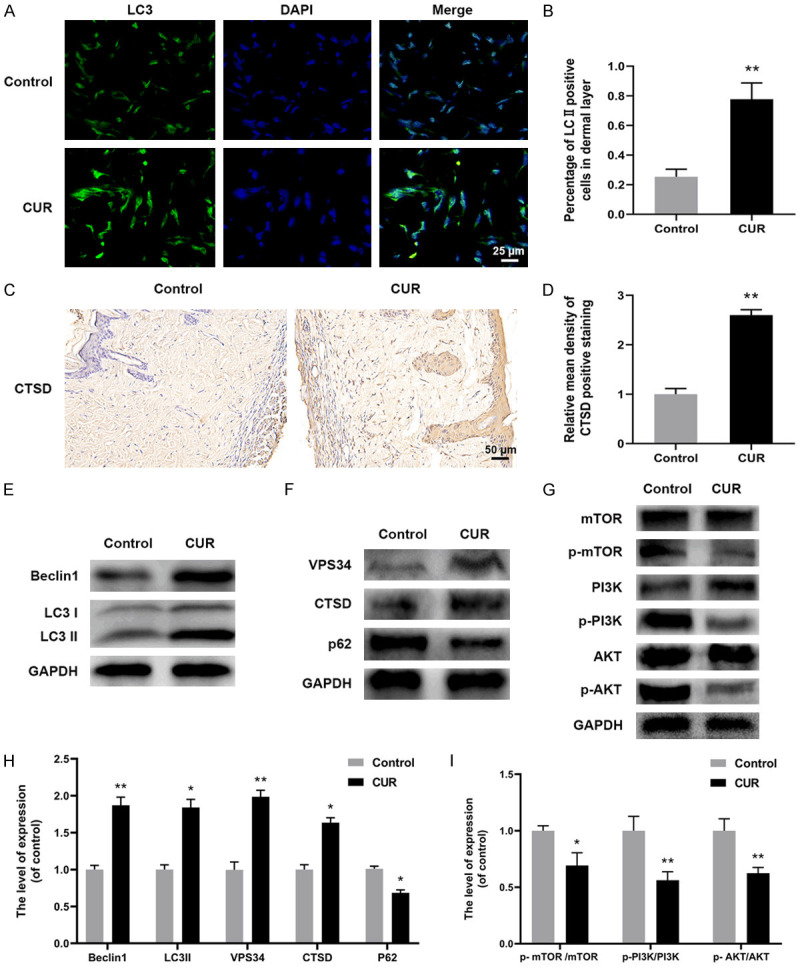
CUR facilitated autophagy and may be medicated by the PI3K/AKT/mTOR pathway in random skin flaps. A, B. Immunostaining results of LC3 showing the autophagosomes (green) in the skin flaps at day 7 (scale bar: 10 µm); C, D. IHC results of CTSD expression in Control and CUR groups (× 200, scale bar: 50 µm); E, F, H. Western blotting results of apoptosis related proteins including LC3, Beclin1, VPS34, CTSD and p62 in two groups; G, I. Western blotting analysis of AKT, p-AKT, PI3K, p-PI3K, mTOR, p-mTOR in two groups. Data were expressed as means ± SD, *P < 0.05 and **P < 0.01 vs Control (n = 6 per group).
Inhibition of autophagy reversed the protective effect of CUR on skin flaps
The autophagy inhibitor 3-MA was applied to investigate the function of autophagy activated by CUR on the survival of flaps. Compared with CUR group, the survival rate significantly decreased in the CUR+3-MA group ((70.89±4.89)% and (42.28±4.17)%, respectively; Figure 5A, 5B). Meanwhile, 3-MA treatment worsened flap edema and subcutaneous venous blood congestion (Figure 5C), resulting in an obviously higher water content than CUR group (Figure 5D). Moreover, the density of subcutaneous blood vessels as well as the blood flow was also decreased by the 3-MA co-administration. The results of H&E staining in Figure 5G, 5H revealed that the vessel density of area II in CUR+3MA group was statistically lower than CUR group. The CD34 immunofluorescence staining results confirmed that the number of vessels was effectively reduced by the 3-MA treatment when compared with CUR group (Figure 5I, 5J). These above results suggested that CUR activated autophagy played a vital part in protecting the viability of the random flaps.
