Abstract
Objective: Exercise intervention can reduce drug dependence of patients with amphetamines addiction by improving dopamine level and immunity, and reducing negative emotions. Methods: Altogether 90 male patients with amphetamines addiction from March 2018 to June 2019 were selected and grouped. The routine rehabilitation group (RG) (30 cases) was given conventional rehabilitation treatment, while group 1 (30 cases) was given conventional rehabilitation treatment+aerobic exercise for 1 hour. Group 2 (30 cases) received routine rehabilitation+aerobic exercise+strength training for 1 hour. Before and after exercise intervention, the three groups were tested for psychological state with self-rating depression scale (SDS). Visual analogue scale (VAS) was used to evaluate the drug craving. Positive and negative syndrome scale (PANSS) and quality of life scale for drug addiction (QOL-DA) were used to detect the mental status and QOL. The immune function, high sensitivity C-reactive protein (hs-CRP) and dopamine (DA) levels were detected. Results: After intervention, the SDS, VAS and PANSS of group 1 and group 2 weresignificantly better than those of RG, while the improvement of scores of group 2 wassignificantly better than that of group 1 (P < 0.05). hs-CRP in group 1 and group 2 were significantly lower than those in RG, while hs-CRP in group 2 weresignificantly lower than those in group 1 (P < 0.05). IgA, IgG and DA in group 1 and group 2 weresignificantly higher than those in RG, and IgA, IgG and DA in group 2 weresignificantly higher than those in group 1 (P < 0.05). QOL-DA in group 1 and 2 weresignificantly higher than those of RG, and the improvement of scores of group 2 wassignificantly better than that of group 1 (P < 0.05). Conclusion: Psychological status, drug craving, immune function, DA and QOL of patients with amphetamines addiction have been improved after exercise intervention.
Keywords: Exercise intervention, dopamine, body immunity, negative emotions, patients with amphetamines addiction, drug dependence
Introduction
Drug abuse is a serious clinical problem and the main cause of health, psychological and social problems [1]. Amphetamines abuse can lead to various physiological and psychological problems of patients, such as chronic skin lesions, serious rise in blood pressure, psychological anxiety, depression, and even suicidal tendencies in serious cases [2]. At present, the most widely used clinical treatment for amphetamines addiction is drug substitution therapy or psychological and behavioral intervention therapy, but drug substitution therapy still causes side effects such as drug dependence of patients, and psychological and behavioral intervention needs extremely high cost which limit its wide applicability [3,4]. Therefore, it is particularly important to find scientific rehabilitation therapy to alleviate the craving of patients for amphetamines.
Physical exercise is a potential non-drug therapy for addiction and can activate the same reward pathway as drug abuse by increasing dopamine concentration and dopamine receptor binding, and it can also affect brain plasticity by focusing on the mechanism of chromatin remodeling in regions related to drug addiction [5]. Aerobic exercise, as a means of rehabilitation, can not only improve the physical health of drug addiction, but also play a role in drug use behavior of drug addiction [6]. For example, Brown RA et al. found that aerobic exercise intervention for sedentary patients with alcohol dependence could further increase the positive effect of exercise on alcohol consumption, indicating that aerobic exercise is an effective auxiliary means for alcohol therapy [7]. Moreover, Trivedi MH et al. found that exercise intervention could adjust the percentage of withdrawal days of the dependent and improve the outcome of stimulant users who comply with the dosage [8]. Dopamine is an important neurotransmitter in the brain [9]. The stimulating effects caused by drug addiction and the patients’ thirst for drugs are all related to the expression level of dopamine in the brain [10]. Studies have shown that the mechanism of amphetamine addiction can be found from the perspective of dopamine, and the causes of negative emotions in patients with amphetamine addiction can be found to alleviate and improve the psychological status of patients [11].
This study mainly analyzed the relationship of exercise intervention with dopamine, body immunity and negative emotions of patients with amphetamines addiction, aiming to provide clinical reference for the treatment of patients with amphetamines addiction.
