Abstract
Objective: To investigate the efficacy of ibandronate sodium and zoledronate sodium in the treatment of senile osteoporosis and their impact on quality of life, and to analyze the cost-effectiveness. Methods: A retrospective study was conducted on 215 patients with senile osteoporosis, who were admitted to our hospital from January 2017 to June 2019. Among them, 115 cases treated with ibandronate sodium were set to group A and 100 cases treated with zoledronate sodium were set to group B. The clinical efficacy, bone mineral density (BMD) before and after treatment, bone metabolic markers (alkaline phosphatase (ALP), blood phosphorus (P), blood calcium ion (Ca2+)), quality of life, adverse reactions, cost-effectiveness indicators (length of hospitalization, cost) and complications were compared between the two groups. Results: Total therapeutic response rate in group A was 96.52% (111/115), which was not statistically different from that of 93.00% (93/100) in group B (P=0.242). After treatment, the BMD, ALP, BGP and Ca2+ levels of the lumbar spine L1-L4, left femoral neck and right femoral neck, as well as quality of life scores in the two groups increased (P < 0.05), while serum ALP levels decreased (P < 0.05), however, there was no statistically significant difference between the two groups (P > 0.05). The incidence of adverse reactions in group A was 3.48% (4/115), which showed no statistical significance with that of 5.00% (5/100) in group B (P=0.830). The length of hospitalization, annual treatment expense, medical insurance expense and out-of-pocket payments in group A were all lower than those in group B (P < 0.05). Conclusion: In the treatment of senile osteoporosis, the efficacy and adverse reactions of ibandronate sodium and zoledronate sodium are similar, both of them can effectively improve the quality of life. However, the cost-effectiveness of ibandronate sodium is better than that of zoledronate sodium.
Keywords: Ibandronate sodium, zoledronate sodium, senile osteoporosis, quality of life, cost-effectiveness, clinical efficacy
Introduction
As the main cause of fracture, osteoporosis is a common systemic metabolic disease in clinical practice, which occurs mostly in elderly men and postmenopausal women [1]. The clinical manifestations of patients with osteoporosis are mainly fatigue, pain, spinal deformation, fractures, etc. The fracture caused by osteoporosis is of great harm, and it is one of the main causes of disability and death in elderly patients and seriously affects patient’s quality of life [2]. The pathogeneses of osteoporosis are complex, which are mainly related to estrogen, physical, genetic, nutritional status, etc. [3]. At present, clinical treatment of osteoporosis is mainly calcium supplementation, which can effectively maintain the stability of the bone internal environment. However, some patients have poor prognosis [4]. Therefore, how to improve the prognosis of patients with osteoporosis is still a challenge in its clinical treatment.
In recent years, with the continuous improvement of medical level, bisphosphonates have been widely used in the clinical treatment of osteoporosis. Bisphosphonates have great advantages, which can effectively inhibit bone destruction and bone resorption, promote patient’s physical recovery [5]. Both ibandronate sodium and zoledronate sodium are the representative drugs of third-generation bisphosphonates. Related research showed that the anti-bone resorption capacity of the third-generation bisphosphonates is 2000 times that of the second-generation bisphosphonates, and the curative effect is significantly better than that of the second-generation bisphosphonates, but the treatment expense of the third-generation bisphosphonates is higher [6]. Therefore, developing a lower-cost for third-generation bisphosphonate is of great significance for reducing the economic pressure on patients. Clinically, there have been many studies on the comparison of the efficacy of ibandronate sodium and zoledronate sodium in the treatment of osteoporosis, but few reports on their cost-effectiveness [7]. This study retrospectively explored the efficacy of ibandronate sodium and zoledronate sodium in the treatment of senile osteoporosis and their impact on quality of life of patients who were treated in The Third Hospital of Hebei Medical University from January 2017 to June 2019, and analyzed the cost-effectiveness, aiming to provide a reference for the clinical development of drug treatment for senile osteoporosis.
