Abstract
Purpose: Currently, there is no favorable treatment plan for inflammatory pain, so exploring new analgesics is still a research hotspot in this area. Cyclin-dependent protein kinase 5 (Cdk5) is a pain-related protein kinase, but its mechanism in inflammatory pain has not been clarified. This research aimed to explore the mechanism of Cdk5-synaptophysin (Syn)-soluble N-ethylmaleimide-sensitivity factor (NSF) attachment protein receptor (SNARE) in acute and chronic inflammatory pain. Methods: Rat models of acute and chronic inflammatory pain were induced by formalin and complete Freund’s adjuvant (CFA), separately, and some rats injected with normal saline through intraplantar injection were divided into a control group. Thirty minutes before modeling, rats were given Cdk5 inhibitor (Roscovitine, Ros), SNARE scavenger (botulinum toxin A, BTTA), glutamate receptor inhibitor (MK801), and dimethyl sulfoxide (DMSO) through spinal canals, and the paw withdrawal threshold (PWT) and thermal withdrawal latency (PWL) at difference time points were compared. Results: Compared with rats in the control group, those in the rat models of acute and chronic inflammatory pain showed lower PWT and PWL, higher Cdk5 enzyme level, tight correlation of Cdk5 with Syn, SNARE, p25 proteins, and higher levels of Cdk5, Syn and SNARE. And the above situation was dramatically reversed under intervention of Ros, BTTA and MK801. Conclusion: Cdk5-Syn-SNARE pathway is a therapeutic target for inflammatory pain. Blocking the activation of this pathway is beneficial to exert analgesic effect.
Keywords: Chronic inflammatory pain, Cdk5, synaptophysin, SNARE
Introduction
Pain is a complex physiological process, mediated by the body’s nociceptors and responded by mechanical and thermal signals. It is also the main basic feature of inflammation [1,2]. Acute and chronic inflammatory pain is the main type of inflammatory pain, causing major public health problems. The current therapy still needs to be upgraded and perfected, because it has not yet solved the problems of complete pain relief and tolerance [3,4]. Therefore, exploring the pathological mechanism of inflammatory pain is still practical for developing new analgesics.
The pathological process of inflammatory pain involves pain signal conduction, nerve transmission and release, and activation of glutamate receptors [5,6]. In this research, we mainly focus on the mechanism of Cdk5-synaptophysin-SNARE pathway in acute and chronic inflammatory pain. Cyclin-dependent protein kinase 5 (Cdk5) belongs to serine/threonine kinase and is responsible for the overall coordination of neuronal cytoskeleton dynamics. Abnormal changes of CDK5 will stimulate neurodegeneration, pain signal transmission and other behaviors [7-9]. Synaptophysin (Syn) is reported to be involved in Cdk5-related thermal hyperalgesia of rats. Activation inhibition of Cdk5-Syn pathway is a new type of sniper point for anti-inflammation and pain relief [10]. It is understood that Syn is a synaptic protein that basically exists at all presynaptic terminals, and its expression in the dorsal root ganglion (DRG) is dramatically positively associated with the severity of neuropathic pain, and it is also correlated with inflammatory state [11-13]. Soluble N-ethylmaleimide-sensitivity factor (NSF) attachment protein receptor (SNARE) and Syn are both related to neurotransmission, responding to neuropathy after traumatic brain injury [14]. SNARE can also coordinate the neurotransmitter homeostasis under pain stimulation and regulate the steady-state in chronic inflammatory pain [15].
Thus, we suspect that Cdk5-Syn-SNARE pathway is involved in the mechanism of acute and chronic inflammatory pain, and it may be a new target for anti-inflammation and pain relief. We verified the correlation mainly through putting Cdk5 inhibitor (Roscovitine, Ros), SNARE scavenger (botulinum toxin type A, BTTA) and glutamate receptor inhibitor (MK801) into the inflammatory pain models of rats.
Material and methods
Animal research
All the animal experimental procedures were approved by the animal research Committee of Hebei General Hospital and were operated in strict accordance with the guidelines. One hundred SD male rats (Junke Bioengineering Co., Ltd., Nanjing, China, J001) were bought, weighing 200-220 g, and they were reared in 12 h-alternation of day and night for 7 days, with natural drink and food.
Establishment and grouping of inflammatory pain models
As mentioned above [16], 30 min before the test, the rats were acclimatized in the plexiglass observation room (10×20×15 cm). Next, 50 μl 2.5% formalin (G-clone Biotechnology Co., Ltd., Beijing, China, RS1920-500 ml) was injected into the left plantars to establish the acute pain models (n=40), and 50 μl physiological saline (Taize Bio (Beijing) Technology Co., Ltd., China, MA0083) was injected into the plantars as the normal group (n=10). Subsequently, they were put back into the observation room. Thirty minutes before model establishment, in the model group, dimethyl sulfoxide (DMSO) (DASF Bio, Nanjing, China, dasf-5310), Ros (100 ug) (Fengce Biotechnology Co., Ltd, Beijing, China, FCBTI02429), BTTA (Institute of Biological Products, Lanzhou, China, S10970037), MK801 (Hanxiang Biotechnology Co., Ltd., Shanghai, China, BCP201706205655) were administered to the spinal canals, and they were divided into DMSO (n=10), Ros (n=10), BTTA (n=10), MK801 (n=10). The time or frequency of licking and biting the injected paw was measured 0-5 min (Phase I) and 10-40 min (Phase II) after formalin injection, and it was considered as an indicator of pain response [17].
