Abstract
We have recently demonstrated that reactive oxygen species (ROS) scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain (CIBP). In the present study, we investigated anti-nociceptive effect of Nox inhibitor apocynin in CIBP in rats. Mechanical allodynia was assessed by Von Frey tests in sham and CIBP group of rats. Western blotting and immunofluorescence technique were conducted to assess the expression levels and cellular localization of Nox2. Results illustrated that after intra-tibial implantation with tumor cells, Nox2 and ROS were both up-regulated in the spinal cord of rats. Injection of apocynin could dose-dependently decrease the abundance of Nox2 and inhibit the development of CIBP. Furthermore, pretreatment with the apocynin could delay the development of CIBP. This study for the first time proved that Nox2 inhibitors could downregulate the production of ROS in CIBP rats, which highlights the fact that Nox inhibitor is an important therapeutic option for CIBP and that, precise targeting inhibitor of different subtypes of Nox enzymes is needed to developed in future.
Keywords: Apocynin, cancer-induced bone pain, NADPH oxidase 2, reactive oxygen species
Introduction
Cancer-induced bone pain (CIBP) is a severe and intractable problem that occurs in as many as 75% of patients with advanced or metastatic cancer [1], which further compromises life quality of breast cancer patients with bone metastasis [2,3]. Main clinical therapy for relief of CIBP is often limited and unsatisfactory [4-7]. Emerging studies have illustrated that reactive oxygen species (ROS) is involved in the pathogenesis of inflammatory and neuropathic pain models [8-10]. Furthermore, our previous study is the first to directly demonstrate the role of reactive oxygen scavengers in alleviating CIBP in rats [11], suggesting ROS plays critical roles in the process of CIBP.
However, given the complexity of various sources of ROS in the etiology of different pain models, identification of the source of ROS for CIBP and clarification of the mechanism by which ROS are generated is important for developing effective and specific therapies [12-14]. It has been demonstrated that Nox enzyme family is a key source for ROS production. Evidence suggests a role of NADPH oxidase 2 (Nox2) in the process of neuropathic pain and other chronic pain [15,16]. It has been reported that Nox2-derived ROS production in microglia/macrophages in DRG contributes to pain hypersensitivity [16]. Moreover, it has also been found that spinal Nox2 activity is involved in the maintenance of opioid-induced antinociceptive tolerance [17,18]. However, no studies to date have elucidated the role of spinal Nox enzymes in the development of CIBP. Nox2 is comprised of membrane-bound subunits p22phox, gp91phox, and cytosolic subunits including the main organizing and activating subunits p47phox and p67phox respectively, as well as the small GTPase RAC1/RAC2 and p40phox [19,20]. The cytosolic subunits are necessary for its activity. However, much less is known about the possible mechanisms. Thus, the present study aimed to examine the precise effect of Nox2 in a CIBP model.
Apocynin (APO), a methoxycatechol extracted from medicinal herb Picrorhiza kurroa, is regarded as an inhibitor of Nox. However, its inhibitory effect requires to be activated by peroxidases [21]. APO has been implicated in neuronal dysfunction and degeneration diseases including ischemic stroke and Alzheimer’s disease [22,23]. Moreover, emerging studies have demonstrated that APO could attenuate diabetic neuropathic pain, spinal nerve transection (SNT), chronic constriction injury (CCI) and the development of morphine-induced hyperalgesia and antinociceptive tolerance [24-26]. Thus, we evaluated analgesic effects of APO used as a Nox inhibitor in CIBP rats.
Material and methods
Animals
Female Sprague-Dawley rats (weighing 200-220 g, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, PR China) were housed under climate-controlled conditions (22°C to 24°C, 12-hr light/dark cycles, with free access to food and water). All experimental protocols and animal handling procedures were conducted in strict accordance with the International Association for the Study of Pain and was approved by the Committee on the Ethics of Animal Experiments of Huazhong University of Science and Technology. The recommendations in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals was also strictly followed [27].