Materials and methods
General data
From March 2018 to June 2019, 90 male patients with amphetamines addiction received treatment in Guangxi No.6 compulsory isolation detoxification center were selected and divided into three groups according to treatment methods. The routine rehabilitation group (30 cases) was given routine rehabilitation treatment, while the experiment 1 group (30 cases) was given conventional rehabilitation treatment+aerobic exercise for 1 hour. Experiment 2 group (30 cases) received routine rehabilitation+aerobic exercise+strength training for 1 hour. Inclusion criteria: The study met ATS drug dependence diagnostic criteria [12]; the patients had complete general clinical data; no antidepressant or anti-inflammatory drugs have been taken in the past 3 months; patients have received detoxification treatment for at least 12 weeks. Exclusion criteria: patients had respiratory, blood and nervous system diseases with inflammation, hearing impairment; drug abusers; patients with severe trauma which was not cured; patients lost to follow; patients dropped out of the study halfway. In this study, patients and their families have signed an informed consent form, and this study has been approved by the Ethics Committee.
Exercise intervention method
Routine rehabilitation group: health education and nutritional support were given to patients, and corrective management on the living habits and behaviors of each patient was carried out.
Experiment 1 group was given conventional rehabilitation therapy+aerobic exercise for 1 hour, and aerobic exercise was added to the conventional rehabilitation group: aerobic exercise (interval exercise and treadmill exercise) for 40 min and warm-up for 5 min, flexibility exercise for 10 min, and finally relaxation exercise for 5 min.
The experiment 2 group was given routine rehabilitation+aerobic exercise+strength training for 1 hour. On the basis of the routine rehabilitation group, aerobic exercise and strength training were added: warm-up for 5 min, aerobic exercise for 40 min (interval exercise, treadmill training), resistance training for 25 min, Swiss ball training for 10 min, flexibility training for 10 min, and finally relaxation exercise for 5 min.
Outcome measures
(1) Self-rating depression scale (SDS) [13]: SDS had 20 items, with a cut-off value of 53 points. A score of 53-62 points was regarded as mild depression, 62-72 as moderate depression, and above 72 as severe depression. SAS had 20 items, with a cut-off value of 50 points. A score of 50-59 points was regarded as mild anxiety, 60-69 points as moderate anxiety and above 70 points as severe anxiety.
(2) Visual analogue scale (VAS) [14]: The patient’s craving for drugs in different time periods was evaluated (Before exercise = T0, during exercise = T1, immediately after exercise = T2, 50 min after exercise = T3, 80 min after exercise = T4). VAS is a 10 cm line segment to indicate the patient’s craving for drugs, with a total of 11 assessment points. The high score was closely related to the high craving for drugs.
(3) Positive and negative syndrome scale (PANSS) [15]: The scale was divided into positive scale, negative scale and neuropathologic scale. The score range of positive scale and negative scale was 7-49 points, and the score range of neuropathologic scale was 16-112 points. The high score was closely related to the more severe symptoms.
(4) Determination of immune function and DA level: A 5 mL venous blood was collected from patients in the three groups between 7:30 and 9:00 in the morning before and after the intervention, centrifuged at 1500 × g at 4°C for 10 min, and placed in a low-temperature refrigerator at -70°C for later use. Enzyme-linked immunosorbent assay (ELISA) [16] was applied to detect the expression level of indicators, including hypersensitive -C reactive protein (hs-CRP), immunoglobulin A (IgA), immunoglobulin G (IgG), dopamine (DA), with referring to the instructions of hs-CRP, IgA, IgG, DA (Shanghai Bluegene Biotech CO.,LTD, Shanghai, China) kits.
(5) Quality of life scale for drug addiction (QOL-DA) [17]: The scale consisted of 4 dimensions and 40 items, with a 5-grade score ranging from 40 to 200 points. The low score after measurement was closely related to the poor quality of life.
Statistical method
SPSS 21.0 (EASYBIO, China) was applied for data analysis. GraphPad 6 was applied for data analysis and picture visualizing. All data were expressed by mean ± SD. Independent sample t test was applied for the comparison of the two groups, and one-way ANOVA for the comparison among multiple groups, which was expressed as F. LSD-t test was applied for post-event pairwise comparison, repeated measurement ANOVA was applied for multi-time point expression, which was expressed as F, Bonferroni was applied for back testing. P < 0.05 was considered as statistical difference.
Results
General data
There was no evident difference among the three groups in general data such as age, BMI, residence, educational level, marital status, occupation, drug consumption and drug use duration (P > 0.05). See Table 1.
Table 1.