Materials and methods
General information
A retrospective study was conducted on 215 patients with senile osteoporosis, who were treated in The Third Hospital of Hebei Medical University from January 2017 to June 2019. The patients were divided into two groups according to different treatment drugs, with 115 cases treated with ibandronate sodium as group A and 100 cases treated with zoledronate sodium as group B. In group A, there were 78 males and 37 females aged 61-88 years, with an average of 72.1±3.5 years; the disease course was 3-19 years, with an average of 7.9±2.2 years; patient’s weight was 43.24-82.21 kg, with an average of 56.28±3.14 kg. In group B, there were 69 males and 31 females aged 62-89 years, with an average of 72.3±3.4 years; the disease course was 4-20 years, with an average of 8.1±2.1 years; patient’s weight was 42.18-81.85 kg, with an average of 56.09±3.52 kg. There was no statistically significant difference in general information between the two groups (P > 0.05). This study was approved by the Ethics Committee of The Third Hospital of Hebei Medical University.
Inclusion and exclusion criteria
Inclusion criteria: Patients met the relevant diagnostic criteria in “Expert consensus on the diagnosis of osteoporosis in Chinese Population” [8]. The clinical manifestations were mainly body ache or low back pain, and the pain worsened when the load increased. Dual-energy X-ray absorptiometry indicated that T ≤ -2.5 SD; patients were above 60 years old and could walk independently; patients did not take any bone calcium supplements in the past month.
Exclusion criteria: Patients were allergic to ibandronate sodium and zoledronate sodium; patient’s secondary osteoporosis was caused by diabetes, hyperthyroidism, rheumatism, rheumatoid disease, etc.; long-term use of glucocorticoid drugs affected patient’s bone metabolism; patients had severe heart, liver and kidney dysfunction; patients had poor compliance, and was unable to cooperate with investigators.
Treatment methods
Patients in group A were treated with ibandronate sodium injection (Pharmaceutical Factory of Hebei Medical University, China): 2 mg of ibandronate sodium was added into 250 mL of 0.9% sodium chloride solution (Fujian Jinshan Biopharmaceutical Co., Ltd., China) for intravenous infusion, with infusion time of ≥ 120 min, frequency of once per 6 months and duration of 12 months. Patients in group B were treated with zoledronate sodium injection (Chia Tai-Tianqing Pharmaceutical Factory, China): 5 mg of zoledronate sodium was added into 100 mL of 0.9% sodium chloride solution for intravenous drip, with infusion time of ≥ 15 min and a one-time dose per year. Patients could be discharged from the hospital when the clinical symptoms such as pain and fatigue disappeared or were significantly relieved, and the functional activities basically returned to normal. After discharge, patients continued to receive drug treatment.
Outcome measures
The clinical efficacy, bone mineral density (BMD) before and after treatment, bone metabolic markers (alkaline phosphatase (ALP), blood phosphorus (P), blood calcium ion (Ca2+)), quality of life, adverse reactions, cost-effectiveness indicators (length of hospitalization, cost) and complications were compared between the two groups.
1) Clinical efficacy [9]. The clinical efficacy was evaluated at 12 months after treatment. Markedly effective: The clinical symptoms such as pain and fatigue disappeared or were significantly relieved, BMD increased by ≥ 2% compared with that of before treatment, and functional activities basically returned to normal; effective: The clinical symptoms such as pain and fatigue were relieved, BMD increased by < 2% than that before treatment, and functional activities significantly improved; invalid: clinical symptoms such as pain and fatigue were not relieved or worsened, BMD didn’t increase or decrease compared to that before treatment, and functional activities didn’t improve or deteriorate. Total therapeutic response rate = markedly effective efficiency + effective efficiency.
2) Cost-effectiveness. The length of hospitalization, treatment expenses, medical insurance expenses, and out-of-pocket payments during the hospitalization were compared between the two groups.
3) BMD. The BMD levels of the lumbar spine L1-L4, left femoral neck and right femoral neck were measured by dual-energy X-ray bone density analyzer before treatment and 12 months after treatment.
4) Bone metabolic markers. Fasting venous blood was extracted in the morning before treatment and 12 months after treatment. The ALP level was determined by enzyme-linked immunosorbent assay; the P level was determined by enzymatic method; the Ca2+ level was determined by colorimetric method. The kits were provided by Shanghai Bangyi Biotechnology Co., Ltd., China.