Preparation of tumor cells
Walker 256 rat mammary gland carcinoma cells (1 × 107 cells/ml, 1 ml) were slowly inoculated into the abdominal cavity of female SD rats as per the previous report [28,29]. After about seven days, tumor cells were extracted from the ascites, rinsed in D-Hank’s solution, and then centrifuged for 10 min at 500 × g at 4°C (3 cycles). The concentration was calibrated to 4 × 107 cells/ml and was maintained on ice until inoculation.
Model of cancer-induced bone pain
CIBP model was performed as previous reported [30-32]. Briefly, after anesthetization with pentobarbital sodium (2%, 50 mg/kg, i.p.), and the posterior right leg of experimental rats was shaved and disinfected. A minimal incision was then made in the skin overlying the top half of the tibia. Walker 256 mammary carcinoma cells (10 µL, 4 × 107) were slowly injected into the intramedullary space of the right tibia of rats in the experimental group, whereas the sham group received injections of 10 μl of D-Hank’s solution alone. After sealing the drill hole with bone wax, the incision was sutured with 4-0 suture. Rats were then placed on a heated pad until they had recovery from anesthesia.
Drug administration
4’-hydroxy-3’-methoxyacetophenone (apocynin, APO) was purchased from Selleckchem (Houston, TX, USA) and was dissolved in dimethylsulfoxide (DMSO) and later diluted in tween 80 and saline to a final concentration of 100 mg/ml (DMSO, 10%; Tween 80, 10%). To determine the analgesic effect of APO in rats, each rat was intraperitoneally injected of 1 ml APO solution in the experimental group on day 14, while in the sham + DMSO group, each rat received a mixture containing DMSO, tween 80 and saline with the same concentration. To determine the antinociceptive effect of repeated injection of APO solution in CIBP rats, APO (200 mg/kg, i.p.) or a mixture of DMSO, tween 80 and saline was given once a day for five consecutive days to CIBP group and sham + DMSO group, respectively. To explore whether the early treatment of APO can prevent the occurrence of CIBP, APO (200 mg/kg, i.p.) or a mixture of DMSO, tween 80 and saline (10%, 2 ml, i.p.) was given to rats once a day from day 1 to day 5 in CIBP and sham group, respectively. APO was used as a Nox inhibitor for the present study.
Behavioral assessment of mechanical allodynia
Paw withdrawal mechanical threshold (PWT) of the right hind paw was determined by von Frey filaments (1, 1.4, 2, 4, 6, 8, 10, 15 g; Stoelting, USA) as previously reported [33,34]. Applied the tip of filaments upright to the plantar surface of each rat, the period of each stimulus was 5 seconds. Once a positive response was observed, the next lower filament was used after 5 minutes. Once no positive response was observed, the hind paw was re-applied with the next higher filament after 5 minutes. The normal locomotor behavior was ignored. PWTs were documented as the lowest force that induced by at least two positive responses after stimulated for three times. The investigator was blinded to the experimental situation.