Comparison of general data of three groups of patients [N (%)] (mean ± SD)
| Classification | Routine rehabilitation group (n = 30) | Experiment 1 group (n = 30) | Experiment 2 group (n = 30) | F/χ2 | P |
|---|---|---|---|---|---|
| Age (years) | 31.85±3.17 | 32.04±3.19 | 31.92±3.14 | 0.027 | 0.972 |
| BMI (kg/m2) | 23.31±1.74 | 23.15±1.73 | 23.28±1.77 | 0.071 | 0.931 |
| Residence | 0.637 | 0.727 | |||
| Urban | 11 (36.67) | 13 (43.33) | 14 (46.67) | ||
| Rural | 19 (63.33) | 17 (56.67) | 16 (53.33) | ||
| Educational level | 3.482 | 0.175 | |||
| ≥ high school | 13 (43.33) | 18 (60.00) | 11 (36.67) | ||
| < high school | 17 (56.67) | 12 (40.00) | 19 (63.33) | ||
| Marital status | 1.867 | 0.393 | |||
| Unmarried | 12 (40.00) | 16 (53.33) | 17 (56.67) | ||
| Married | 18 (60.00) | 14 (46.67) | 13 (43.33) | ||
| Occupation | 3.015 | 0.555 | |||
| Unemployed | 9 (30.00) | 15 (50.00) | 13 (43.33) | ||
| Individual management | 13 (43.33) | 9 (30.00) | 12 (40.00) | ||
| Enterprises and institutions | 8 (26.67) | 6 (20.00) | 5 (16.67) | ||
| Drug abuse (mg/d) | 0.83±0.08 | 0.86±0.05 | 0.85±0.06 | 1.680 | 0.192 |
| Drug use duration/years | 3.95±0.57 | 3.87±0.54 | 4.01±0.63 | 0.438 | 0.646 |
Comparison of SDS scores
There was no evident difference in SDS of the three groups of patients before intervention (P > 0.05). SDS scores of the three groups of patients decreased significantly after intervention (P < 0.05). SDS scores of the patients in experiment 1 group and experiment 2 group were significantly lower than those in routine rehabilitation group (P < 0.05), while SDS scores of the patients in experiment 2 group were significantly lower than those in experiment 1 group (P < 0.05). See Figure 1.
Figure 1.
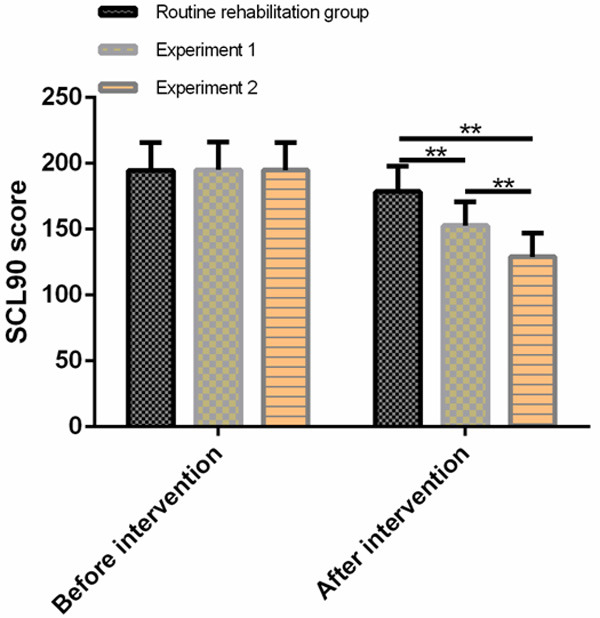
Comparison of SDS scores of three groups of patients before and after intervention. There was no evident difference in SDS scores among the three groups before treatment. After treatment, SDS scores in experiment 1 group and experiment 2 group were significantly lower than those in routine rehabilitation group, and the improvement degree in experiment 2 group was better than that in experiment 1 group. Note: compared with routine rehabilitation group or comparison between the two groups, *P < 0.05, **P < 0.01.
Comparison of VAS scores
There was no evident difference in the VAS scores in T0 among the three groups of patients (P > 0.05). With the extension of the intervention time, the VAS scores in T1, T2 and T3 decreased significantly (P < 0.05), but recovered in T4 period (P < 0.05). VAS scores in T1, T2, T3 and T4 of the patients in experiment 1 group and experiment 2 group were significantly lower than those in the routine rehabilitation group (P < 0.05), while the VAS scores in T1, T2, T3 and T4 of experiment 2 group were also significantly lower than those in experiment 1 group (P < 0.05). See Figure 2.