As mentioned above [18], a chronic inflammatory pain model (n=40) was established by injecting 100 μl of Freund’s complete adjuvant (CFA) (Chreagen Biotechnology Co., Ltd., Beijing, China, YZ0152) into the left plantars of rats, and normal saline was injected into their plantars (n=10). Other steps and the grouping method of model groups were the same as before.
Behavioral research (PWT, PWL)
The mechanical and thermal hyperalgesia of rat models with chronic inflammatory pain were investigated by recording the paw withdrawal threshold (PWT) and thermal withdrawal latency (PWL). The measurement method was as follows [19]: each rat was measured 3 times, and the average value was taken as the final result. The main recorded time points were 0 h, 1 d, 3 d and 5 d after surgery.
PWT: The rats were placed 30 min in a transparent plastic cage with the bottom of wire mesh. At first, the plantar surface of their left hind paws was pressed for 5-6 s by 2 g von Frey filament. If the rats withdrew their paws or licked their feet, 1.4 g filament was used instead. If they did not have the aforementioned reaction, we employed 4.0 g filament. Among them, 50% withdrawal threshold was determined according to Dixon’s up-down method.
PWL: The rats were placed 30 min in a transparent plastic box and acclimatized. The left paw of each rat was heated and injured by the radiant heat beam from the glass plate. If the rats withdrew their paws or licked their feet, we cut off the radiation heat source and recorded the total irradiation time. If they did not have the above reaction until 30 s, to prevent potential skin injury, the radiant heat source would be automatically cut off and would be recorded as “30 s”.
Western blotting (WB)
The test samples were mainly taken from lumbar enlargement (L4-L5) of DRG ipsilateral segment and spinal cord tissue of rats. The total protein of the sample was extracted by RIPA lysis method, and the concentration was detected by BCA kit (Chreagen Biotechnology Co., Ltd., Beijing, China, 120982). The dilution ratio of all primary antibodies was 1:1000. We used rabbit polyclonal antibody to detect all primary antibodies bought from Xiamen Huijia Biotechnology Co., Ltd. The membrane was rinsed and incubated with horseradish peroxidase labeled goat anti-rabbit secondary antibody. Excess liquid on the membrane was absorbed by filter paper. Afterwards, it developed in a dark room using the enhanced chemiluminescence (ECL). Finally, we analyzed the gray value.
Co-immunoprecipitation
Totally 50 µg protein lysate from L4-L5 was incubated 1 h with 2 µg CDK5 or SYN antibody as control at 4°C. It was mixed well with 40 μl protein G agarose gel (Baiaolaibo Technology Co., Ltd., Beijing, China, WE0270) pre-washed with 1X PBS at 4°C. Being washed with lysis buffer (SenBeiJia Biological Technology Co., Ltd., Nanjing, China, SBJ-0161), the immunoprecipitated and related proteins were analyzed by WB.
Real-time quantitative PCR
Firstly, the total RNA in cells was extracted with Trizol (Baiaolaibo Technology Co., Ltd., Beijing, China, QN2070-ZOG). Then, 5 μg was taken respectively to reverse transcripted cDNA in view of the instructions of reverse transcription kit (Huada Protein Research and Development Center Co., Ltd., Beijing, China, BPI01030), and 1 μL synthesized cDNA was taken for amplification. We employed β-Actin as internal reference, and the data were analyzed via 2-ΔΔct. Thereinto, Cdk5 sense sequence 5’-ATTAGCAGGTTCTGGGGCTT-3’, antisense sequence 5’-AATGGTGACCTCGATCCTGA 3’; SYN sense sequence 5’-CAACACCTCGGTGGTGTTCG-3’, antisense sequence 5’-CCTGAGGCCCGTAGGAATC-3’; SNARE sense sequence 5’-ACCAGTTGGCTGATGAGTCG-3’, antisense sequence 5’-CAAAGTCCTGATACCAGCATCTT-3’; β-Actinsense sequence 5’-CTTACGGCAATCAGGAAAGC-3’; antisense sequence 5’-GACAGACAGCACCTTCAGC-3’.
Statistical analysis
All the data were analyzed and pictures were drawn via GraphPad 6. The comparison between both groups was assessed via independent-samples T test, and that between multiple groups was assessed via one-way analysis of variance (ANOVA) and expressed as F. Post hoc pairwise comparison was analyzed through LSD-t test, multi-time point expression was analyzed through repeated measures ANOVA and expressed as F, and back testing adpoted Bonferroni. There were statistical differences when P < 0.05.