Immunofluorescence
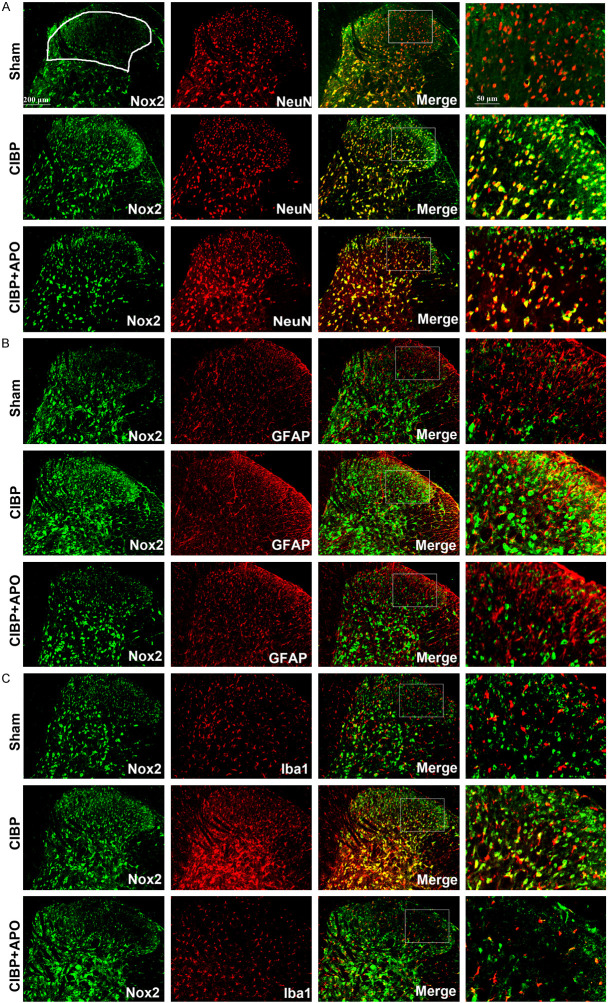
Under deep anesthetization with sodium pentobarbital (2%, 50 mg/kg, i.p.), rats were intracardially perfused with 500 ml PBST and 500 ml 4% PFA in 0.1 mol/l phosphate buffer saline (PBS). The L4-5 spinal cord segments were collected and post-fixed with 4% PFA for 6 h and subsequently equilibrates with 20% and 30% sucrose in PBS for at least 24 h at 4°C. Then, the segments were cut into 20-μm sections on a freezing microtome and consecutive six sections were collected on a slide. Free-floating sections were washed 3 times (3 × 10 min) with PBST under gentle shaking, and the spinal sections were blocked with 0.3% Triton for 10 min and followed by blocking with 5% donkey serum for 45 minutes. Then, the sections were then incubated for 24 h at 4°C with primary antibodies: mouse-anti 8-OHdG antibody (1:100, ab 62623, Abcam, Cambridge, MA, USA), rabbit anti-Nox2 antibody (1:50; 19013-1-AP, Proteintech, Wuhan, China), with mouse anti-NeuN antibody (NeuN marker; 1:100; MAB377; Millipore), mouse anti-GFAP antibody (astrocytes marker; 1:300; 3670; Cell Signaling Technology), or goat anti-Iba1 antibody (microglia marker; 1:100; ab5076; Abcam). After washing with PBST, the sections were incubated with a complex of Alexa 488-conjugated donkey anti-rabbit (1:100, Millipore, Billerica, MA, USA) and Alexa 594-conjugated donkey anti-mouse (1:200; Millipore, Billerica, MA, USA) or Alexa 594-conjugated donkey anti-goat (1:200; 705-585-003; Jackson ImmunoResearch) at room temperature (22-24°C) for 2 hours. Images were then captured using a laser-scanning confocal microscope. The Nox2/8-OHdG/NeuN/GFAP/Iba1 immunolabeled areas were calculated in laminae I-IV (as depicted in Figure 5A) of the spinal cord dorsal horn using Image J software. Quantification of average percentage of Nox2/8-OHdG/NeuN/GFAP/Iba1 immunoreactive surface area relative to the total measured picture area.
Figure 5.
Expression and cellular localization of Nox2 in the ipsilateral spinal cord. A-C. Nox2 (green) double fluorescence labeling with NeuN (red) for neurons, GFAP (red) for astrocytes and Iba1 (red) for microglia in the spinal cord dorsal horn at day 21 after tumor cell implantation. Amplified pictures showed the co-localization of Nox2 (green) and NeuN (red), GFAP (red) or Iba1 (red). B. The results showed that Nox2 was upregulated in CIBP + DMSO group compared with sham + DMSO group. C. Repeated injection of APO (200 mg/kg, i.p.) for 5 consecutive days notably decreased the expression of Nox2. The results showed that Nox2 was co-localized mostly with microglia (yellow) and neurons (yellow). (***P < 0.001 compared with the sham + DMSO group. ###P < 0.001 compared with the CIBP + DMSO group. n = 3 in each group). Scale bar = 200 μm.