Figure 5.
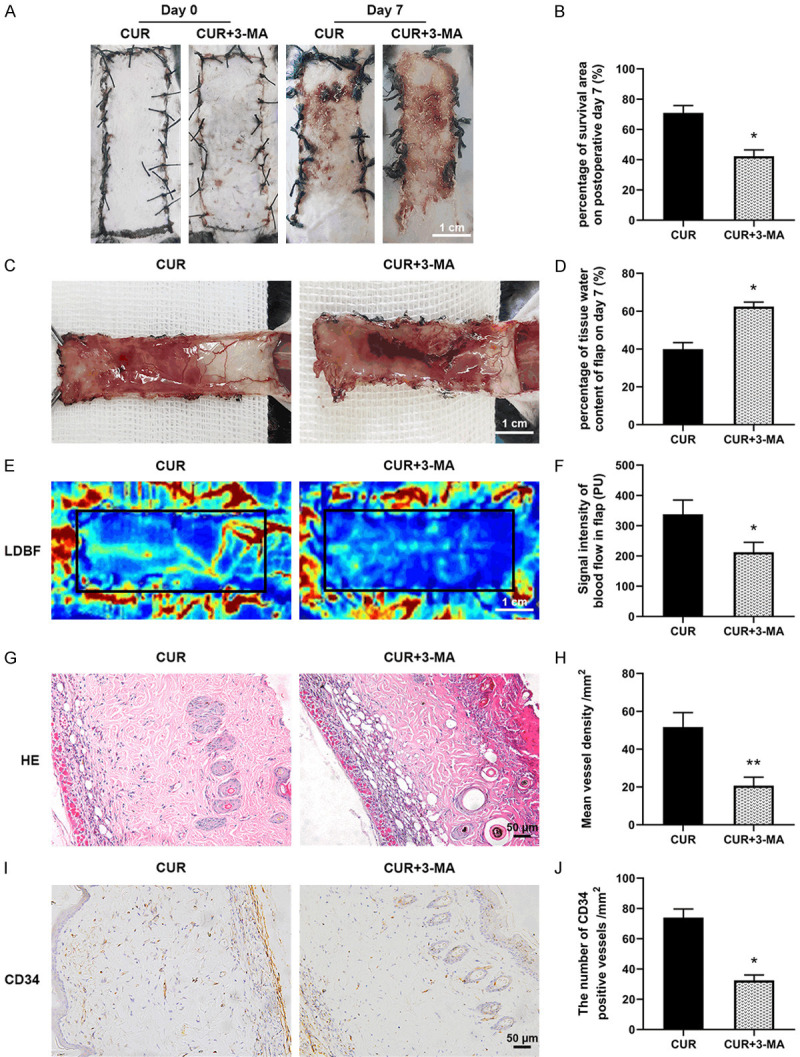
Inhibition of autophagy reversed the protective effect of CUR on skin flaps. A. Representative images of the flaps in control and CUR+3-MA groups at day 7 (scale bar: 1.0 cm); B. The percentage of survival area at day 7; C. Representative images of the inner surface tissue showing necrosis, edema and vascular network of the flaps in the two groups (scale bar: 1.0 cm); D. The percentage of tissue water content in each group; E. LDBF scanned photographs of subcutaneous vascular flow and blood supply in control and CUR+3-MA groups (scale bar: 1.0 cm); F. Quantitative data of blood flow signal intensity of the flaps; G. Representative H&E staining images in each group (× 200, scale bar: 50 µm); H. Quantitative data of mean vessel density in the flaps (/mm2); I. Representative CD34 immunostaining images in each group (× 200, scale bar: 50 µm); J. Quantitative data of the density of CD34 positive stained blood vessels (/mm2). Data are mean ± SD, *P < 0.05 and **P < 0.01 vs Control (n = 6 per group).