Results
Pain response in acute pain models of rats improves dramatically under the intervention of Ros, BTTA and MK801
In order to investigate the effects of Ros, BTTA and MK801 on the pain response in acute pain models of rats, we recorded their effects on the licking time and biting times of the models in phases I and II. The results showed that the rats in the DMSO group showed longer licking time and more shaking times compared with those in the normal group (P < 0.05), and the licking and biting in the treatment group were dramatically improved under the intervention of the above three (P < 0.05). However, the phase II acute pain models of rats seem to show relatively more licking time and biting times that those of phase I. These results indicate that Ros, BTTA and MK801 can markedly reduce the pain response of acute pain model in rats, and there are effective in both phases I and II (Figure 1).
Figure 1.
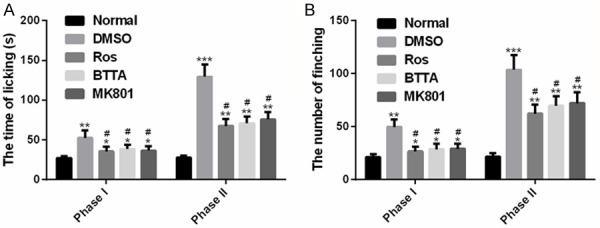
Effects of Ros, BTTA, MK801 on pain response in acute pain models of rats. A. Effects of Ros, BTTA, MK801 on licking time in acute pain models of rats; B. Effects of Ros, BTTA, MK801 on frequency of biting in acute pain models of rats. Note: compared with the normal group, *means P < 0.05, **means P < 0.01, ***means P < 0.001; compared with DMSO, #means P < 0.05.
Changes of Cdk5 enzyme in acute pain models of rats
To investigate the effects of Ros, BTTA and MK801 on Cdk5 enzyme in acute pain models of rats, we detected the changes of protein levels of p-Cdk5 and Cdk5 in phases I and II. The results manifested that the rats in the DMSO group showed a higher level of p-Cdk5 than those in the normal group in phases I and II (P < 0.05), and the p-Cdk5 levels in the treatment group decreased markedly under the intervention of the above three (P < 0.05). Besides, a higher level of p-Cdk5 was found in acute pain models of rats in phase II. These data indicate that Ros, BTTA and MK801 can improve the pain response of acute pain model by down regulating the p-Cdk5 level (Figure 2).
Figure 2.
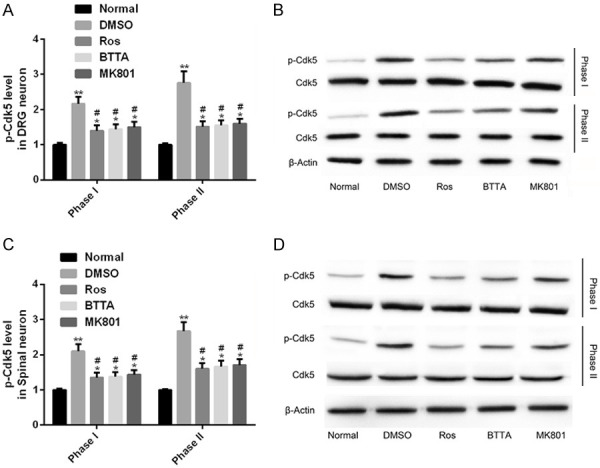
Changes of Cdk5 enzyme in acute pain models of rats. A, B. Effects of Ros, BTTA, MK801 on p-Cdk5 in DRG neurons of acute pain models of rats and its protein map; C, D. Effects of Ros, BTTA, MK801 on p-Cdk5 in spinal cord neurons of acute pain models of rats and its protein map; Note: compared with the normal group, *means P < 0.05, **means P < 0.01; compared with DMSO, #means P < 0.05.
Protein relationship between Cdk5-Syn-SNARE in acute pain models of rats
In order to understand the reasons behind the effects of Ros, BTTA and MK801 on Cdk5 enzyme in acute pain models of rats, we analyzed the potential relationship between Cdk5-Syn-SNARE proteins in the models. The results showed that the levels of Cdk5-SYN, SYN-SNARE and Cdk5-p35 in the DMSO group were dramatically higher than those in the normal group (P < 0.05); and under the intervention of Ros, BTTA and MK801, the level of immunocoprecipitation protein decreased obviously (P < 0.05). There was no remarkable difference in Cdk5-p25 coprecipitation protein levels among the three groups (P > 0.05). Thus, there was interaction between Cdk5 and Syn, Syn and SNARE, Cdk5 and p35 proteins, but there was no obvious interaction between Cdk5 and p25 proteins; the interaction between Cdk5 and Syn, Syn and SNARE, Cdk5 and p35 proteins could be obviously weakened by Ros, BTTA and MK801 (Figure 3).
Figure 3.