Western blot analysis
After deep anesthetization with pentobarbital (2%, 100 mg/kg, i.p.), rats were euthanized at days 0, 3, 7, 14, and 21 after tumor injection and 2 h after drug administration. The spinal cord of L4-L5 was quickly removed and collected on ice, followed by homogenization in sodium dodecyl sulphate (SDS) sample buffer (10 ml/mg tissue) containing a mixture of proteinase and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO, USA). The protein concentration of supernatants was measured using the bicinchoninic acid assay kit (AR0146, Boster, Wuhan, China). After being heated in boiling water for 15 min at 100°C, protein (40 μg) samples were subjected to 10% SDS-polyacrylamide and eletransferred onto a polyvinylidene difluoride membrane (IPVH00010, Millipore, Billerica, MA, USA).
The membranes were blocked with 5% non-fat dry milk dissolved in Tris-buffered saline and Tween-20 (TBST) buffer for 1.5 h at room temperature (22-24°C), and were subsequently incubated with following primary antibodies overnight at 4°C: Nox2 (1:1000; A1636, abclonol, Wuhan, China) and GAPDH (1:5000, Proteintech, Wuhan, China). The membranes were then rinsed with TBST for three times (5 minutes each) and incubated with the secondary antibody secondary (1:5000; A21020; Abbkine) for 2 h. After being washed for three times with TBST (5 min each), images were visualized using Pierce ECL Western Blotting Substrate (K22030; Abbkine).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.01. The behavioral responses to mechanical stimuli over time among groups were tested using two-way analysis of variance with repeated measures followed by post hoc tests (Bonferroni test). Alterations of expression of the NOX2, 8-OHdG, NeuN, GFAP and Iba1 were tested with one -way analysis of variance with repeated measures followed by Bonferroni test. Data are presented as mean ± SEM, and P < 0.05 was considered as the level of statistical significance.
Results
Mechanical allodynia in cancer-induced pain model
Mechanical PWTs were conducted before the test and at day 3, 7, 14 and 21 following carcinoma cells inoculation. There was no significant difference in PWTs at day 0 among all groups (P > 0.05). After carcinoma cells injection, PWT of the CIBP groups showed a dramatic decrease from 14.29 ± 0.71 g to 3.71 ± 0.29 g at day 14 and further deteriorated to 2.49 ± 0.40 g at day 21. In contrast, there was no significant difference in naïve and sham rats until day 21, indicating a successful CIBP model was established in the present study (Figure 1).
Figure 1.

The time course of mechanical paw withdrawal threshold (PWT). The ipsilateral PWTs were tested using von Frey filaments at 3, 7, 10, 14, and 21 days after surgery in naïve, sham and CIBP group. The results are expressed as mean ± SEM, showing that PWTs decreased from 14.29 ± 0.71 g to 3.71 ± 0.29 g at day 14 and further deteriorated to 2.49 ± 0.40 g at day 21. In contrast, naïve and sham rats showed no significant change until day 21 (***P < 0.001 compared with the naive group at each corresponding time point, n = 6 per group).
Spinal expression of Nox2 in CIBP models
Previously, it has been demonstrated that Nox2 played an important role in neuropathic pain models. To determine whether Nox2 is involved in the process of CIBP, we measured the spinal levels of Nox2 by western blot analysis and immunofluorescence. As shown in Figure 2, the levels of Nox2 was time-dependently increased in the L4-5 spinal cord in CIBP models, beginning at day 7 after tumor cells implantation in the tibia and remaining until day 21. The results suggested that expression levels of Nox2 were involved in the downregulation of tumor-induced hyperalgesia.
Figure 2.
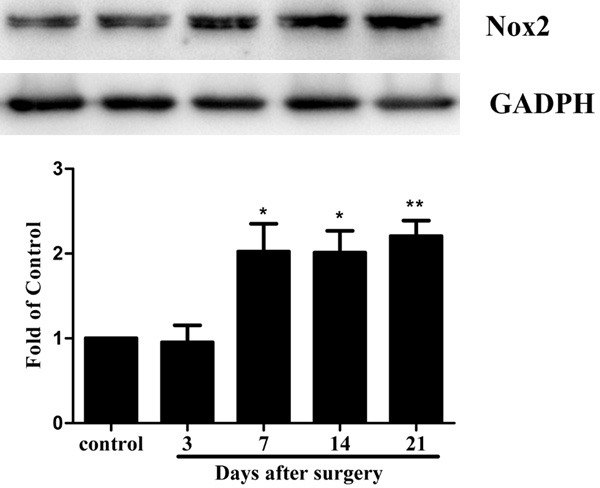
Time-course of in Nox2 expression in the spinal cord after tumor cells implantation. Western blot assay detected the time course of Nox2 expression in control and CIBP rats (n = 6 per group). Fold change for the density of Nox2 was standardized to GAPDH. The fold change of Nox2 in the control (sham) group was set at 1 for quantification. (*P < 0.05 compared with sham-operated group, n = 6 per group).