Suppression of autophagy reversed the proangiogenic, anti-oxidative stress and anti-apoptosis effect of CUR on skin flaps
To further demonstrate the function of autophagy on the skin flaps survival, the effect of 3-MA combined CUR administration on the angiogenesis, oxidative stress and apoptosis of the random skin flaps were explored. The results of Figure 6A, 6B showed that significant lower ratio of LC3 positive stained cells can be found in CUR+3-MA group than CUR group. In addition, the protein levels of Beclin1, LC3II, VPS34, CTSD and p62 were effectively reversed by the 3-MA treatment (Figure 6C-E). Meanwhile, compared with CUR group, 3-MA treatment significantly downregulated the levels of angiogenic proteins including MMP9, VEGF and Cadherin5 (Figure 6F, 6I). The levels of protective proteins against oxidative stress including SOD1, eNOS and HO1 were also decreased (Figure 6G, 6J), whereas higher expression of CYC, Bax, CASP3 was observed in CUR+3-MA group (Figure 6H, 6K). These data suggested that CUR can activate autophagy in ischemic skin flaps, resulting in faster angiogenesis, lower oxidative stress level and reduced apoptosis, together promote the survival of random skin flaps.
Figure 6.
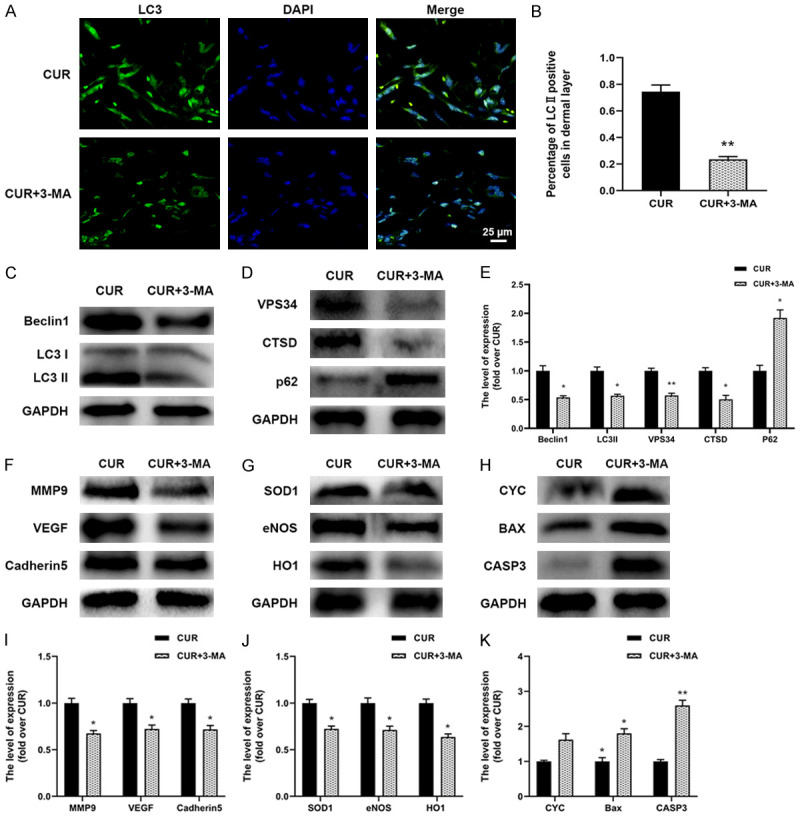
Suppression of autophagy reversed the proangiogenic, anti-oxidative stress and anti-apoptosis effect of CUR on skin flaps. A, B. Immunostaining results of LC3 showing the autophagosomes (green) in CUR and CUR+3-MA groups at day 7 (scale bar: 10 µm); C-E. Western blotting results of autophagy related proteins including LC3, Beclin1, VPS34, CTSD and p62 in the two groups; F, I. Western blotting results of angiogenic proteins including MMP9, VEGF and Cadherin5; G, J. Western blotting results of oxidative stress related proteins including SOD1, eNOS and HO1; H, K. Western blotting results of apoptosis related proteins including CYC, Bax, and CASP3. Data were expressed as means ± SD, *P < 0.05 and **P < 0.01 vs Control (n = 6 per group).
Discussion
Necrosis in distal area is a common complication of flap transplantation in clinic. Although many surgical approaches and drug studies have been conducted to prevent these adverse outcomes, it remains a challenge in the reconstructive and plastic surgery field [1]. As an natural polyphenol herb, CUR has attracted enormous research attention over the past ten years, which shows multiple biological activities including inhibiting oxidative stress and cell apoptosis, protecting the microcirculation and promoting autophagy [12,21,22]. The data in this study displayed that CUR elevated the survival of random skin flaps through promoting angiogenesis, inhibiting oxidative stress and apoptosis in mice, which should be mediated by increasing the autophagy process through regulating the PI3K/AKT/mTOR pathway.