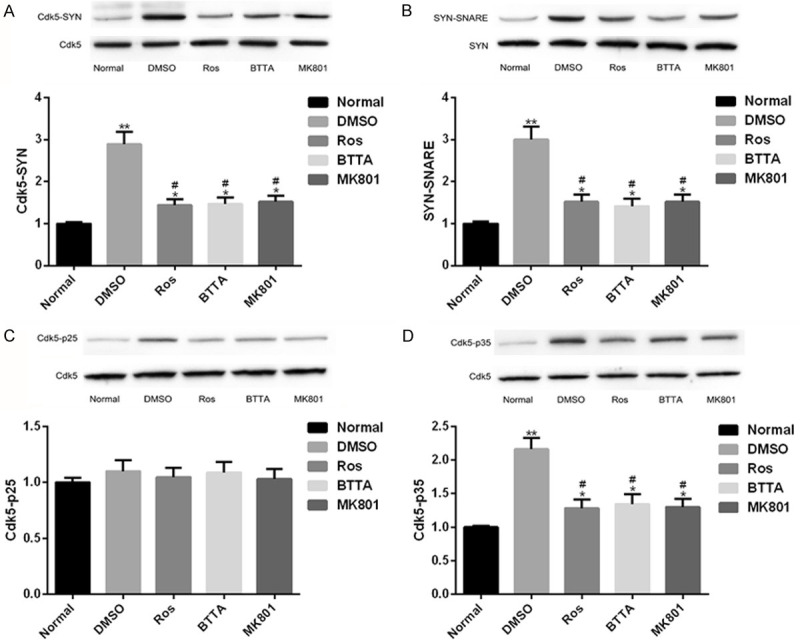
Protein connection between Cdk5-Syn-SNARE in rat acute pain model. A. Close relationship between Cdk5-Syn protein in acute pain models of rats and its protein map; B. Close relationship between SYN-SNARE protein in acute pain models of rats and its protein map; C. Close relationship between Cdk5-p25 protein in acute pain models of rats and its protein map; D. Close relationship between Cdk5-p35 protein in acute pain models of rats and its protein map. Note: compared with the normal group, *means P < 0.05, **means P < 0.01; compared with DMSO, #means P < 0.05.
Cdk5, Syn and SNARE levels are up-regulated in acute pain models of rats
We further detected the Cdk5, Syn and SNARE expression in acute pain models of rats, and found that compared with the normal group, the levels of the three in the DMSO group were dramatically up-regulated (P < 0.05), while Ros, BTTA and MK801 could obviously down-regulate the levels of the three in varying degrees. The above data indicate that the abnormal expression of Cdk5, SYN and SNARE in the rat acute pain model can be reversed from the three to nearly normal levels, which may be used as a potential target for improving acute pain (Figure 4).
Figure 4.
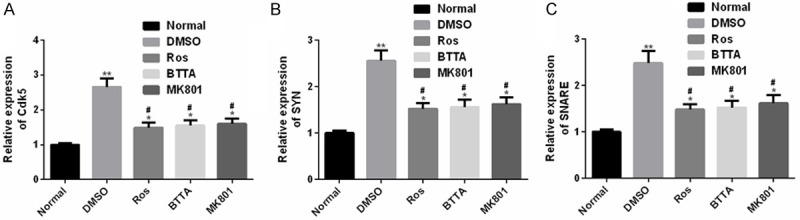
Cdk5, Syn and SNARE levels in acute pain models of rats. A. Cdk5 is dramatically up-regulated in acute pain models of rats; B. SYN is dramatically up-regulated in acute pain models of rats; C. SNARE is dramatically up-regulated in acute pain models of rats. Note: compared with the normal group, *means P < 0.05, **means P < 0.01; compared with DMSO, #means P < 0.05.
PWT and PWL in chronic pain models of rats improve dramatically under the intervention of Ros, BTTA and MK801
We induced the chronic pain models of rats by CFA and conducted similar studies. The results manifested that PWT and PWL in the DMSO group reduced dramatically in 1-5 d compared with the normal group (P < 0.05), while the two increased obviously under the intervention of Ros, BTTA and MK801 (P < 0.05). These data reveal that the three can improve PWT and PWL of chronic pain model in rats (Figure 5).
Figure 5.
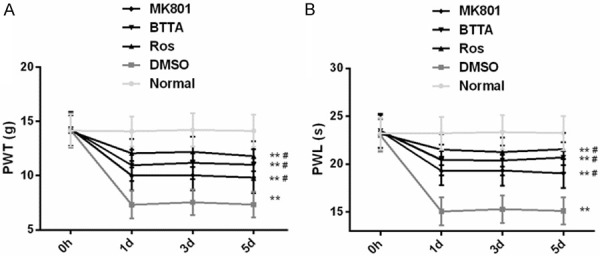
Effects of Ros, BTTA, MK801 on PWT, PWL in chronic pain models of rats. A. Effects of Ros, BTTA, MK801 on PWT in chronic pain models of rats; B. Effects of Ros, BTTA, MK801 on PWL in chronic pain models of rats. Note: compared with the normal group, **means P < 0.01; compared with DMSO, #means P < 0.05.