5) Quality of life. Before treatment and 12 months after treatment, the world health organization quality of life (WHOQOL-BREF) questionnaire was used for evaluation [10]. The contents of the scale included psychological state, physiological function, environment and social relations. Each item was scored from 0 to 100 points. The higher the score, the better the quality of life is.
6) Adverse reactions. The occurrence of adverse reactions such as fever, low back pain and dizziness during treatment were compared.
7) Complications. The occurrence of secondary fractures, bone injury and other complications during the follow-up visit were compared between the two groups.
Statistical analysis
SPSS 22.0 statistical software was used to analyze the data. Measurement data (BMD, ALP, P, Ca2+, quality of life, length of hospitalization and cost) were expressed as mean ± standard deviation (x̅ ± sd). Independent sample t-test was used between groups, and paired t-test was used within groups. Count data (clinical efficacy, adverse reactions) was expressed as percentage, and chi-square test (χ2) was used. Rank sum test was used for ranked data. P < 0.05 was considered statistically significant.
Results
Clinical efficacy
The total therapeutic response rate in group A was 96.52% (111/115), which was not statistically different from that of 93.00% (93/100) in group B (P > 0.05). It can be seen that zoledronate sodium and ibandronate sodium has the same clinical efficacy in the treatment of senile osteoporosis. See Table 1.
Table 1.
Comparison of clinical efficacy (n, %)
| Group A (n=115) | Group B (n=100) | Z/χ2 | P | |
|---|---|---|---|---|
| Markedly effective | 64 (55.65) | 49 (49.00) | 1.756 | 0.103 |
| Effective | 47 (40.87) | 44 (44.00) | ||
| Invalid | 4 (3.48) | 7 (7.00) | ||
| Total therapeutic response rate (%) | 111 (96.52) | 93 (93.00) | 1.367 | 0.242 |
Cost-effectiveness
The length of hospitalization, treatment expenses during the observation, medical insurance expenses, and out-of-pocket payments of group A were lower than those of group B (P < 0.001). It can be seen that compared with zoledronate sodium, the use of ibandronate sodium could significantly reduce the cost-effectiveness in the treatment of senile osteoporosis. See Table 2.
Table 2.
Comparison of cost-effectiveness (x̅ ± sd)
| Items | Group A (n=115) | Group B (n=100) | t | P |
|---|---|---|---|---|
| Length of hospitalization (day) | 17.25±3.25 | 22.14±2.24 | 12.656 | 0.000 |
| Treatment expenses (CNY) | 15632.85±1855.15 | 20624.84±2142.52 | 18.311 | 0.000 |
| Medical insurance expenses (CNY) | 9252.36±2121.25 | 12425.63±2048.25 | 11.119 | 0.000 |
| Out-of-pocket payments (CNY) | 6354.81±1524.84 | 8426.85±1482.74 | 10.066 | 0.000 |
Note: CNY: China yuan.
BMD level
There was no significant difference in the BMD levels of the lumbar spine L1-L4, left femoral neck and right femoral neck between the two groups before treatment (P > 0.05). After treatment, the BMD levels of the lumbar spine L1-L4, left femoral neck and right femoral neck in both groups increased (P < 0.001), and there was no statistically significant difference between group A and group B (P > 0.05). The bone density of patients with senile osteoporosis elevated significantly after treating with ibandronate sodium and zoledronate sodium. See Table 3 and Figure 1.
Table 3.
Comparison of BMD levels before and after treatment (x̅ ± sd, g/cm3)
| Items | Group A (n=115) | Group B (n=100) | t | P |
|---|---|---|---|---|
| Lumbar spine L1-L4 | ||||
| Before treatment | 0.61±0.12 | 0.62±0.09 | 0.683 | 0.496 |
| After treatment | 0.82±0.11*** | 0.83±0.13*** | 0.611 | 0.542 |
| Left femoral neck | ||||
| Before treatment | 0.69±0.12 | 0.68±0.10 | 0.658 | 0.511 |
| After treatment | 0.88±0.15*** | 0.87±0.14*** | 0.503 | 0.616 |
| Right femoral neck | ||||
| Before treatment | 0.42±0.08 | 0.40±0.11 | 1.538 | 0.126 |
| After treatment | 0.59±0.11*** | 0.57±0.12*** | 1.275 | 0.204 |
Note: Compared with the same group before treatment;
P < 0.001.