Figure 2.
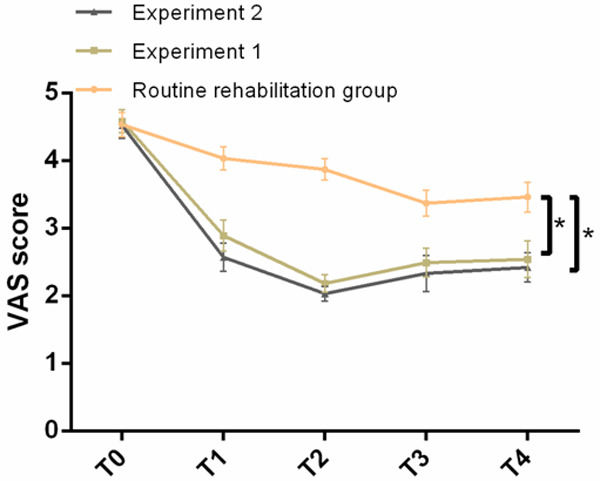
Comparison of VAS scores of three groups of patients at different time periods before and after intervention. There was no evident difference in the VAS scores of the three groups of patients in T0. The VAS scores of the patients in T1, T2, T3 and T4 in experiment 1 group and experiment 2 group were significantly lower than those in the routine rehabilitation group, while the improvement in the experiment 2 group was significantly better than that in the experiment 1 group. Note: * indicates compared with routine rehabilitation group or comparison between the two groups, P < 0.05.
Comparison of PANSS scores before and after intervention in three groups of patients
There was no evident difference in positive symptoms, negative symptoms, psychopathology and total score of PANSS scores of the three groups before intervention (P > 0.05). After intervention, the positive symptoms, negative symptoms, psychopathology and total score of the three groups of patients were significantly decreased (P < 0.05). The positive symptoms, negative symptoms, psychopathology and total score of experiment 1 group and experiment 2 group were significantly lower than that of routine rehabilitation group (P < 0.05), and the positive symptoms, negative symptoms, psychopathology and total score of experiment 2 group were significantly lower than that of experiment 1 group (P < 0.05). See Table 2.
Table 2.
Comparison of PANSS scores before and after intervention in three groups of patients (mean ± SD)
| Group | n | Positive symptoms | Negative symptoms | Psychopathology | Total score | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Before intervention | After intervention | Before intervention | After intervention | Before intervention | After intervention | Before intervention | After intervention | ||
| Routine rehabilitation group | 30 | 24.38±4.23 | 18.67±4.19 | 21.69±3.83 | 14.78±3.72 | 39.96±3.26 | 34.76±3.18 | 86.03±9.47 | 68.21±8.47 |
| Experiment 1 group | 30 | 24.76±4.27 | 14.87±4.26 | 21.42±3.56 | 11.75±3.32 | 39.47±3.23 | 28.67±3.06 | 85.65±9.45 | 55.29±8.21 |
| Experiment 2 group | 30 | 24.95±4.26 | 11.65±4.13 | 21.08±3.62 | 9.36±3.16 | 40.02±3.21 | 22.74±3.01 | 86.05±9.42 | 43.75±8.03 |
| F | - | 0.139 | 21.060 | 0.207 | 19.060 | 0.261 | 113.900 | 0.139 | 21.060 |
| P | - | 0.869 | < 0.001 | 0.812 | < 0.001 | 0.771 | < 0.001 | 0.869 | < 0.001 |
Comparison of immune function indexes
The expression levels of hs-CRP, IgA and IgG in the three groups of patients had no evident difference before intervention (P > 0.05). After intervention, hs-CRP in the three groups of patients reduced significantly (P < 0.05), while IgA and IgG enhanced significantly (P < 0.05). The improvement of hs-CRP, IgA and IgG in the experiment 1 group and experiment 2 group after intervention was significantly better than that in the routine rehabilitation group (P < 0.05), while the improvement of hs-CRP, IgA and IgG in the experiment 2 group after intervention was significantly better than that in the experiment 1 group (P < 0.05). See Table 3.