Changes of Cdk5 enzyme in chronic pain models of rats
Our research found that Cdk5 enzyme also had similar changes in chronic pain models of rats. That was, after one-day CFA induction, the DMSO group showed a higher level of p-Cdk5 than the normal group (P < 0.05), while the p-Cdk5 level recovered obviously under the intervention of Ros, BTTA and MK801 (P < 0.05). These data manifest that the three can reduce the p-Cdk5 level to improve the PWT and PWL of chronic pain model in rats (Figure 6).
Figure 6.
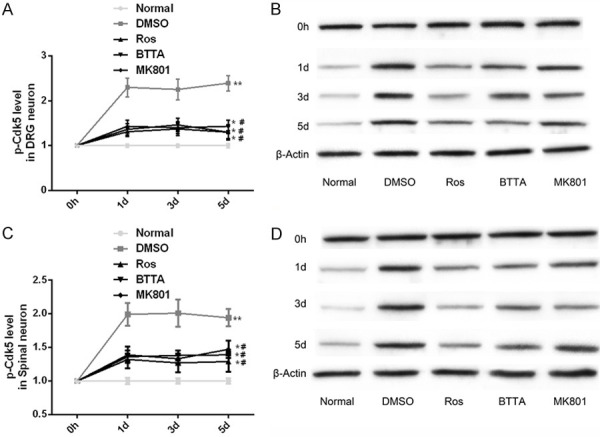
Effects of Ros, BTTA, MK801 on Cdk5 enzyme in chronic pain models of rats. A, B. Effects of ROS, BTTA, MK801 on Cdk5 enzyme in DRG neurons of chronic pain models of rats and its protein map; C, D. Effects of ROS, BTTA, MK801 on Cdk5 enzyme in spinal cord neurons of chronic pain model of rats and its protein map. Note: compared with the normal group, *means P < 0.05, **means P < 0.01; compared with DMSO, #means P < 0.05.
Protein relationship between Cdk5-Syn-SNARE in chronic pain models of rats
Similarly, the protein connection between Cdk5-Syn-SNARE in chronic pain models of rats also showed similar results. Compared with the normal group, the levels of Cdk5-SYN, SYN-SNARE and Cdk5-p35 in the DMSO group increased (P < 0.05), while the levels of the three decreased in the intervention of Ros, BTTA, MK801 (P < 0.05). There was no marked difference in the level of Cdk5-p25 immunoprecipitation protein among the three groups (P > 0.05). Hence, Cdk5 and Syn, Syn and SNARE, Cdk5 and p35 also showed interaction relationship; and this connection was inhibited by Ros, BTTA, MK801, while Cdk5 and p25 had no remarkable connection (Figure 7).
Figure 7.
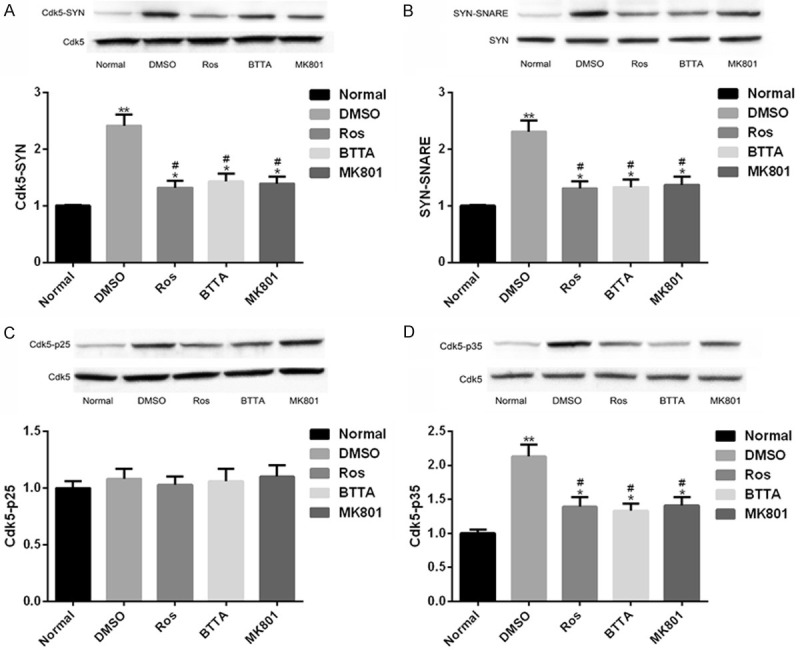
Protein connection between Cdk5-Syn-SNARE in chronic pain models of rats. A. Close interaction relation of Cdk5-Syn protein in chronic pain models of rats and its protein map; B. Close interaction between Syn-SNARE protein in chronic pain models of rats and its protein map; C. Close interaction between Cdk5-p25 protein in chronic pain models of rats and its protein map; D. Close interaction between Cdk5-p35 protein in chronic pain models of rats and its protein map. Note: compared with the normal group, *means P < 0.05, **means P < 0.01; compared with DMSO, #means P < 0.05.