Angiogenesis is one of the determining factors for the survival of transplanted skin flaps as blood vessels provide the necessary nutrients and oxygen for the tissue [30]. Recent studies showed that curcumin, as a potent blocker of NF-κB activation, could link with proliferation, invasion, and angiogenesis as well as suppression of apoptosis in tumor [31,32]. Interestingly, as for wound healing, topical use of curcumin improved impaired cutaneous wound healing through enhancement of angiogenesis via increased expression of VEGF, transforming growth factor β1 (TGF-β1), and hypoxia-inducible growth factor 1α (HIF-1α) [33,34]. Moreover, Previous study showed that CUR loaded hydrogel significantly accelerated wound healing by augmenting the VEGF expression in a full-thickness skin defect model [35], and CUR can also heal gastric ulcers by stimulating angiogenesis via VEGF and MMP-2 mediated signaling [25]. In our study, the effect of CUR on angiogenesis was also evaluated, and results showed CUR improved the blood vessel density in flaps (Figure 1G, 1I). Furthermore, compared with control, blood flow in the skin flaps was also higher in CUR group. The increased surviving area and improved vascular network distribution suggested that CUR is a potent promoter for skin flap viability. Meanwhile, the effect of CUR on modulating angiogenic proteins including VEGF, MMP9 and Cadherin5 was tested. Here, the results showed that with CUR treatment, the expression of these three proteins were all increased in the ischemic flaps (Figure 2). VEGF is a well-known promoter for angiogenesis that can stimulate the mobilization, proliferation and migration of endothelial cells and finally promote blood vessels formation [36,37]. MMP9 can contribute to the angiogenic process via migrating endothelial cell and releasing of sequestered angiogenic factors [38], while Cadherin5 could promote the formation and maturation of neovascularization process [39]. Therefore, based on the above analysis, it can be concluded that CUR promoted the angiogenesis and neovascularization process by upregulating the expression of VEGF, MMP9 and cadherin5, ultimately benefit survival of random skin flaps.
Under condition of partial or total necrosis of the flaps, mitigating oxidative stress and cell apoptosis are key determinants of tissue survival [40]. During ischemic reperfusion such as in the situation of random skin flaps, high concentration or long-time exposure of ROS will damage the cellular molecules including DNA, proteins and lipids, eventually leading to cell necrosis and apoptosis [41]. Antioxidant enzymes, in particularly the superoxide dismutases (SOD), compose the ubiquitous antioxidant defenses against oxidative injury that occurs in various conditions [42]. HO1 and eNOS are important protective genes that can significantly attenuate the damage of ischemic and oxidative stress conditions [43]. In this study, compared with Control, CUR treatment effectively increased the expression of HO1 and eNOS as well as the SOD1 in the ischemic skin flaps (Figure 3C). Therefore, these results showed that CUR is a potent protector against oxidative stress and further promote random skin flaps’ survival.
Balance the apoptosis level is vital for promoting the survival of ischemic skin flaps. Upon apoptotic stimuli, Bax is activated in the outer mitochondrial membrane (MOM) and oligomerized to mediate its permeability, leading to mitochondrial swelling [44]. Then CYC is released from the mitochondria into the intermembrane and activates caspase-3 through a cascade reaction [45], which is a crossing point of two apoptotic pathways and last step of cell death initiation [46]. Previous study has reported that CUR inhibited apoptosis through activating PI3K/Akt/FoxO1 pathway [47]. In this study, the levels of apoptosis related proteins including CYC, Bax, and CASP3 in ischemic area of random skin flaps were significantly reduced by the CUR treatment (Figure 3G), suggesting that CUR effectively inhibited the apoptosis in random skin flaps and can benefit survival of the flaps.