A single dose of APO attenuated pain behavior in CIBP model
To investigate whether APO inhibited the response to mechanical stimulus in CIBP model, a single dose of APO (10, 100, 200 mg/kg) or a mixture of DMSO, tween 80 and saline (10%, 2 ml) was injected i.p. once daily to CIBP and sham rats at day 14. PWTs were performed at 0, 0.5, 1, 2, 4 and 8 h after injection of APO. As shown in Figure 3A, PWTs were inhibited by 100 or 200 mg/kg of APO, while 10 mg/kg APO could not affect PWTs of rats (P > 0.05). Upregulation of PWTs in CIBP model started at 0.5 h, peaked at 1 h and was maintained for 8 h. Moreover, it showed that 200 mg/kg APO had a better analgesic effect in comparison with 100 mg/kg APO, indicating APO could dose-dependently inhibit the mechanical hyperalgesia in a rat model of CIBP.
Figure 3.
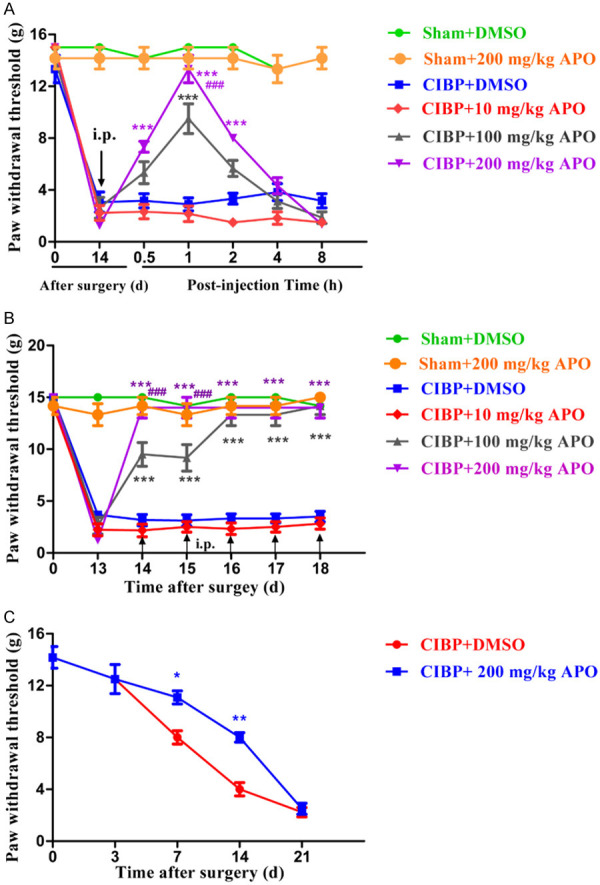
Analgesic effect of APO on established CIBP models. A. A single dose of APO (10, 100, 200 mg/kg, i.p.) was injected on day 14 after surgery. PWTs were performed at 0, 0.5, 1, 2, 4 and 8 h after injection of APO. Downregulation of PWTs was greatly inhibited by APO (100 and 200 mg/kg, i.p.) in CIBP rats. Upregulation of PWT began at 0.5 h, peaked at 1 h and lasted for 8 h. (*P < 0.05, **P < 0.01, ***P < 0.001 compared with the CIBP + 200 mg/kg APO group, ###P < 0.001 compared with the CIBP + 100 mg/kg APO group, n= 6 in each group). However, 10 mg/kg APO had no significant effect on the PWTs compared with CIBP + 200 mg/kg APO group (P > 0.05). B. Chronic treatment with APO (200 mg/kg, i.p.) once daily for 5 consecutive days after surgery. PWTs were performed at day 13 and 1 h after APO injection each day. Repeated injection of APO (200 mg/kg, i.p.) markedly inhibited the mechanical allodynia in CIBP rats without tolerance (***P < 0.001 compared with the CIBP + DMSO group, ###P < 0.001 compared with the CIBP + 100 mg/kg APO group, n = 6 per group). C. Preventive effect of early treatment with APO on the development of CIBP. APO (i.p., 200 mg/kg once a day) or a mixture of DMSO, tween 80 and saline (2 ml, i.p.) was injected once daily from day 1 to day 5 after surgery. PWTs were performed before the test on day 3, 7, 14 and day 21. Treatment with 200 mg/kg APO showed significant downregulation in PWTs at day 7 and day 14 in CIBP rats compared with CIBP + DMSO group. However, PWTs showed no significant difference between these two groups at day 21. (*P < 0.5, **P < 0.001 compared with the CIBP + DMSO group. n = 6 per group).