BMD: bone mineral density.
Figure 1.
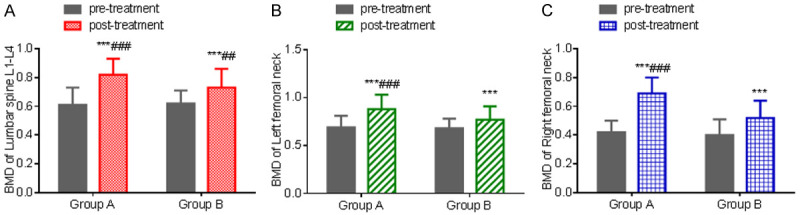
Comparison of BMD levels before and after treatment. A: BMD level of lumbar spine L1-L4; B: BMD level of left femoral neck; C: BMD level of right femoral neck. Compared with the same group before treatment, ***P < 0.001; compared with group B, ###P < 0.001. BMD: bone mineral density.
Bone metabolism
There was no significant difference in serum ALP level between the two groups before treatment (P > 0.05). After treatment, the serum ALP level of the two groups decreased (P < 0.001), and there was no statistically significant difference between group A and group B (P > 0.05). There was no significant difference between the two groups in serum P and Ca2+ levels before and after treatment (P > 0.05). Serum ALP level can be significantly improved with ibandronate sodium and zoledronate sodium in the treatment of elderly osteoporosis. See Table 4 and Figure 2.
Table 4.
Comparison of serum ALP, P and Ca2+ levels before and after treatment (x̅ ± sd)
| Items | Group A (n=115) | Group B (n=100) | t | P |
|---|---|---|---|---|
| ALP (U/L) | ||||
| Before treatment | 75.25±6.17 | 76.06±7.05 | 0.898 | 0.370 |
| After treatment | 60.36±10.29*** | 59.47±11.52*** | 0.598 | 0.550 |
| P (mmol/L) | ||||
| Before treatment | 1.12±0.20 | 1.13±0.28 | 0.304 | 0.761 |
| After treatment | 1.11±0.21*** | 1.10±0.24*** | 0.326 | 0.745 |
| Ca2+ (mmol/L) | ||||
| Before treatment | 2.35±0.48 | 2.37±0.51 | 0.296 | 0.768 |
| After treatment | 2.26±0.52*** | 2.31±0.39*** | 0.788 | 0.432 |
Note: Compared with the same group before treatment;
P < 0.001.
ALP: alkaline phosphatase; P: blood phosphorus; Ca2+: calcium ion.
Figure 2.
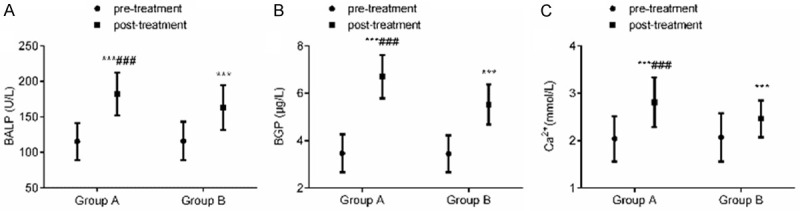
Comparison of serum ALP, P and Ca2+ levels before and after treatment. A: serum ALP level; B: serum P level; C: serum Ca2+ level. Compared with the same group before treatment, ***P < 0.001; compared with group B, ###P < 0.001. ALP: alkaline phosphatase; P: blood phosphorus; Ca2+: calcium ion.
Quality of life
There was no significant difference in quality of life scores between the two groups before treatment (P > 0.05). After treatment, the quality of life scores in both groups increased (P < 0.001), and there was no significant difference between the two groups (P > 0.05). The quality of life in patients with senile osteoporosis significantly improved after treating with ibandronate sodium and zoledronate sodium. See Table 5 and Figure 3.