Autophagy is a natural subcellular self-degradative process that is critical for mammalian cell survival [48]. Through degradation of the accumulated proteins and molecules and recycling damaged organelles [49], autophagy showed a positive effect on preventing tissue damage. Activating PI3K/Akt autophagy pathway also positively regulated angiogenesis process in EPCs [50]. It was reported that CUR activated autophagy and protected against diabetic cardiomyopathy [22]. Thus, the role of autophagy in random skin flaps’ survival was evaluated. Once autophagy initiated, the expression of autophagosomal proteins of LC3II, Beclin1, VPS34 and lysosomal related proteins of CTSD increased, while the autophagosomal substrate protein p62 expression that reflects autophagy flux declined (Figure 4A-F) [51,52]. In our present work, increased expression of LC3II, Beclin1, VPS34 and CTSD but a lower level of p62 were detected in CUR group (Figure 4G), which indicated that autophagic flux was promoted by CUR treatment. Previous studies mainly focused on CUR’s anti-cancer function through inhibiting PI3K/Akt/mTOR signaling pathway [53,54]. The present work clearly showed that CUR inhibited the PI3K/Akt/mTOR pathway in the random skin flaps (Figure 4G), together with the change of autophagy markers, it suggested that CUR may facilitate autophagy through PI3K/AKT/mTOR pathway in random skin flaps.
Autophagy generally has a housekeeping function; however, excessive autophagy activity may trigger cell death due to excessive consumption of critical cellular components [55]. To confirm the role of CUR-induced autophagy in the survival of random skin flaps, 3-MA, a class III PI3K inhibitor, was administered together with CUR. Our study demonstrated that the CUR-mediated enhancement of flaps survival was reversed by the 3-MA administration. The 3-MA treatment worsened the edema and reduced blood vessel density in skin flaps (Figure 5C, 5G). Moreover, the expression of angiogenic proteins (VEGF, MMP9 and Cadherin5) was also decreased, which indicated that CUR promoted angiogenesis in random skin flaps through stimulating autophagy. Meanwhile, the upregulated levels of apoptosis markers (Bax, CYC, CASP3) but decreased expression of oxidative stress indicators (SOD, HO-1, eNOS) were also detected in the CUR+3-MA group, indicating that the anti-apoptosis and anti-oxidant stress function of CUR was also achieved by inducing autophagy in the flaps. With the 3-MA treatment, the levels of autophagy indicators (LC3II, Beclin1, VPS34, CTSD and p62) was also reversed. Together with the promoted angiogenesis, anti-oxidative stress and anti-apoptosis effect that may be the secondary response following autophagy, it can be confirmed that autophagy may play a critical part in promoting random skin flaps’ survival.
In summary, the present study found that the CUR treatment can enhance the survival of random skin flaps through promoting the neovascularization, reducing oxidative stress and suppressing apoptosis process, which should be modulated through activating autophagy via PI3K/AKT/mTOR signaling pathway. The present work provided a potent and efficient therapy for the survival of random skin flaps.
Acknowledgements
This study was financially supported by the Natural Science Foundation of China (51802227), Zhejiang Provincial Basic Public Welfare Research program (2017C01054 and LGF18H150008), Medical Health Science and Technology Project of Zhejiang Province (2019KY464), and Wenzhou Municipal Science and Technology Bureau (ZY2019003, Y20190123).
Disclosure of conflict of interest
None.