Repeated administration of APO attenuated PWT in CIBP model
To assess the antinociceptive effect of repeated treatment with APO in CIBP model, APO (100, 200 mg/kg, i.p.) was injected once a day for 5 consecutive days. As shown in Figure 3B, PWTs were examined at 14 days and 1 h after injection of APO (100, 200 mg/kg, i.p.) from 14 days to 18 days. Repeated treatment of APO (200 mg/kg, i.p.) significantly reversed PWTs in CIBP without tolerability issues. Moreover, injection of APO (100 mg/kg) could produce accumulative analgesic effect in CIBP rats. In contrast, both bone cancer pain group injected with vehicle (a mixture of DMSO, tween 80 and saline, 2 ml, i.p.) and sham rats treated with APO (200 mg/kg, i.p.) displayed no significant changes in PWTs at any time point (P > 0.05). These results suggested that repetitive treatment with APO could produce accumulative antinociceptive effect without tolerance.
Repeated treatment with APO decreased the expression of Nox2, downregulated the production of ROS and reduced the activation of microglia in CIBP models
To confirm the role of Nox2 in the development of CIBP, 200 mg/kg APO was intraperitoneally injected in CIBP rats for 5 consecutive days. As shown in Figure 4, level of Nox2 in the L4-5 spinal cord was significantly increased in CIBP rats compared with that of sham + DMSO group (P < 0.001). Treatment with APO (200 mg/kg, i.p.) once a day for 5 consecutive days from day 14 to day 18 could significantly decrease Nox2 level induced by tumor cells implantation. In contrast, expression of Nox2 showed no significant differences in sham + DMSO group compared with sham + APO group. Consistent with our western blot results, our immunofluorescence staining confirmed that increased immunoreactivity of Nox2 in the spinal cord of CIBP could be attenuated by APO (Figure 5A-C). Previously, our group has demonstrated that ROS play a key role in CIBP models. To determine whether Nox2 inhibitors are involved in the production of ROS, we measured the spinal levels of 8-OHdG by immunofluorescence analysis. As shown in Figure 7, the level of 8-OHdG was increased in the L4-5 spinal cord in CIBP models. Treatment with APO (200 mg/kg, i.p.) once daily from day 14 to day 18 notably reduced the production of 8-OHdG in the spinal cord after treatment. To determine the cellular expression of Nox2 in L4-L5 spinal dorsal horn, we further performed double immunofluorescence staining of Nox2 (green) with NeuN (red) for neurons, GFAP (red) for astrocytes or Iba1 (red) for microglia, respectively. As shown in Figure 5, Nox2 was mainly co-expressed with NeuN-positive cells and Iba1-positive cells under CIBP conditions in the superficial dorsal horn of spinal cord. Treatment with APO (200 mg/kg, i.p.) once daily from day 14 to day 18 notably reduced the activity of microglia in the spinal cord. Our immunofluorescence data also confirmed that tumor cells transplantation produced a significant increase of Nox2 in the spinal cord compared with sham + DMSO rats (Figure 6A). Moreover, tumor cells transplantation produced a significant increase of astrocytes and microglia in the spinal cord, treatment with APO (200 mg/kg, i.p.) notably reduced the activity of microglia in the spinal cord without affecting the abundance of the neuron in the spinal cord (Figure 6). These results suggested that spinal expression of Nox2 is involved in the development of tumor-induced hyperalgesia in CIBP rats; APO as an inhibitor of Nox enzymes, could modulate tumor-induced hyperalgesia, downregulated ROS levels in the spinal cord in CIBP rats.