Cdk5, Syn and SNARE levels are up-regulated in chronic pain models of rats
In addition, the Cdk5, Syn and SNARE levels in chronic pain models of rats in the DMSO group were dramatically up-regulated compared with the normal group (P < 0.05), but the above levels were dramatically suppressed under the intervention of Ros, BTTA and MK801 (P < 0.05). And there was still obvious difference between the two groups (P < 0.05). These results signify that Ros, BTTA and MK801 can cut off the mechanism of Cdk5 pain signal transmission by down-regulating the levels of Cdk5, Syn and SNARE in rat chronic pain model, and then relieve chronic pain (Figure 8).
Figure 8.
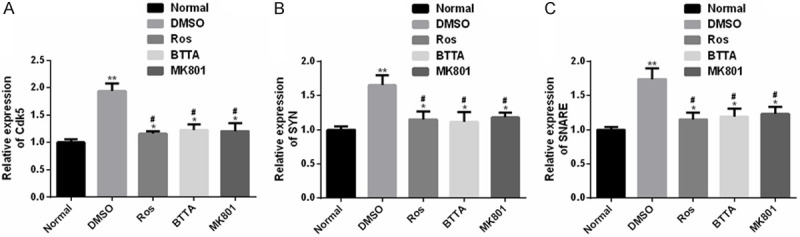
Cdk5, Syn and SNARE expression levels in chronic pain models of rats. A. The Cdk5 expression in chronic pain models of rats is obviously up-regulated; B. The Syn expression in chronic pain models of rats is obviously up-regulated; C. The SNARE expression in chronic pain models of rats is obviously up-regulated. Note: compared with the normal group, *means P < 0.05, **means P < 0.01; compared with DMSO, #means P < 0.05.
Discussion
Acute inflammatory pain is a self-limiting host protection state, while chronic inflammatory pain is a potentially extremely harmful disease manifestation. Acute inflammation that has not been solved promptly or effectively can cause chronic inflammatory pain, which can further develop into more serious neurological diseases, and it may cause great medical burden to individuals and even society [20,21]. Therefore, it is vital to explore the pathological mechanism of inflammatory pain and potential anti-inflammatory and pain-relieving targets.
In this research, we mainly propose that Cdk5-Syn-SNARE pathway may play a key role in acute and chronic inflammatory pain as a pain conduction signal, and Ros, BTTA and MK801 are used for related interventions, which are inhibitors of Cdk5, SNARE and glutamate receptors respectively. We aimed to analyze whether the block of Cdk5-synaptophysin-SNARE pathway affects the pain conduction mechanism of acute and chronic inflammatory pain models, so as to probe into the dual therapeutic targets of the two inflammatory pain models. It is understood that Cdk5, Syn and SNARE all mediate the release of excitatory neurotransmitter glutamic acid directly or indirectly. The mechanism of the three is that Cdk5 adjusts phosphorylated gated voltage calcium channels and different vesicle proteins acting on presynaptic sites, while Syn mediates the formation of SNARE [22-25]. We induced acute inflammatory pain and chronic inflammatory pain models in rats by formalin and CFA respectively. First of all, we tested the pain response or hyperalgesia. The results showed that the acute inflammatory pain model group had more frequent licking or biting after coping with injury stimulation, especially in formalin phase II. However, the above phenomenon was dramatically alleviated under the intervention of the three inhibitors. The chronic inflammatory pain model group showed dramatically lower PWT and PWL in the later stage of injury (1-5 d), and also showed varying degrees of improvement under the action of the three inhibitors. Li and others [26] pointed out that rats in the inflammatory pain models established by ischemia/reperfusion also showed lower PWT and PWL in the late stage of injury. All the above results suggest that the three inhibitors may have analgesic effects, and Cdk5-Syn-SNARE pathway may be involved in the pathological mechanism of two inflammatory pains. At the same time, the pain response time of the two models also showed similar Cdk5 enzyme levels, and the time showed higher levels of Cdk5 enzyme, mainly reflected in the increase of p-Cdk5 in DRG and spinal cord neurons; but under the action of the three inhibitors, this up-regulation was markedly weakened, suggesting that Cdk5 enzyme might respond to pain actively in DRG or spinal cord neurons in acute or chronic inflammatory pain models. Zhang and others [27] reported that p-Cdk5 had also been obviously up-regulated in two inflammatory pain models, which had a positive effect on peripheral inflammatory pain hypersensitivity and is a crucial trigger mechanism for activation of Cdk5 in spinal cord neurons, similar to our results.
