Abstract
Objective: This study aimed to confirm the association of miR-151-3p with nephrotic syndrome (NS) in children and to explore the molecular mechanisms by which glucocorticoid-induced transcript 1 gene (GLCCI1) targets cellular biological functions in children with nephrotic syndrome. Methods: miR-151-3p levels were detected in 20 children with hormone-sensitive nephrotic syndrome (SSNS), 15 children with steroid-dependent nephrotic syndrome (SDNS) and 20 children with steroid-resistant nephrotic syndrome (SRNS), using qRT-PCR before and after glucocorticoid treatment, and TargetScan information software was used to predict the biological targets between miR-151-3p and GLCCI1 gene. The change in albumin-to-creatinine ratio (ACR) before and after treatment in children with NS was determined to judge the treatment efficacy. Results: Compared with healthy controls, pediatric patients with NS had significantly increased serum miR-151-3p levels before treatment (P<0.01). After glucocorticoid treatment, children with SSNS/SDNS had significantly decreased serum miR-151-3p levels (P<0.01), with no significant difference from healthy controls. The ACR of children with SSNS/SDNS was significantly lower than that before treatment (P<0.05), and the symptoms of proteinuria were significantly relieved. The serum miR-151-3p levels and ACR of children with SRNS did not change significantly from that before treatment (P>0.05), and the symptoms of proteinuria were also not improved. Targetscan prediction results showed that miR-151-3p has well-matched sites with GLCCI13’UTR. Conclusion: miR-151-3p directly influences the onset and progression of NS through targeted regulation of GLCCI1 expression in podocytes. miR-151-3p may be a biological marker for the diagnosis, treatment and prognosis of NS.
Keywords: NS, GLCCI1, SSNS, SDNS, SRNS
Introduction
Childhood nephrotic syndrome is not a disease in itself; rather, it is a group of symptoms that indicate kidney damage, -particularly damage to the glomeruli, the tiny units within the kidney where blood is filtered and this results in the release of too much protein from the body into the urine. It’s typical symptoms include progressive edema, severe proteinuria, hyperlipidemia and hypoproteinemia. Glucocorticoid steroids remain the first-line drugs of treatment for nephrotic syndrome, but the response to treatment varies among patients. Due to the lack of clinical predictors for response to hormone therapy, Saudi scholar Abdulla proposed the classification of NS into hormone-sensitive and hormone-resistant categories based on the responsiveness of patients to hormone therapy during treatment [1]. In the last decade, numerous studies have been devoted to finding specific serum cytokines [2] or urinary markers patients with NS [3], but with little success. There has been a lack of specific and non-invasive molecular markers for the diagnosis of NS or the prediction of hormonal response; and if not treated in a timely manner, NS will seriously affect the living state of children [4]. Currently, renal biopsy is still the only way to clarify NS pathology, but compliance with this invasive procedure is poor in children.
miRNAs are endogenous non-coding single-stranded small RNAs of approximately 22-25 nucleotides in length. miRNAs are highly conserved in evolution and play an important role in a variety of physiological processes through complementary pairing with the 3’ end non-coding region of target gene mRNA molecules to regulate gene and protein expression at the transcriptional and post-transcriptional levels [5]. Studies have found that multiple miRNAs are present in human serum and the serum miRNA expression profile varies in different diseases [6,7]. Some miRNA have been recognized as a class of potential biological markers and therapeutic targets. A study used low-density microarrays to analyze the serum of children with NS and found multiple miRNAs were differentially expressed, such as miR-151-3p and miR-30a-5p [8].