Table 5.
Comparison of quality of life before and after treatment (x̅ ± sd, score)
| Group A (n=115) | Group B (n=100) | t | P | |
|---|---|---|---|---|
| Psychological state | ||||
| Before treatment | 58.36±5.63 | 58.12±5.74 | 0.309 | 0.758 |
| After treatment | 86.74±6.24*** | 87.24±6.05*** | 0.594 | 0.553 |
| Physiological function | ||||
| Before treatment | 52.89±5.24 | 52.48±5.61 | 0.554 | 0.580 |
| After treatment | 88.14±6.60*** | 89.24±6.72*** | 1.209 | 0.228 |
| Environment | ||||
| Before treatment | 60.25±6.01 | 59.52±6.22 | 0.874 | 0.383 |
| After treatment | 80.24±7.39*** | 81.62±6.84*** | 1.414 | 0.159 |
| Social relations | ||||
| Before treatment | 61.35±5.48 | 61.42±5.43 | 0.094 | 0.925 |
| After treatment | 80.65±6.27*** | 81.54±6.39*** | 1.029 | 0.305 |
Note: Compared with the same group before treatment;
P < 0.001.
Figure 3.
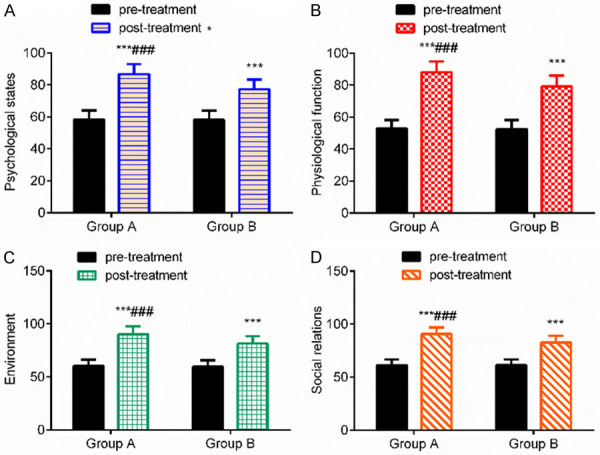
Comparison of quality of life before and after treatment. A: comparison of psychological state score; B: comparison of physiological function score; C: comparison of environment score; D: comparison of social relation score. Compared with the same group before treatment, ***P < 0.001; compared with group B, ###P < 0.001.
Adverse reactions
The incidence of adverse reactions in group A was 3.48% (4/115), which showed no statistical significance with that of 5.00% (5/100) in group B (P > 0.05). It can be seen that the incidence of adverse reactions of ibandronate sodium and zoledronate sodium in the treatment of senile osteoporosis were similar and at a low level. See Table 6.
Table 6.
Comparison of adverse reactions (n, %)
| Group A (n=115) | Group B (n=100) | t | P | |
|---|---|---|---|---|
| Fever | 2 (1.74) | 3 (3.00) | 0.025 | 0.874 |
| Low back pain | 1 (0.87) | 0 (0.00) | 0.000 | 1.000 |
| Dizziness | 1 (0.87) | 2 (2.00) | 0.015 | 0.903 |
| Total | 4 (3.48) | 5 (5.00) | 0.046 | 0.830 |
Complications
There were no complications such as secondary fractures and bone injury in the two groups.