Figure 4.
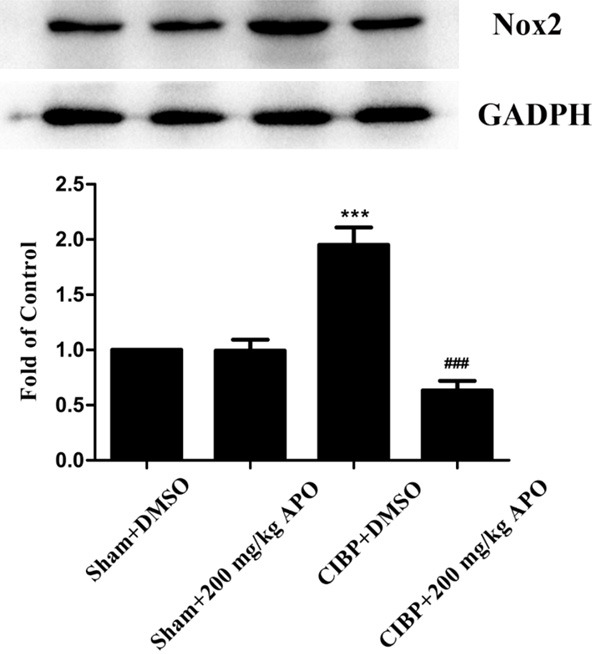
Expression of Nox2 in the spinal cord. APO (i.p., 200 mg/kg once a day) or a mixture of DMSO, tween 80 and saline (2 ml, i.p.) was injected on five consecutive days (from day 14 to day 18). Western blot analysis showed that inoculation of Walker 256 carcinoma cells significantly increased the expression of Nox2. Repeated injection of APO (200 mg/kg, i.p.) for 5 consecutive days significantly reversed the increasing of Nox2 after the tumor cells implantation (***P < 0.001 compared with the sham + DMSO group. ###P < 0.001 compared with the CIBP + DMSO group. n = 6 in each group).
Figure 7.
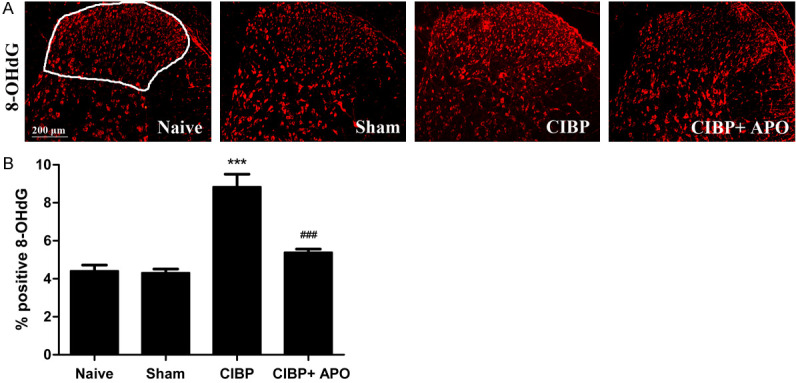
Expression of 8-OHdG in the ipsilateral spinal cord of rats. The levels of 8-OHdG were increased in the L4-5 spinal cord in CIBP models. Treatment with APO (200 mg/kg, i.p.) once daily from day 14 to day 18 notably reduced the production of 8-OHdG in the spinal cord after treatment. 8-OHdG immunolabeled surface area (laminae I-IV; as depicted in Naïve group) were quantified using Image J software. (***P < 0.001 compared with the sham + DMSO group. ###P < 0.001 compared with the CIBP + DMSO group. n = 3 in each group). Scale bar = 200 μm.