The glomerular filtration barrier consists of 3 components: fenestrated endothelial cells, glomerular basement membrane, and podocyte foot processes with their interconnecting slit diaphragm. It is now well established that damage to the glomerular filtration barrier leads to proteinuria and the onset of NS, which will eventually lead to end-stage renal disease (ESRD) if not effectively reversed [9,10]. Glomerular filtration damage may be caused by mutations in genes of glomerular cells or the glomerular basement membrane [10]. Little is known about the details of the development of proteinuria, and the causes of proteinuria may vary. Considering the correlation between glomerular filtration function and proteinuria, the podocytes associated with the slit-diaphragm region have been of great interest [11-13]. In recent years, studies have reported several glomerular-associated proteins that may be involved in the development of glomerular diseases, but the biological roles and potential functions of these proteins are still unknown [14]. GLCCI1 is one of the highly specific glomerular-associated proteins, whose level is 14-fold higher in the glomerulus than in the rest of the kidney, and even 2.8-fold higher in podocytes than in glomerular cells [11]. Such specific expression suggests that it has a significant role in ensuring the normal structure and function of the glomerulus.
In this study, qRT-PCR was used to confirm the correlation between miR-151-3p and childhood NS, and to investigate the molecular mechanism by which miR-151-3p targets GLCCl1 to regulate cell biological functions in children with SSNS/SDNS following hormone therapy, so as to provide a more convenient molecular marker for the diagnosis and treatment of childhood NS.
Materials and methods
Study subjects
Fifty-five children with NS diagnosis in our hospital, aged 3-14 years, with a male to female ratio of approximately 2:1, were enrolled. According to the Guidelines for Diagnosis and Treatment of Kidney Diseases of the Chinese Medical Association of Pediatrics on the classification of glucocorticoid-sensitive, relapsing or dependent nephrotic syndrome [15], there were 20 cases of SSNS, 15 cases of SDNS and 20 cases of SRNS. Children with immune diseases, other types of renal diseases, severe infections, and cardiovascular diseases were excluded. Meanwhile, 30 healthy controls were enrolled as the control group, and the age composition and sex ratio were comparable to that of the group of children with NS. Children with SSNS, SDNS, and SRNS had 3 ml of fasting venous blood drawn before and after hormone therapy, and the control group had 3 ml of fasting venous blood drawn. The hormone therapy [16] was administered at an initial dose of 60 mg/m2/d for 4 weeks as recommended by the International Study Group on Kidney Diseases in Children (ISKDC). After centrifuged at 4000 rpm to separate serum we added TRIzol reagent (1:3), all blood samples were maintained at -80°C until RNA extraction. This study was approved by the Ethics Committee of Wuwei Liangzhou Hospital. The subjects’ families signed an informed consent voluntarily.
Methodology
(1) Predicted match between miR-151-3p and GLCCl1 gene: TargetScan was used to analyze the target matching relationship between miR-151-3p and GLCCl1.
(2) qRT-PCR detection of serum miR-151-3p: The total RNA of the samples from each group was extracted according to the instruction of the Trizol kit. The reverse transcription reaction was performed using U6 as an internal reference, and miR-151-3p was reverse transcribed using stem-loop primers (Figure 1) in a 20 μl reverse transcription reaction system at following conditions: 55°C for 60 min, 85°C for 5 min. Twenty min after RNase H treatment at 37°C, the RNA template was removed and the products were stored at 4°C. The above products were used as templates for qPCR amplification of miR-151-3p and U6. A 50 μl qPCR reaction system was used, and 3 tubes were prepared for each reverse transcription products, and the primers are listed in Table 1. Reaction conditions were Pre-denaturation 95°C for 2 minutes, over 40 cycles (95°C for 15 seconds, 60°C for 45 seconds), warming up 1°C every 20 seconds.
Figure 1.

miR-151-3p reverse transcription stem-loop primer.
Table 1.
Sequences of each primer in qPCR experiments
| Primer | Sequence |
|---|---|
| U6 forward | CGCTTCGGCAGCACATATAC |
| U6 reverse | TTCACGAATTTGCGTGTCAT |
| miR-151-3p forward | TCGGCAGCCTAGACTGAAGCT |
| miR-151-3p reverse | CTCAACTGGTGTCGTGGA |
(3) Measurement of urine protein before and after treatment: Five ml of morning urine was sampled from children in the NS group before treatment and after 4 weeks of hormone therapy. Quantitative determination of urine protein was measured by biuret method and albumin-to-creatinine ratio (ACR) was calculated.