Discussion
In recent years, with the continuous acceleration of the aging process of the population, the number of people with osteoporosis in China has been increasing, and osteoporosis has caused serious negative effects on the lives of patients. Osteoporosis is mainly caused by the imbalance of bone remodeling due to bone deformation of osteoblast and bone resorption of osteoclast, which leads to the decrease of BMD, destruction of bone microstructure, and increase of bone fragility. The third-generation bisphosphonates are common drugs in the treatment of senile osteoporosis, which can inhibit bone destruction and bone resorption; however, different bisphosphonates lead to different therapeutic effects [11]. In this study, the third-generation bisphosphonates of ibandronate sodium and zoledronate sodium were adopted in the treatment of patients with senile osteoporosis. The results showed that the total therapeutic response rate in both groups was similar. After treatment, the quality of life score in both groups increased, and there was no significant difference between the two groups. There was no significant difference in adverse reactions between the two groups. The results suggested that the therapeutic effect of ibandronate sodium in the treatment of senile osteoporosis was better than that of zoledronate sodium, which could significantly improve patient’s quality of life with similar adverse reactions. But study has shown that the total effective rate in the treatment of postoperative osteoporosis of breast cancer with ibandronate sodium was significantly higher than that with zoledronate sodium, and the difference of adverse reactions between the two groups was not significant, the result of above study is consistent with that of this study [12]. The reason may be related to the small number of samples in this study.
As third-generation bisphosphonates, ibandronate sodium and zoledronate sodium are widely used in the clinical treatment of osteoporosis, which can effectively relieve the clinical symptoms and promote physical rehabilitation [13,14]. Ibandronate sodium is a type of bone resorption inhibitor, which can be combined with hydroxyapatite in bone to inhibit the formation and dissolution of hydroxyapatite, and thus play a role in the formation of osteoclast and inhibition of bone resorption [15]. In addition, ibandronate sodium can also promote morphological changes in osteoclasts and inhibit osteoblast-mediated cytokines [16]. Zoledronic acid is a diphosphate compound that specifically acts on bone, which can effectively inhibit bone resorption caused by increased osteoclast activity, reduce bone injury and protect bone substance [17,18].
Different from most clinical reports at this stage, this study analyzed the cost-effectiveness of ibandronate sodium and zoledronate sodium in the treatment of senile osteoporosis by comparing the length of hospitalization and expenses. The results of the study showed that the length of hospitalization, annual treatment expense and out-of-pocket payments in group A were lower than those in group B, suggesting that the cost-effectiveness of ibandronate sodium in the treatment of senile osteoporosis was better than that of zoledronate sodium. The reasons may be related to two aspects: on the one hand, the price of ibandronate sodium is lower than that of zoledronate sodium; on the other hand, the length of hospitalization of patients treated with zoledronate sodium was longer, which increased the treatment expenses [19]. In this study, the medical insurance expenses of group A was lower than that of group B, which may be related to the higher treatment expenses of group B.
Clinically, BMD is an indicator of bone strength, which can effectively reflect the degree of osteoporosis and predict the risk of osteoporotic fractures [20]. Although BMD can reveal the changes of bone mass in patients with osteoporosis, it cannot reflect the status of bone metabolism in real time. Corresponding metabolites will be produced during the process of bone remodeling, and the status of bone metabolism is reflected in the changes of the content of these metabolites [21,22]. ALP is mainly synthesized by liver and bone, which can reflect bone formation [23]. Ca2+ level can reflect the situation of bone calcium [24]. The results of this study indicated that BMD and serum ALP improved significantly in both groups after treatment. The study of Wang et al. showed that BMD significantly improved 6 months and one year after treatment with ibandronate sodium in male patients with senile osteoporosis [25]. The study by Wang et al. suggested that the bone metabolism indicators improved significantly after treating with ibandronate sodium in patients with postmenopausal osteoporosis patients combined with type 2 diabetes [26]. The results of above reports are basically consistent with those of this study, indicating that ibandronate sodium and zoledronate sodium could significantly improve BMD and bone metabolism of patients with senile osteoporosis.
To sum up, ibandronate sodium and zoledronate sodium in the treatment of patients with elderly osteoporosis has similar clinical efficacy and adverse reactions, and can effectively improve patient’s quality of life, but the cost-effectiveness of ibandronate sodium is better than that of zoledronate sodium. Therefore, ibandronate sodium is recommended as the first choice of the third-generation bisphosphonate in the treatment of senile osteoporosis. However, due to the small and single number of samples collected in this study, the results may be biased. The credibility of the study conclusions needs to be verified by more in-depth correlation studies in the future.
Acknowledgements
This work was supported by the Medical Science Research Project of Hebei Health Commission in 2020 (20202004).
Disclosure of conflict of interest
None.