References
- 1.Park IS, Mondal A, Chung PS, Ahn JC. Prevention of skin flap necrosis by use of adipose-derived stromal cells with light-emitting diode phototherapy. Cytotherapy. 2015;17:283–292. doi: 10.1016/j.jcyt.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Martignago CCS, Tim CR, Assis L, Andrade ALM, Brassolati P, Bossini PS, Leiebano RE, Parizotto NA. Preemptive treatment with photobiomodulation therapy in skin flap viability. J Photochem Photobiol B. 2019;201:111634. doi: 10.1016/j.jphotobiol.2019.111634. [DOI] [PubMed] [Google Scholar]
- 3.Lin R, Lin J, Li S, Ding J, Wu H, Xiang G, Li S, Huang Y, Lin D, Gao W, Kong J, Xu H, Zhou K. Effects of the traditional Chinese medicine baicalein on the viability of random pattern skin flaps in rats. Drug Des Devel Ther. 2018;12:2267–2276. doi: 10.2147/DDDT.S173371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Shaer WM, Ahmed AEE, Sakr WM, Hawas EM, Fathi MZ. Effect of perivascular injection of botulinum toxin type A versus lidocaine in survival of random pattern flaps in a rat model. Plast Reconstr Surg. 2019;143:527e–533e. doi: 10.1097/PRS.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 5.Seyed Jafari SM, Shafighi M, Beltraminelli H, Geiser T, Hunger RE, Gazdhar A. Improvement of flap necrosis in a rat random skin flap model by in vivo electroporation-mediated HGF gene transfer. Plast Reconstr Surg. 2017;139:1116e–1127e. doi: 10.1097/PRS.0000000000003259. [DOI] [PubMed] [Google Scholar]
- 6.Basu G, Downey H, Guo S, Israel A, Asmar A, Hargrave B, Heller R. Prevention of distal flap necrosis in a rat random skin flap model by gene electro transfer delivering VEGF(165) plasmid. J Gene Med. 2014;16:55–65. doi: 10.1002/jgm.2759. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Lin R, Li S, Wu H, Ding J, Xiang G, Li S, Wang Y, Lin D, Gao W, Kong J, Xu H, Zhou K. Protective effects of resveratrol on random-pattern skin flap survival: an experimental study. Am J Transl Res. 2019;11:379–392. [PMC free article] [PubMed] [Google Scholar]
- 8.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 9.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 10.Deheng C, Kailiang Z, Weidong W, Haiming J, Daoliang X, Ningyu C, Huazi X. Salidroside promotes random skin flap survival in rats by enhancing angiogenesis and inhibiting apoptosis. J Reconstr Microsurg. 2016;32:580–586. doi: 10.1055/s-0036-1584205. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Zhou K, Chen H, Li S, Lin D, Zhou D. Calcitriol promotes survival of experimental random pattern flap via activation of autophagy. Am J Transl Res. 2017;9:3642–3653. [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Q, Ke ZQ, Guo S, Yang XS, Zhang FX, Liu XF, Chen X, Chen HG, Ke HY, Liu C. Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J Mol Cell Cardiol. 2018;124:26–34. doi: 10.1016/j.yjmcc.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Han X, Kou J, Zheng Y, Liu Z, Jiang Y, Gao Z, Cong L, Yang L. ROS generated by upconversion nanoparticle-mediated photodynamic therapy induces autophagy via PI3K/AKT/mTOR signaling pathway in M1 peritoneal macrophage. Cell Physiol Biochem. 2019;52:1325–1338. doi: 10.33594/000000093. [DOI] [PubMed] [Google Scholar]
- 14.Liang P, Jiang B, Li Y, Liu Z, Zhang P, Zhang M, Huang X, Xiao X. Autophagy promotes angiogenesis via AMPK/Akt/mTOR signaling during the recovery of heat-denatured endothelial cells. Cell Death Dis. 2018;9:1152. doi: 10.1038/s41419-018-1194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin J, Zhou J, Dai X, Zhou H, Pan X, Wang X, Zhang F, Rao J, Lu L. Short-term starvation attenuates liver ischemia-reperfusion injury (IRI) by Sirt1-autophagy signaling in mice. Am J Transl Res. 2016;8:3364–3375. [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Ma A, Liu K. Geniposide alleviates lipopolysaccharide-caused apoptosis of murine kidney podocytes by activating Ras/Raf/MEK/ERK-mediated cell autophagy. Artif Cells Nanomed Biotechnol. 2019;47:1524–1532. doi: 10.1080/21691401.2019.1601630. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Correa M, Hylind LM, Marrero JH, Zahurak ML, Murray-Stewart T, Casero RA Jr, Montgomery EA, Iacobuzio-Donahue C, Brosens LA, Offerhaus GJ, Umar A, Rodriguez LM, Giardiello FM. Efficacy and safety of curcumin in treatment of intestinal adenomas in patients with familial adenomatous polyposis. Gastroenterology. 2018;155:668–673. doi: 10.1053/j.gastro.2018.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 21.Jeong CW, Yoo KY, Lee SH, Jeong HJ, Lee CS, Kim SJ. Curcumin protects against regional myocardial ischemia/reperfusion injury through activation of RISK/GSK-3β and inhibition of p38 MAPK and JNK. J Cardiovasc Pharmacol Ther. 2012;17:387–394. doi: 10.1177/1074248412438102. [DOI] [PubMed] [Google Scholar]