Statistical analysis
Each specimen was assayed three times, and the Ct values of qPCR experiments were averaged. The miR-151-3p level was determined by relative quantification, and the multiplicity of gene expression was expressed as 2-ΔCt. ΔCt = Ct(miR-151-3p) - Ct(U6).
SPSS 23.0 was used to process the data. Measurement data was expressed as (x̅±s) and analyzed by t-test. P<0.05 was considered statistically significant.
Results
Matching relationship between miR-151-3p and GLCCI1
TargetScan showed that miR-151-3p had a conservative, 7mer-type matching site in the GLCCI1 mRNA3’UTR (Table 2).
Table 2.
Matching relationship of miR-151-3p and GLCCI1
| Predicted consequential pairing of target region (top) and miRNA (bottom) | Site type | Context++ score | Context++ score percentile | Weighted context++ score | Conserved branch length | |
|---|---|---|---|---|---|---|
| Position 520-526 of GLCCI1 3’ UTR | 5’ ...AGGUACUUCAGUUGAAGUCUAAA... | 7mer-A1 | -0.11 | 67 | -0.11 | 2.621 |
| hsa-miR-151a-3p | 3’ GGAGUUCCUCGAAGUCAGAUC |
Relative levels of serum miR-151-3p before glucocorticoid treatment
The relative level of serum miR-151-3p was significantly increased in all NS children before treatment, which was different from healthy controls (P<0.01). Serum miR-151-3p in children with SRNS was slightly higher than that in children with SSNS/SSDS (P>0.05) (Figure 2).
Figure 2.
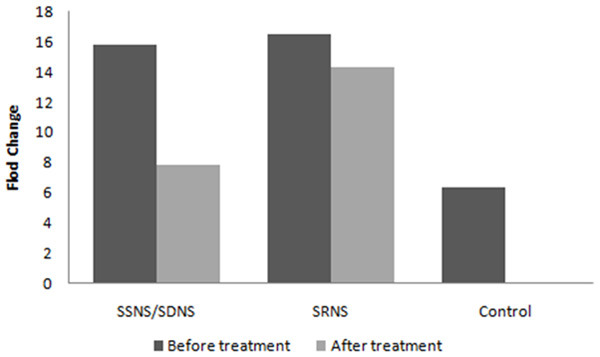
Changes in serum miR-151-3p levels in children with NS before and after treatment. After hormone therapy, the miR-151-3p level in the serum of children with SSDS/SDNS decreased significantly and was close to the level of healthy control (P<0.01).
Relative level of serum miR-151-3p in children with NS after glucocorticoid treatment
After 4 weeks of hormone treatment, the serum miR-151-3p level in children with SSNS/SSDS was significantly lower than that before treatment (P<0.01) and was not different from that of healthy controls (P>0.05). After the hormone treatment in SRNS children, the serum miR-151-3p levels were not significantly different from those before treatment (P>0.05) (Figure 2).
Changes in urinary protein levels before and after treatment
The urinary protein levels in children with NS met the criteria for NS, e.g. ACR ≥200 mg/mmol before treatment. After 4 weeks of treatment, the ACR of children with SSNS/SDNS decreased significantly to ACR<20 mg/mmol [16]. The urinary protein levels of SSNS/SDNS children were significantly different from those before and after treatment (P<0.01). The ACR of children with SRNS after treatment did not change significantly (P>0.05) compared with that before treatment (Figure 3).
Figure 3.

Change in ACR before and after treatment in children with NS. Children with SSDS/SDNS were treated with hormone therapy, the ACR was greatly reduced and met the criteria for remission of proteinuria symptoms, and the difference between before and after treatment was significant (P<0.01); children with SRNS were treated with hormone therapy, the change in ACR was not significant, and proteinuria symptoms did not improve (P>0.05).