References
- 1.Piao CD, Li ZW, Ding J, Kong DL. Bone viscoelastic properties in an animal model with osteoporosis after BMSC-alendronate sodium intervention. J Hard Tissue Biol. 2019;28:315–320. [Google Scholar]
- 2.Hoefert S, Sade Hoefert C, Munz A, Schmitz I, Grimm M, Yuan A, Northoff H, Reinert S, Alexander D. Effect of bisphosphonates on macrophagic THP-1 cell survival in bisphosphonate-related osteonecrosis of the jaw (BRONJ) Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:222–232. doi: 10.1016/j.oooo.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Popp AW, Senn R, Curkovic I, Senn C, Buffat H, Popp PF, Lippuner K. Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int. 2017;28:1995–2002. doi: 10.1007/s00198-017-3992-5. [DOI] [PubMed] [Google Scholar]
- 4.Shima K, Tsuchiya M, Oizumi T, Takano-Yamamoto T, Sugawara S, Endo Y. Inflammatory effects of nitrogen-containing bisphosphonates (N-BPs): modulation by Non-N-BPs. Biol Pharm Bull. 2017;40:25–33. doi: 10.1248/bpb.b16-00521. [DOI] [PubMed] [Google Scholar]
- 5.Ataoğlu B, Kaptan AY, Eren TK, Yapar AE, Berkay AF. Atypical femoral fracture following zoledronic acid treatment. Eklem Hastalik Cerrahisi. 2016;27:54–57. doi: 10.5606/ehc.2016.11. [DOI] [PubMed] [Google Scholar]
- 6.Laroche M. Denosumab: lifetime treatment of osteoporosis? Presse Med. 2016;45:4–6. doi: 10.1016/j.lpm.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Shao ZW. Clinical effect analysis of alendronate sodium and zoledronic acid in the treatment of primary osteoporosis. Chin J Pract Med. 2018;26:15–17. [Google Scholar]
- 8.Zhang ZH, Liu ZH, Li N, Zhang MM, Huang QR, Ma YZ, Wang L, Liu Y, Liu XY, Zhu J, Lan X, Li SC, Yang HB, Yu HG, Tang GY, Zhang W, Yao WW, Li SL, Peng JH, Zhou S, Zhou JS. Expert consensus on the diagnosis of osteoporosis in Chinese Population. Chin J Osteoporosis. 2014;20:1007–1010. [Google Scholar]
- 9.Zhou C, Fang HJ, Gu N, Yu X, Wu YQ. Effect of Gushukang Granule on the efficacy and toxicity of alendronate sodium in the treatment of osteoporosis. Eval Anal Drug-Use Hosp China. 2019;19:679–682. [Google Scholar]
- 10.Gou J, Yue JB, Li X, Suo G. Clinical effect of ibandronate sodium combined with zoledronic acid in the treatment of postmenopausal osteoporosis and its influence on quality of life. Chin J Difficult Complicated Cases. 2019;18:65–69. [Google Scholar]
- 11.Tan W, Sun J, Zhou L, Li Y, Wu X. Randomized trial comparing efficacies of zoledronate and alendronate for improving bone mineral density and inhibiting bone remodelling in women with post-menopausal osteoporosis. J Clin Pharm Ther. 2016;41:519–523. doi: 10.1111/jcpt.12429. [DOI] [PubMed] [Google Scholar]
- 12.Liu K, Liu Z, Gao J, Liu YQ, Liu ZF. Therapeutic effects of three kinds of bisphosphonates on postoperative osteoporosis in patients with breast cancer. Hebei Med J. 2019;41:687–690. [Google Scholar]
- 13.Aref MW, McNerny EM, Brown D, Jepsen KJ, Allen MR. Zoledronate treatment has different effects in mouse strains with contrasting baseline bone mechanical phenotypes. Osteoporos Int. 2016;27:3637–3643. doi: 10.1007/s00198-016-3701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massey AS, Pentlavalli S, Cunningham R, McCrudden CM, McErlean EM, Redpath P, Ali AA, Annett S, McBride JW, McCaffrey J, Robson T, Migaud ME, McCarthy HO. Potentiating the anticancer properties of bisphosphonates by nanocomplexation with the cationic amphipathic peptide, RALA. Mol Pharm. 2016;13:1217–1228. doi: 10.1021/acs.molpharmaceut.5b00670. [DOI] [PubMed] [Google Scholar]