Table 3.
Comparison of immune function indexes before and after intervention in three groups of patients (mean ± SD)
| Group | n | hs-CRP (mg/L) | IgA (g/L) | IgG (g/L) | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Before intervention | After intervention | Before intervention | After intervention | Before intervention | After intervention | ||
| Routine rehabilitation group | 30 | 2.78±0.27 | 2.13±0.19 | 1.88±0.16 | 1.95±0.17 | 9.31±0.57 | 10.48±0.47 |
| Experiment 1 group | 30 | 2.73±0.29 | 1.83±0.16 | 1.93±0.13 | 2.16±0.19 | 9.46±0.53 | 12.37±0.49 |
| Experiment 2 group | 30 | 2.67±0.23 | 1.75±0.18 | 1.91±0.18 | 2.28±0.21 | 9.47±0.59 | 14.07±0.55 |
| F | - | 1.301 | 38.380 | 0.761 | 23.020 | 0.757 | 380.200 |
| P | - | 0.277 | < 0.001 | 0.470 | < 0.001 | 0.471 | < 0.001 |
Comparison of DA expression levels
There was no evident difference in DA among the three groups before intervention (P > 0.05). DA expression level in the three groups of patients after intervention was significantly increased (P < 0.05). DA in experiment 1 group and experiment 2 group was significantly higher than that in the routine rehabilitation group (P < 0.05), while DA in experiment 2 group was significantly higher than that in experiment 1 group (P < 0.05). See Table 4.
Table 4.
Comparison of DA expression levels of three groups of patients after intervention (mean ± SD)
| Group | n | DA (pg/mg) | |
|---|---|---|---|
|
| |||
| Before intervention | After intervention | ||
| Routine rehabilitation group | 30 | 75.04±2.76 | 78.36±1.15 |
| Experiment 1 group | 30 | 75.16±2.68 | 91.34±1.09 |
| Experiment 2 group | 30 | 75.86±2.91 | 96.54±1.02 |
| F | - | 0.758 | 2222.000 |
| P | - | 0.471 | < 0.001 |
Comparison of QOL-DA scores before and after intervention in three groups of patients
There was no evident difference in physical function, psychological function, withdrawal symptom and social function of QOL-DA scale before intervention in the three groups of patients (P > 0.05). After intervention, the physical function, psychological function, withdrawal symptom and social function of the patients in experiment 1 group and experiment 2 group were significantly higher than those in the routine rehabilitation group (P < 0.05), and those in experiment 2 group were significantly higher than those in experiment 2 group (P < 0.05). See Table 5.
Table 5.
Comparison of QOL-DA scores before and after intervention in three groups of patients (mean ± SD)
| Group | n | Physical functioning | Psychological function | Withdrawal symptom | Social functioning | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Before intervention | After intervention | Before intervention | After intervention | Before intervention | After intervention | Before intervention | After intervention | Before intervention | After intervention | ||
| Routine rehabilitation group | 30 | 25.87±4.38 | 29.83±4.51 | 24.53±4.03 | 27.31±3.23 | 30.48±3.87 | 38.02±3.84 | 32.19±3.63 | 36.38±3.78 | 113.07±12.47 | 131.54±12.59 |
| Experiment 1 group | 30 | 25.31±4.32 | 33.68±4.56 | 24.16±4.06 | 29.48±3.76 | 30.73±3.68 | 40.39±3.97 | 32.67±3.61 | 39.83±3.92 | 112.87±12.95 | 143.38±13.46 |
| Experiment 2 group | 30 | 25.62±4.33 | 37.21±4.13 | 24.37±4.02 | 31.72±3.57 | 31.04±3.85 | 43.04±3.27 | 32.74±3.66 | 40.76±3.95 | 113.77±12.58 | 152.73±13.57 |
| F | - | 0.125 | 21.070 | 0.063 | 11.730 | 0.163 | 13.780 | 0.203 | 10.590 | 0.041 | 32.290 |
| P | - | 0.882 | < 0.001 | 0.938 | < 0.001 | 0.849 | < 0.001 | 0.816 | < 0.001 | 0.959 | < 0.001 |
Discussion
Amphetamine abuse is very common in clinic. Repeated use of such drugs will lead to addiction, which is a neuropsychiatric disease and is believed to be caused by neural adaptation in cells, tissues and molecules after repeated use of drugs [18]. Clinical research has shown that amphetamine addiction have serious mental and medical consequences. At present, there is no effective auxiliary treatment and rehabilitation intervention for amphetamine addiction [19]. Therefore, finding an early and effective treatment method is undoubtedly an urgent problem to be solved.