To further understand the principle behind the above pain phenomenon, we explored the underlying mechanism of Cdk5-Syn-SNARE pathway in the two inflammatory pain models. Our co-immunoprecipitation results showed that Cdk5-Syn, Syn-SNARE, and Cdk5-p25 proteins were closely linked in both inflammatory pain models, but the interaction between Cdk5-p35 was not obvious, and the above-mentioned link would be weakened remarkably under the influence of the three inhibitors. Tang and others [18] also pointed out that the thermal hyperalgesia of Cdk5 in chronic inflammatory pain was related to p25, but had nothing to do with p35, and Ros had no marked effect on p35. Then, we also found that rats in the model group all had abnormal up-regulated Cdk5, Syn, SNARE, and the inhibitor intervention could dramatically prevent this abnormal disorder. All our above research results point to that Cdk5-Syn-SNARE pathway mediates the mechanism in acute and chronic inflammatory pain. Cdk5 may regulate the movement of vesicles and the release of excitatory neurotransmitter glutamic acid by mediating the interaction of synaptic vesicles protein, thus regulating the pain response of acute inflammatory pain and the mechanical or thermal pain threshold of chronic inflammatory pain. Hence, blocking the transitional activation and coupling of Cdk5 and synaptic vesicles protein will become a new therapeutic target for chronic inflammatory pain.
The innovation of this study lies in: 1. We have confirmed that Cdk5 can mediate inflammatory pain through presynaptic SNARE complex, and further explore the pain signal transmission mechanism of Cdk5. 2. We have proved that Cdk5-synaptophysin-SNARE pathway is a dual therapeutic target for acute and chronic inflammatory pain models. Blocking this pathway can inhibit inflammatory pain signal transmission and relieve inflammatory pain. Although we have confirmed the potential of Cdk5-Syn-SNARE pathway in anti-inflammatory and analgesic, there is still some room for improvement. In the first place, we can increase the research on the effect of this pathway on glutamate release from synaptic space, further confirming our guess. In the second place, we can also supplement its effect on electrophysiological responses of the two models, which is helpful to understand the underlying mechanism of this pathway in depth. We will further improve our research from the above two points.
To make a long story short, we first propose that blocking Cdk5-Syn-SNARE pathway is beneficial to treat acute and chronic inflammatory pain, and we clarify the mechanism of this pathway in acute and chronic inflammatory pain, thereby providing a new direction for the development of anti-inflammatory and analgesic preparations in inflammatory pain.
Conclusion
To make a long story short, we first propose that blocking Cdk5-Syn-SNARE pathway is beneficial to treat acute and chronic inflammatory pain, and we clarify the mechanism of this pathway in acute and chronic inflammatory pain, thereby providing a new direction for the development of anti-inflammatory and analgesic preparations in inflammatory pain.
Disclosure of conflict of interest
None.
References
- 1.Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39:240–255. doi: 10.1016/j.it.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest. 2018;128:3568–3582. doi: 10.1172/JCI99888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afridi R, Khan AU, Khalid S, Shal B, Rasheed H, Ullah MZ, Shehzad O, Kim YS, Khan S. Anti-hyperalgesic properties of a flavanone derivative Poncirin in acute and chronic inflammatory pain models in mice. BMC Pharmacol Toxicol. 2019;20:57. doi: 10.1186/s40360-019-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Ahmad N, Subhan F, Islam NU, Shahid M, Rahman FU, Fawad K. A novel pregabalin functionalized salicylaldehyde derivative afforded prospective pain, inflammation, and pyrexia alleviating propensities. Arch Pharm (Weinheim) 2017;350 doi: 10.1002/ardp.201600365. [DOI] [PubMed] [Google Scholar]
- 5.Liao HY, Hsieh CL, Huang CP, Lin YW. Electroacupuncture attenuates induction of inflammatory pain by regulating opioid and adenosine pathways in mice. Sci Rep. 2017;7:15679. doi: 10.1038/s41598-017-16031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zussy C, Gomez-Santacana X, Rovira X, De Bundel D, Ferrazzo S, Bosch D, Asede D, Malhaire F, Acher F, Giraldo J, Valjent E, Ehrlich I, Ferraguti F, Pin JP, Llebaria A, Goudet C. Dynamic modulation of inflammatory pain-related affective and sensory symptoms by optical control of amygdala metabotropic glutamate receptor 4. Mol Psychiatry. 2018;23:509–520. doi: 10.1038/mp.2016.223. [DOI] [PubMed] [Google Scholar]