- 15.Wei K, Bao ZM, Zhang K. Alfacalcidol soft capsules and calcium carbonate D3 tablets combined with zoledronic acid in the treatment of 53 elderly patients with osteoporosis: a short-term follow-up study. Drug Eval. 2018;15:10–13. [Google Scholar]
- 16.Cheng HL, Lin CW, Yang JS, Hsieh MJ, Yang SF, Lu KH. Zoledronate blocks geranylgeranylation not farnesylation to suppress human osteosarcoma U2OS cells metastasis by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways. Oncotarget. 2016;7:9742–9758. doi: 10.18632/oncotarget.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly CC, Raynor WY, Hong AL, Kargilis DC, Lee JS, Alecxih AG, Gupta N, Lim MK, Al-Zaghal A, Werner TJ, Rhodes SS, Alavi A, Rajapakse CS. Diagnosis and monitoring of osteoporosis with (18) f-sodium fluoride PET: an unavoidable path for the foreseeable future. Semin Nucl Med. 2018;48:535–540. doi: 10.1053/j.semnuclmed.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Popp AW, Senn R, Curkovic I, Senn C, Buffat H, Popp PF, Lippuner K. Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int. 2017;28:1995–2002. doi: 10.1007/s00198-017-3992-5. [DOI] [PubMed] [Google Scholar]
- 19.Nagy DI, Grün A, Lévay K, Garadnay S, Keglevich G. Efficient syntheses of zoledronic acid as an active ingredient of a drug against osteoporosis. Synth Commun. 2018;48:1–9. [Google Scholar]
- 20.Lawson MA, Ebetino FH, Mazur A, Chantry AD, Paton-Hough J, Evans HR, Lath D, Tsoumpra MK, Lundy MW, Dobson RL, Quijano M, Kwaasi AA, Dunford JE, Duan X, Triffitt JT, Jeans G, Russell RGG. The pharmacological profile of a novel highly potent bisphosphonate, OX14 (1-Fluoro-2-(Imidazo-(1,2-α) Pyridin-3-yl)-Ethyl-Bisphosphonate) J Bone Miner Res. 2017;32:1860–1869. doi: 10.1002/jbmr.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swallow EA, Aref MW, Chen N, Byiringiro I, Hammond MA, McCarthy BP, Territo PR, Kamocka MM, Winfree S, Dunn KW, Moe SM, Allen MR. Skeletal accumulation of fluorescently tagged zoledronate is higher in animals with early stage chronic kidney disease. Osteoporos Int. 2018;29:2139–2146. doi: 10.1007/s00198-018-4589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamy O, Gonzalez-Rodriguez E, Stoll D, Aubry-Rozier B. Denosumab in clinical practice: beware before, during and after. Rev Med Suisse. 2017;13:863–866. [PubMed] [Google Scholar]
- 23.Albert SG, Reddy S. Clinical evaluation of cost efficacy of drugs for treatment of osteoporosis: a meta-analysis. Endocr Pract. 2017;23:841–856. doi: 10.4158/EP161678.RA. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Ni JL, Hu M, Qiu XP. Comparison of the efficacy of Xianling Gubao Capsule combined with calcium D 600 and alendronate tablet in the treatment of senile osteoporosis. Shanxi Med Pharm J. 2019;48:284–287. [Google Scholar]
- 25.Wang P, Liu JQ, Tian LM, Wang YZ, Wang CR. Clinical efficacy and safety of ibandronate injection and alendronate sodium tables in treatment of senile male osteoporosis. Smart Health. 2019;5:58–61. [Google Scholar]
- 26.Wang Y. Clinical observation of ibandronate sodium and calcitonin in postmenopausal osteoporosis patients with type 2 diabetes mellitus. Guide China Med. 2016;14:126–127. [Google Scholar]