Studies have shown that amphetamine addiction will have various physical withdrawal symptoms during treatment, which will further aggravate the psychological burden of abstainers and lead to various psychological problems or emotional disorders [20]. However, studies by Gimenez-Meseguer J et al. have shown that physical exercise is of great significance to people who rely on or abuse drugs. It can improve the physical function of patients, eliminate daily problems, improve emotions, and relieve psychological stress and anxiety [21]. This study found that SCL90, SDS and SAS scores of patients with amphetamines addiction were significantly increased. After exercise intervention, the scores in experiment 1 group and experiment 2 group were significantly lower than those in the routine rehabilitation group, and the improvement of scores in experiment 2 group was significantly better than that in experiment 1 group, indicating that aerobic exercise+strength exercise could reduce the anxiety and depression of amphetamines addiction, and exercise intervention could be used as a treatment method to assist drug addiction treatment and to improve the mental health of amphetamines addiction abstainers. Smith MA et al. indicated that aerobic exercise could reduce the self-management of drugs and other measures of drug seeking behavior, reduce the self-administration of methamphetamine, heroin and other drugs on exercise wheels or treadmills, and also reduce drug seeking recovery after abstinence [22]. Therefore, in this study, VAS scores were used to measure the degree of drug craving of the three groups of patients in different time periods during the intervention. It was found that the VAS scores in T1, T2, T3 and T4 of the patients in the experiment 1 group and the experiment 2 group were significantly lower than those in the routine rehabilitation group, while those in the experiment 2 group were significantly lower than those in the experiment 1 group. This showed that exercise intervention could better reduce the craving for amphetamines addiction, while strengthening exercise could better reduce the craving for drugs. After observing the PANSS scores of the three groups of patients after intervention treatment, it was found that the positive symptoms, negative symptoms, psychopathology and total scores of the experiment 1 group and the experiment 2 group were significantly lower than those of the routine rehabilitation group, and the improvement of the experiment 2 group was significantly better than that of the experiment 1 group, indicating that exercise intervention could improve the mental health of patients with mental disorder caused by amphetamine addiction.
Studies have shown that long-term use of amphetamine drugs can affect the central nervous system and peripheral immune function of patients, change immune homeostasis, and thus promote inflammation [23]. In the study of Heidarianpour A, moderate exercise can improve the immune system during morphine withdrawal syndrome [24]. The results of this research showed that the improvement of hs-CRP, IgA and IgG after intervention in experiment 1 group and experiment 2 group was significantly better than that in routine rehabilitation group, while the improvement of experiment 2 group after intervention was significantly better than that in experiment 1 group, indicating that exercise intervention activated the immune system of patients, thus improving the immune function. Dopamine is an important neurotransmitter in the brain, plays an important physiological function in brain motion control, neuroendocrine and other aspects, and has become an important target for research and development for the treatment of drug dependence, schizophrenia and other diseases [25]. In the study of London ED, amphetamine type stimulants users will show neurochemical evidence of dopamine system dysfunction and impulsive behavior, which will interfere with the success of treatment for addiction patients, and increased exercise training can be used as a means of treatment for addiction behavior [26]. The results of this research revealed that dopamine in patients with amphetamine drug addiction was significantly reduced, but after exercise intervention, the level of dopamine was significantly enhanced, and the expression level in experiment 2 group was higher than that in experiment 1 group. This showed that aerobic exercise+strength exercise could better increase the dopamine content in patients, improve the mental health of patients and reduce the patients’ drug craving. Clinical research showed that the improvement of QOL was one of the main purposes of treating drug-dependent patients, and the relationship between life therapy and dependence needs to be better explored [27]. In this study, QOL-DA scale was used to evaluate the QOL of the three groups of patients. It was found that the QOL-DA scores of experiment 1 group and experiment 2 group were significantly higher than that of the routine rehabilitation group after intervention treatment, while the QOL-DA scores of experiment 2 group were significantly higher than that of experiment 1 group. This showed that the exercise intervention of aerobic exercise+strength exercise could better improve the withdrawal discomfort during the withdrawal of amphetamine drug addiction, and improve the physical and mental health and social functions of patients.