- 7.Chernov AV, Remacle AG, Hullugundi SK, Cieplak P, Angert M, Dolkas J, Shubayev VI, Strongin AY. Amino acid sequence conservation of the algesic fragment of myelin basic protein is required for its interaction with CDK5 and function in pain. FEBS J. 2018;285:3485–3502. doi: 10.1111/febs.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhardt L, Kordes S, Reinhardt P, Glatza M, Baumann M, Drexler HCA, Menninger S, Zischinsky G, Eickhoff J, Fröb C, Bhattarai P, Arulmozhivarman G, Marrone L, Janosch A, Adachi K, Stehling M, Anderson EN, Abo-Rady M, Bickle M, Pandey UB, Reimer MM, Kizil C, Schöler HR, Nussbaumer P, Klebl B, Sterneckert JL. Dual inhibition of GSK3beta and CDK5 protects the cytoskeleton of neurons from neuroinflammatory-mediated degeneration in vitro and in vivo. Stem Cell Reports. 2019;12:502–517. doi: 10.1016/j.stemcr.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moutal A, Luo S, Largent-Milnes TM, Vanderah TW, Khanna R. Cdk5-mediated CRMP2 phosphorylation is necessary and sufficient for peripheral neuropathic pain. Neurobiol Pain. 2019;5:100022. doi: 10.1016/j.ynpai.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang HH, Zhang XQ, Wang WY, Xue QS, Lu H, Huang JL, Gui T, Yu BW. Increased synaptophysin is involved in inflammation-induced heat hyperalgesia mediated by cyclin-dependent kinase 5 in rats. PLoS One. 2012;7:e46666. doi: 10.1371/journal.pone.0046666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung J, Franklin JF, Lee HJ. Central expression of synaptophysin and synaptoporin in nociceptive afferent subtypes in the dorsal horn. Sci Rep. 2019;9:4273. doi: 10.1038/s41598-019-40967-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang CY, Sheu ML, Cheng FC, Chen CJ, Su HL, Sheehan J, Pan HC. Comprehensive analysis of neurobehavior associated with histomorphological alterations in a chronic constrictive nerve injury model through use of the CatWalk XT system. J Neurosurg. 2014;120:250–262. doi: 10.3171/2013.9.JNS13353. [DOI] [PubMed] [Google Scholar]
- 13.Chiang CY, Liu SA, Sheu ML, Chen FC, Chen CJ, Su HL, Pan HC. Feasibility of human amniotic fluid derived stem cells in alleviation of neuropathic pain in chronic constrictive injury nerve model. PLoS One. 2016;11:e0159482. doi: 10.1371/journal.pone.0159482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson SW, Yan H, Dixon CE. Lithium increases hippocampal SNARE protein abundance after traumatic brain injury. Exp Neurol. 2017;289:55–63. doi: 10.1016/j.expneurol.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi C, Guo B, Ren K, Yao H, Wang M, Sun T, Cai G, Liu H, Li R, Luo C, Wang W, Wu S. Chronic inflammatory pain decreases the glutamate vesicles in presynaptic terminals of the nucleus accumbens. Mol Pain. 2018;14:1744806918781259. doi: 10.1177/1744806918781259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Q, Sun L, Wang XM, Lo ACY, Wong KL, Gu P, Wong SCS, Cheung CW. Propofol produces preventive analgesia via GluN2B-containing NMDA receptor/ERK1/2 signaling pathway in a rat model of inflammatory pain. Mol Pain. 2017;13:1744806917737462. doi: 10.1177/1744806917737462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Zhang W, Zhou J, Li M, Zhong F, Zhang Y, Liu Y, Wang Y. Involvement of endoplasmic reticulum stress in formalin-induced pain is attenuated by 4-phenylbutyric acid. J Pain Res. 2017;10:653–662. doi: 10.2147/JPR.S125805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Y, Peng Z, Tao S, Sun J, Wang W, Guo X, Liu G, Luo X, Chen Y, Shen Y, Ma H, Xu P, Li Q, Zhang H, Feng Z. VGLUT2/Cdk5/p25 signaling pathway contributed to inflammatory pain by complete Freund’s adjuvant. Pain Res Manag. 2020;2020:4807674. doi: 10.1155/2020/4807674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YR, Xu H, Tao M, Xu LH, Fu XC. Ligustilide relieves complete Freund’s adjuvant-induced mechanical hyperalgesia through inhibiting the activation of spinal c-Jun N-terminal kinase/c-Jun pathway in rats. Pharmacogn Mag. 2017;13:634–638. doi: 10.4103/pm.pm_546_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang SM, Chung G, Kim YH, Park CK. The role of maresins in inflammatory pain: function of macrophages in wound regeneration. Int J Mol Sci. 2019;20:5849. doi: 10.3390/ijms20235849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren J, Liu N, Sun N, Zhang K, Yu L. Mesenchymal stem cells and their exosomes: promising therapeutics for chronic pain. Curr Stem Cell Res Ther. 2019;14:644–653. doi: 10.2174/1574888X14666190912162504. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Ryan TA. CDK5 serves as a major control point in neurotransmitter release. Neuron. 2010;67:797–809. doi: 10.1016/j.neuron.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomizawa K, Ohta J, Matsushita M, Moriwaki A, Li ST, Takei K, Matsui H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J Neurosci. 2002;22:2590–2597. doi: 10.1523/JNEUROSCI.22-07-02590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin RC, Scheller RH. Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- 26.Li XQ, Zhang ZL, Tan WF, Sun XJ, Ma H. Down-regulation of CXCL12/CXCR4 expression alleviates ischemia-reperfusion-induced inflammatory pain via inhibiting glial TLR4 activation in the spinal cord. PLoS One. 2016;11:e0163807. doi: 10.1371/journal.pone.0163807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Zhang H, Shao H, Xue Q, Yu B. ERK MAP kinase activation in spinal cord regulates phosphorylation of Cdk5 at serine 159 and contributes to peripheral inflammation induced pain/hypersensitivity. PLoS One. 2014;9:e87788. doi: 10.1371/journal.pone.0087788. [DOI] [PMC free article] [PubMed] [Google Scholar]