Discussion
The causes of NS in children are diverse, with different types of pathology. Empirical treatment with high-dose administration of glucocorticoids is the current standard treatment [17], with a high risk of adverse effects. In pediatric patients with SRNS, SRNS can only be clinically diagnosed when there is persistent proteinuria after 8 weeks of hormone therapy at 60 mg/m2 or 2 mg/kg [16], which undoubtedly increases risk of the abuse of hormones. Renal biopsy helps to detect underlying pathology and can predict the response to hormone therapy; therefore, the pathological pattern remains the gold standard for the diagnosis of SRNS [18]. However, renal biopsy was not often performed in children. Kidney biopsies require invasive procedures and show poor compliance in children, and continuous monitoring is not possible as well. Studies have shown that the most common type of NS in children is microscopic lesion, which cannot be detected under light microscopy.
Research on miRNAs mostly focused on cancer, and abnormal expression of miRNAs has been found in a variety of tumors [19]. However, miRNAs that play a mediating role in metabolism and cellular life cycle, were differently expressed in non-neoplastic diseases [20] and have the potential to be diagnostic and therapeutic targets. In this study, miR-151-3p levels were significantly increased in the serum of children with NS and decreased in the serum of children with SSNS/SDNS after glucocorticoid treatment, suggesting that there is a link between serum miR-151-3p levels and NS. Blood tests are easily performed for a real-time “fluid biopsy” to monitor the dynamics of the disease. miR-151-3p can be used not only as a molecular marker for the diagnosis of NS, but also as an indicator to predict the response of patients to hormone therapy, thus avoiding unnecessary hormone therapy as much as possible.
Studies have suggested that GLCCI1 is a thymocyte-specific protein that is rapidly upregulated after dexamethasone treatment [21] and represents an early response to hormone-regulated apoptosis [22]. Studies have suggested that GLCCI1 is a highly specific protein present in the cytoplasm of podocytes and may be associated with glucocorticoid resistance in nephrotic syndrome. GLCCI1 is essential for the maturation of glomerular cells and maintaining structure and function of normal glomerular cells. The GLCCI1 knockdown could lead to proteinuria and morphological and pathological changes in the glomerular filtration barrier, suggesting that this protein is involved in the pathogenesis of glomerular diseases [23]. In a study on responses to inhaled glucocorticosteroids in asthmatic patients, two imbalance-linked SNP sites in the GLCCI1 promoter region were associated with a poor response to glucocorticoid therapy [24]. We speculated that altered GLCCI1 expression in children with NS may also influence the occurrence of NS or the response to glucocorticoid therapy.
A central purpose of miRNA study is to explore the effects of miRNAs on target genes. miRNAs regulate gene expression at the translational level by inhibiting translation or degrading target gene mRNAs, which is the basis for miRNAs to exert biological functions [25,26]. Using bioinformatics prediction tools, it is possible to predict the possible targets between a segment of miRNA and a functional gene. miR-151-3p is a 21-nucleotide single-stranded small RNA with the sequence: CUAGACUGAAGCUCCUUGAGG. Targetscan predictions revealed that miR-151-3p and the 3’UTR untranslated region of GlCCI1 have base complementation sites suggesting that GLCCI1 may be one of the target genes of miR-151-3p, and the two genes down-regulate GLCCI1 expression by non-specific binding at the post-transcriptional level to inhibit gene expression of GLCCI1 [27].