Although this study confirmed that exercise intervention can improve the drug dependence of amphetamine-type drug addicts, there is still room for improvement in this study. For example, we can further analyze the risk factors affecting the adverse prognosis of amphetamine-type drug addicts, and we can also refer to different exercise intervention methods to observe the improvement degree of amphetamine-type drug addicts. In the future, supplementary research will be carried out gradually from the above perspectives.
To sum up, psychological status, drug craving, immune function, DA level and QOL of patients with amphetamine drug addiction have been improved after exercise intervention.
Disclosure of conflict of interest
None.
References
- 1.Shrot S, Poretti A, Tucker EW, Soares BP, Huisman TA. Acute brain injury following illicit drug abuse in adolescent and young adult patients: spectrum of neuroimaging findings. Neuroradiol J. 2017;30:144–150. doi: 10.1177/1971400917691994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shakeri J, Ahmadi SM, Maleki F, Hesami MR, Parsa Moghadam A, Ahmadzade A, Shirzadi M, Elahi A. Effectiveness of group narrative therapy on depression, quality of life, and anxiety in people with amphetamine addiction: a randomized clinical trial. Iran J Med Sci. 2020;45:91–99. doi: 10.30476/IJMS.2019.45829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariani JJ, Levin FR. Psychostimulant treatment of cocaine dependence. Psychiatr Clin North Am. 2012;35:425–439. doi: 10.1016/j.psc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Crescenzo F, Ciabattini M, D’Alo GL, De Giorgi R, Del Giovane C, Cassar C, Janiri L, Clark N, Ostacher MJ, Cipriani A. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLoS Med. 2018;15:e1002715. doi: 10.1371/journal.pmed.1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nygard M, Mosti MP, Brose L, Flemmen G, Stunes AK, Sorskar-Venaes A, Heggelund J, Wang E. Maximal strength training improves musculoskeletal health in amphetamine users in clinical treatment. Osteoporos Int. 2018;29:2289–2298. doi: 10.1007/s00198-018-4623-5. [DOI] [PubMed] [Google Scholar]
- 7.Brown RA, Abrantes AM, Minami H, Read JP, Marcus BH, Jakicic JM, Strong DR, Dubreuil ME, Gordon AA, Ramsey SE, Kahler CW, Stuart GL. A preliminary, randomized trial of aerobic exercise for alcohol dependence. J Subst Abuse Treat. 2014;47:1–9. doi: 10.1016/j.jsat.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivedi MH, Greer TL, Rethorst CD, Carmody T, Grannemann BD, Walker R, Warden D, Shores-Wilson K, Stoutenberg M, Oden N, Silverstein M, Hodgkins C, Love L, Seamans C, Stotts A, Causey T, Szucs-Reed RP, Rinaldi P, Myrick H, Straus M, Liu D, Lindblad R, Church T, Blair SN, Nunes EV. Randomized controlled trial comparing exercise to health education for stimulant use disorder: results from the CTN-0037 stimulant reduction intervention using dosed exercise (STRIDE) study. J Clin Psychiatry. 2017;78:1075–1082. doi: 10.4088/JCP.15m10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aumann TD. Environment- and activity-dependent dopamine neurotransmitter plasticity in the adult substantia nigra. J Chem Neuroanat. 2016;73:21–32. doi: 10.1016/j.jchemneu.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Chukwueke CC, Kowalczyk WJ, Di Ciano P, Gendy M, Taylor R, Heishman SJ, Le Foll B. Exploring the role of the Ser9Gly (rs6280) dopamine D3 receptor polymorphism in nicotine reinforcement and cue-elicited craving. Sci Rep. 2020;10:4085. doi: 10.1038/s41598-020-60940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA. Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur J Neurosci. 2016;43:1661–1673. doi: 10.1111/ejn.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Lu C, Zhang J, Hu L, Song H, Li J, Kang L. Gender differences in abusers of amphetamine-type stimulants and ketamine in southwestern China. Addict Behav. 2013;38:1424–1430. doi: 10.1016/j.addbeh.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Liu X, Li H, Yu L, Shen M, Lou Y, Xie S, Chen J, Zhang R, Yuan TF. Chronic repetitive transcranial magnetic stimulation (rTMS) on sleeping quality and mood status in drug dependent male inpatients during abstinence. Sleep Med. 2019;58:7–12. doi: 10.1016/j.sleep.2019.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Ivanets NN, Vinnikova MA, Ezhkova EV, Titkov MS, Bulatova RA. Aripiprazole and quetiapine in the treatment of patients with ‘dual diagnosis’ of schizophrenia and drug addiction. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119:52–61. doi: 10.17116/jnevro201911909152. [DOI] [PubMed] [Google Scholar]