- 22.Feng K, Ge Y, Chen Z, Li X, Liu Z, Li X, Li H, Tang T, Yang F, Wang X. Curcumin inhibits the PERK-eIF2α-CHOP pathway through promoting SIRT1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid Med Cell Longev. 2019;2019:8574386. doi: 10.1155/2019/8574386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Quiroz J, García-Becerra R, Santos-Cuevas C, Ramírez-Nava GJ, Morales-Guadarrama G, Cárdenas-Ochoa N, Segovia-Mendoza M, Prado-Garcia H, Ordaz-Rosado D, Avila E, Olmos-Ortiz A, López-Cisneros S, Larrea F, Díaz L. Synergistic antitumorigenic activity of calcitriol with curcumin or resveratrol is mediated by angiogenesis inhibition in triple negative breast cancer xenografts. Cancers (Basel) 2019;11:1739. doi: 10.3390/cancers11111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohanty C, Pradhan J. A human epidermal growth factor-curcumin bandage bioconjugate loaded with mesenchymal stem cell for in vivo diabetic wound healing. Mater Sci Eng C Mater Biol Appl. 2020;111:110751. doi: 10.1016/j.msec.2020.110751. [DOI] [PubMed] [Google Scholar]
- 25.Sharma AV, Ganguly K, Paul S, Maulik N, Swarnakar S. Curcumin heals indomethacin-induced gastric ulceration by stimulation of angiogenesis and restitution of collagen fibers via VEGF and MMP-2 mediated signaling. Antioxid Redox Signal. 2012;16:351–362. doi: 10.1089/ars.2011.4232. [DOI] [PubMed] [Google Scholar]
- 26.Huang SJ, Huang J, Yan YB, Qiu J, Tan RQ, Liu Y, Tian Q, Guan L, Niu SS, Zhang Y, Xi Z, Xiang Y, Gong Q. The renoprotective effect of curcumin against cisplatin-induced acute kidney injury in mice: involvement of miR-181a/PTEN axis. Ren Fail. 2020;42:350–357. doi: 10.1080/0886022X.2020.1751658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He XL, Yang L, Wang ZJ, Huang RQ, Zhu RR, Cheng LM. Solid lipid nanoparticles loading with curcumin and dexanabinol to treat major depressive disorder. Neural Regen Res. 2021;16:537–542. doi: 10.4103/1673-5374.293155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Chen H, Zheng Z, Li J, Ding J, Huang Z, Jia C, Shen Z, Bao G, Wu L, Mamun AA, Xu H, Gao W, Zhou K. Trehalose promotes the survival of random-pattern skin flaps by TFEB mediated autophagy enhancement. Cell Death Dis. 2019;10:483. doi: 10.1038/s41419-019-1704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou K, Chen H, Lin J, Xu H, Wu H, Bao G, Li J, Deng X, Shui X, Gao W, Ding J, Xiao J, Xu H. FGF21 augments autophagy in random-pattern skin flaps via AMPK signaling pathways and improves tissue survival. Cell Death Dis. 2019;10:872. doi: 10.1038/s41419-019-2105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun XM, Lang Q, Zhang HB, Cheng LY, Zhang Y, Pan GQ, Zhao X, Yang HL, Zhang YG, Santos HA, Cui WG. Electrospun photocrosslinkable hydrogel fibrous scaffolds for rapid in vivo vascularized skin flap regeneration. Advanced Functional Materials. 2017;27:1–12. [Google Scholar]
- 31.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK, Aggarwal BB, Sood AK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 32.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alibolandi M, Mohammadi M, Taghdisi SM, Abnous K, Ramezani M. Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int J Pharm. 2017;532:466–477. doi: 10.1016/j.ijpharm.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 34.Kant V, Gopal A, Kumar D, Pathak NN, Ram M, Jangir BL, Tandan SK, Kumar D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015;193:978–988. doi: 10.1016/j.jss.