Due to the limitations of the experimental equipment and biopsy materials, the expression of GLCCI1 in the kidney tissue of pediatric patients could not be detected. However, it is speculated that the miR-151-3 level was up-regulated, thereby inhibiting the expression of GLCCI1 in the podocytes, destroying the glomerular barrier and resulting in proteinuria. For children with SSNS/SDNS, miR-151-3p decreased following hormone therapy, and the expression of GLCCI1 in podocytes was no longer inhibited and returned to normal, indicating hormone therapy is effective, and symptoms of proteinuria are relieved. For children with SRNS, there was no significant change in miR-151-3p level before and after hormone treatment. Excessive miR-151-3p inhibited the expression of GLCCI1 and the ACR was not significantly different from that before treatment, and the children showed no improvement after hormone treatment. However, the expression of GLCCI1 is likely to be affected by multifactorial regulation, and miR-151-3p is only one of the regulatory factors. The correlation between the GLCCI1 expression and the diagnosis, treatment and prognosis of NS needs to be studied in more detail.
In summary, the changes in serum miR-151-3p levels are inherently related to the progression of NS. After treatment, the levels of miR-151-3p in children with SSNS/SSDS are significantly reduced, and the symptoms of proteinuria are significantly alleviated. Through targeting and regulating the expression of GLCCI1 by miR-151-3p in podocytes, it directly affects the development of NS. miR-151-3p may be a good serum molecular marker for the diagnosis, treatment and prognosis of NS.
Acknowledgements
This work was supported by the Science and Technology Planning Project of Wuwei City, Gansu Province [grant number WW170326].
Disclosure of conflict of interest
None.
References
- 1.Alharthi AA. Patterns of childhood steroid-sensitive and steroid-resistant nephrotic syndrome in Saudi children. Clin Pediatr (Phila) 2017;56:177–183. doi: 10.1177/0009922816645521. [DOI] [PubMed] [Google Scholar]
- 2.Araya CE, Wasserfall CH, Brusko TM, Mu W, Segal MS, Johnson RJ, Garin EH. A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2006;21:603–610. doi: 10.1007/s00467-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 3.Traum AZ. Urine proteomic profiling to identify biomarkers of steroid resistance in pediatric nephrotic syndrome. Expert Rev Proteomics. 2008;5:715–719. doi: 10.1586/14789450.5.5.715. [DOI] [PubMed] [Google Scholar]
- 4.Deschênes G, Dossier C, Hogan J. Treating the idiopathic nephrotic syndrome: are steroids the answer? Pediatr Nephrol. 2019;34:777–785. doi: 10.1007/s00467-018-3963-x. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang WT, Zhao YN, Yan JX, Weng MY, Wang Y, Chen YQ, Hong SJ. Differentially expressed microRNAs in the serum of cervical squamous cell carcinoma patients before and after surgery. J Hematol Oncol. 2014;7:6. doi: 10.1186/1756-8722-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina-Villaamil V, Martínez-Breijo S, Portela-Pereira P, Quindós-Varela M, Santamarina-Caínzos I, Antón-Aparicio LM, Gómez-Veiga F. Circulating MicroRNAs in blood of patients with prostate cancer. Actas Urol Esp. 2014;38:633–639. doi: 10.1016/j.acuro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Luo Y, Wang C. Increased serum and urinary miR-30a-5p levels and clinical significance in pediatric patients with primary nephrotic syndrome. Journal of Modern Laboratory Medicine. 2013;28:14–18. [Google Scholar]