- 15.Alisauskiene R, Loberg EM, Gjestad R, Kroken RA, Jorgensen HA, Johnsen E. The influence of substance use on the effectiveness of antipsychotic medication: a prospective, pragmatic study. Nord J Psychiatry. 2019;73:281–287. doi: 10.1080/08039488.2019.1622152. [DOI] [PubMed] [Google Scholar]
- 16.Hornbeck PV. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 2015;110:2.1.1–2.1.23. doi: 10.1002/0471142735.im0201s110. [DOI] [PubMed] [Google Scholar]
- 17.Wan C, Fang J, Jiang R, Shen J, Jiang D, Tu X, Messing S, Tang W. Development and validation of a quality of life instrument for patients with drug dependence: comparisons with SF-36 and WHOQOL-100. Int J Nurs Stud. 2011;48:1080–1095. doi: 10.1016/j.ijnurstu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godino A, Jayanthi S, Cadet JL. Epigenetic landscape of amphetamine and methamphetamine addiction in rodents. Epigenetics. 2015;10:574–580. doi: 10.1080/15592294.2015.1055441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morais APD, Pita IR, Fontes-Ribeiro CA, Pereira FC. The neurobiological mechanisms of physical exercise in methamphetamine addiction. CNS Neurosci Ther. 2018;24:85–97. doi: 10.1111/cns.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calpe-Lopez C, Garcia-Pardo MP, Aguilar MA. Cannabidiol treatment might promote resilience to cocaine and methamphetamine use disorders: a review of possible mechanisms. Molecules. 2019;24:2583. doi: 10.3390/molecules24142583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giménez-Meseguer J, Tortosa-Martínez J, de los Remedios Fernández-Valenciano M. Benefits of exercise for the quality of life of drug-dependent patients. J Psychoactive Drugs. 2015;47:409–416. doi: 10.1080/02791072.2015.1102991. [DOI] [PubMed] [Google Scholar]
- 22.Smith MA, Fronk GE, Abel JM, Lacy RT, Bills SE, Lynch WJ. Resistance exercise decreases heroin self-administration and alters gene expression in the nucleus accumbens of heroin-exposed rats. Psychopharmacology (Berl) 2018;235:1245–1255. doi: 10.1007/s00213-018-4840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magrone T, Jirillo E. Drugs of abuse induced-subversion of the peripheral immune response and central glial activity: focus on novel therapeutic approaches. Endocr Metab Immune Disord Drug Targets. 2019;19:281–291. doi: 10.2174/1871530319666181129104329. [DOI] [PubMed] [Google Scholar]
- 24.Heidarianpour A, Vahidian Rezazadeh M, Zamani A. Effect of moderate exercise on serum interferon-gamma and interleukin-17 levels in the morphine withdrawal period. Int J High Risk Behav Addict. 2016;5:e26907. doi: 10.5812/ijhrba.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meszaros J, Cheung T, Erler MM, Kang UJ, Sames D, Kellendonk C, Sulzer D. Evoked transients of pH-sensitive fluorescent false neurotransmitter reveal dopamine hot spots in the globus pallidus. Elife. 2018;7:e42383. doi: 10.7554/eLife.42383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.London ED. Impulsivity, stimulant abuse, and dopamine receptor signaling. Adv Pharmacol. 2016;76:67–84. doi: 10.1016/bs.apha.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Campelo SR, Barbosa MA, Dias DR, Caixeta CC, Leles CR, Porto CC. Association between severity of illicit drug dependence and quality of life in a psychosocial care center in Brazil: cross-sectional study. Health Qual Life Outcomes. 2017;15:223. doi: 10.1186/s12955-017-0795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