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Qu J, Zhao X, Liang Y, Zhang T, Ma PX, Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 37.Bousseau S, Vergori L, Soleti R, Lenaers G, Martinez MC, Andriantsitohaina R. Glycosylation as new pharmacological strategies for diseases associated with excessive angiogenesis. Pharmacol Ther. 2018;191:92–122. doi: 10.1016/j.pharmthera.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Bellafiore M, Battaglia G, Bianco A, Farina F, Palma A, Paoli A. The involvement of MMP-2 and MMP-9 in heart exercise-related angiogenesis. J Transl Med. 2013;11:283. doi: 10.1186/1479-5876-11-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmeliet P, Collen D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann N Y Acad Sci. 2000;902:249–262. doi: 10.1111/j.1749-6632.2000.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Bao G, E AL, Ding J, Li S, Sheng S, Shen Z, Jia Z, Lin C, Zhang C, Lou Z, Xu H, Gao W, Zhou K. Betulinic acid enhances the viability of random-pattern skin flaps by activating autophagy. Front Pharmacol. 2019;10:1017. doi: 10.3389/fphar.2019.01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24:1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonetta R. Potential therapeutic applications of MnSODs and SOD-mimetics. Chemistry. 2018;24:5032–5041. doi: 10.1002/chem.201704561. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Gu X, Yu M, Zi Y, Yu H, Wang Y, Xie Y, Xiang L. Effects of ginsenoside Rb1 on oxidative stress injury in rat spinal cords by regulating the eNOS/Nrf2/HO-1 signaling pathway. Exp Ther Med. 2018;16:1079–1086. doi: 10.3892/etm.2018.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 2018;285:416–431. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 45.Zhuang Z, Lian P, Wu X, Shi B, Zhuang M, Zhou R, Zhao R, Zhao Z, Guo S, Ji Z, Xu K. Abate cytochrome C induced apoptosome to protect donor liver against ischemia reperfusion injury on rat liver transplantation model. Am J Transl Res. 2016;8:1738–1747. [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson A, Francis M, DiPietro LA. Differential apoptosis in mucosal and dermal wound healing. Adv Wound Care (New Rochelle) 2014;3:751–761. doi: 10.1089/wound.2012.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao F, Kang J, Cao Y, Fan S, Yang H, An Y, Pan Y, Tie L, Li X. Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic β-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. Apoptosis. 2015;20:1420–1432. doi: 10.1007/s10495-015-1150-0. [DOI] [PubMed] [Google Scholar]
- 48.He Z, Yang Y, Xing Z, Zuo Z, Wang R, Gu H, Qi F, Yao Z. Intraperitoneal injection of IFN-γ restores microglial autophagy, promotes amyloid-β clearance and improves cognition in APP/PS1 mice. Cell Death Dis. 2020;11:440. doi: 10.1038/s41419-020-2644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 50.Li WD, Zhou DM, Sun LL, Xiao L, Liu Z, Zhou M, Wang WB, Li XQ. LncRNA WTAPP1 promotes migration and angiogenesis of endothelial progenitor cells via MMP1 through MicroRNA 3120 and Akt/PI3K/autophagy pathways. Stem Cells. 2018;36:1863–1874. doi: 10.1002/stem.2904. [DOI] [PubMed] [Google Scholar]
- 51.Miki Y, Shimoyama S, Kon T, Ueno T, Hayakari R, Tanji K, Matsumiya T, Tsushima E, Mori F, Wakabayashi K, Tomiyama M. Alteration of autophagy-related proteins in peripheral blood mononuclear cells of patients with Parkinson’s disease. Neurobiol Aging. 2018;63:33–43. doi: 10.1016/j.neurobiolaging.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Jiang P, Mizushima N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods. 2015;75:13–18. doi: 10.1016/j.ymeth.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Tian B, Zhao Y, Liang T, Ye X, Li Z, Yan D, Fu Q, Li Y. Curcumin inhibits urothelial tumor development by suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway. J Drug Target. 2017;25:626–636. doi: 10.1080/1061186X.2017.1306535. [DOI] [PubMed] [Google Scholar]
- 54.Badr G, Gul HI, Yamali C, Mohamed AAM, Badr BM, Gul M, Abo Markeb A, Abo El-Maali N. Curcumin analogue 1,5-bis(4-hydroxy-3-((4-methylpiperazin-1-yl)methyl)phenyl)penta-1,4-dien-3-one mediates growth arrest and apoptosis by targeting the PI3K/AKT/mTOR and PKC-theta signaling pathways in human breast carcinoma cells. Bioorg Chem. 2018;78:46–57. doi: 10.1016/j.bioorg.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]