- 9.Farquhar MG, Wissig SL, Palade GE. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J Exp Med. 1961;113:47–66. doi: 10.1084/jem.113.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 11.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C. Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J. 2006;25:1160–1174. doi: 10.1038/sj.emboj.7601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L, Sun Y, Patrakka J, Mostad P, Norlin J, Xiao Z, Andrae J, Tryggvason K, Samuelsson T, Betsholtz C, Takemoto M. Glomerulus-specific mRNA transcripts and proteins identified through kidney expressed sequence tag database analysis. Kidney Int. 2007;71:889–900. doi: 10.1038/sj.ki.5002158. [DOI] [PubMed] [Google Scholar]
- 13.He L, Sun Y, Takemoto M, Norlin J, Tryggvason K, Samuelsson T, Betsholtz C. The glomerular transcriptome and a predicted protein-protein interaction network. J Am Soc Nephrol. 2008;19:260–268. doi: 10.1681/ASN.2007050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrakka J, Xiao Z, Nukui M, Takemoto M, He L, Oddsson A, Perisic L, Kaukinen A, Szigyarto CA, Uhlén M, Jalanko H, Betsholtz C, Tryggvason K. Expression and subcellular distribution of novel glomerulus-associated proteins dendrin, ehd3, sh2d4a, plekhh2, and 2310066E14Rik. J Am Soc Nephrol. 2007;18:689–697. doi: 10.1681/ASN.2006060675. [DOI] [PubMed] [Google Scholar]
- 15.Ass S. Evidence-based guidelines on diagnosis and treatment of childhood common renal diseases. (I) Evidence-based guideline on diagnosis and treatment of steroid-sensitive, relapsing/steroid-dependent nephrotic syndrome (for trial implementation) Chin J Pediatr. 2009;47:167–170. [PubMed] [Google Scholar]
- 16.Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet. 2018;392:61–74. doi: 10.1016/S0140-6736(18)30536-1. [DOI] [PubMed] [Google Scholar]
- 17.Gipson DS, Massengill SF, Yao L, Nagaraj S, Smoyer WE, Mahan JD, Wigfall D, Miles P, Powell L, Lin JJ, Trachtman H, Greenbaum LA. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124:747–757. doi: 10.1542/peds.2008-1559. [DOI] [PubMed] [Google Scholar]
- 18.Kemper MJ, Valentin L, van Husen M. Difficult-to-treat idiopathic nephrotic syndrome: established drugs, open questions and future options. Pediatr Nephrol. 2018;33:1641–1649. doi: 10.1007/s00467-017-3780-7. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi RU, Miyazaki H, Ochiya T. The role of microRNAs in the regulation of cancer stem cells. Front Genet. 2014;4:295. doi: 10.3389/fgene.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haider BA, Baras AS, McCall MN, Hertel JA, Cornish TC, Halushka MK. A critical evaluation of microRNA biomarkers in non-neoplastic disease. PLoS One. 2014;9:e89565. doi: 10.1371/journal.pone.0089565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman MS, Qu N, Pascoe S, Chen WX, Apostol C, Gordon D, Miesfeld RL. Isolation of differentially expressed sequence tags from steroid-responsive cells using mRNA differential display. Mol Cell Endocrinol. 1995;108:R1–7. doi: 10.1016/0303-7207(95)03481-l. [DOI] [PubMed] [Google Scholar]
- 22.Chapman MS, Askew DJ, Kuscuoglu U, Miesfeld RL. Transcriptional control of steroid-regulated apoptosis in murine thymoma cells. Mol Endocrinol. 1996;10:967–978. doi: 10.1210/mend.10.8.8843413. [DOI] [PubMed] [Google Scholar]
- 23.Nishibori Y, Katayama K, Parikka M, Oddsson A, Nukui M, Hultenby K, Wernerson A, He B, Ebarasi L, Raschperger E, Norlin J, Uhlén M, Patrakka J, Betsholtz C, Tryggvason K. Glcci1 deficiency leads to proteinuria. J Am Soc Nephrol. 2011;22:2037–2046. doi: 10.1681/ASN.2010111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, Lange C, Lazarus R, Sylvia J, Klanderman B, Duan QL, Qiu W, Hirota T, Martinez FD, Mauger D, Sorkness C, Szefler S, Lazarus SC, Lemanske RF Jr, Peters SP, Lima JJ, Nakamura Y, Tamari M, Weiss ST. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011;365:1173–1183. doi: 10.1056/NEJMoa0911353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 26.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 27.Chua JH, Armugam A, Jeyaseelan K. MicroRNAs: biogenesis, function and applications. Curr Opin Mol Ther. 2009;11:189–199. [PubMed] [Google Scholar]