Figure 6.
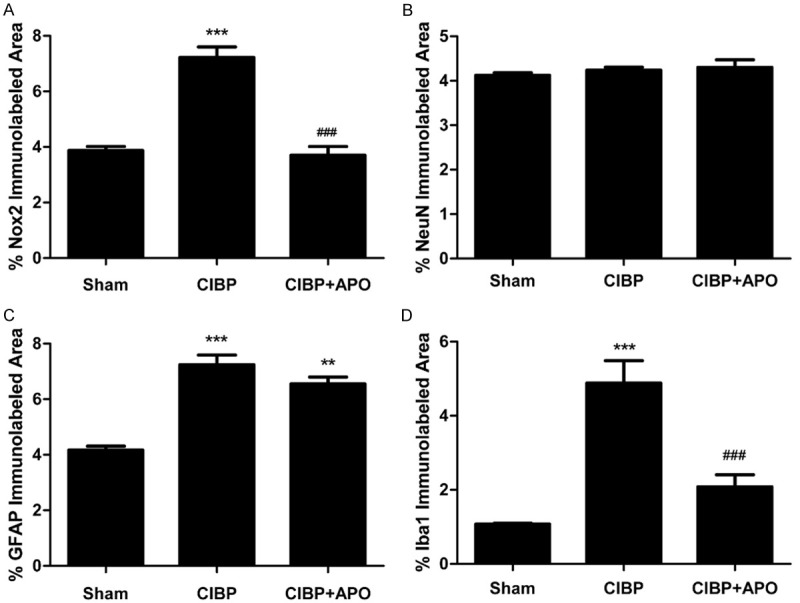
(A) Nox2 immunolabeled surface area (laminae I-IV; as depicted in A) were quantified using Image J software. (B) NeuN immunolabeled surface area (laminae I-IV; as depicted in A) were quantified using Image J software. (C) GFAP immunolabeled surface area (laminae I-IV; as depicted in A) were quantified using Image J software. (D) Iba1 immunolabeled surface area (laminae I-IV; as depicted in A) were quantified using Image J software. (**P < 0.01, ***P < 0.001 compared with the sham + DMSO group. ###P < 0.001 compared with the CIBP + DMSO group. n = 3 in each group).
Preventive effect of APO on the development of CIBP
To investigate whether early treatment of APO could inhibit or delay the development of CIBP, 200 mg/kg APO was given intraperitoneally once a day for 5 consecutive days from day 1 to day 5. PWTs were performed before the test and on day 3, 7, 14 and day 21. As shown in Figure 3C, PWTs were upregulated significantly in CIBP + 200 mg/kg APO group compared with CIBP + DMSO group at day 7 and day 14. However, there was no difference at day 21 between these two groups. These data illustrated that early treatment with 200 mg/kg APO from day 1 to day 5 could delay or slow, but inhibit the development of tumor-induced mechano-allodynia in rats.
Discussion
The current study first demonstrated that (1) tumor cells increased the expression of Nox2 in a dose-dependent manner; (2) chronic treatment with APO decreased the production of ROS, which plays an important role in the development of CIBP; (3) chronic treatment with APO exhibited accumulative antinociceptive effects without tolerance, which might be attributed to the suppression of microglia in the spinal cords of CIBP rats; (4) early treatment with APO could delay but failed to prevent the development of CIBP.
Among Nox enzyme family members, Nox2 is the most responsive to pain. Nox2 is known to play a major role as the enzyme carries out a variety of oxidative reactions [35]. In the present study, we first showed Nox2 protein expression was time dependently elevated as early as day 7 after tumor cells inoculation in the L4-5 spinal cord in rats, maintaining elevated until 21 days (Figure 2). These results demonstrated that the upregulation of Nox2 in the spinal cord might be involved in the occurrence and maintenance of CIBP. Chronic administration of 200 mg/kg APO for consecutive 5 days from day 14 to day 18 abolished PWTs in bone cancer pain rats without tolerance and reduced the expression of Nox2. These results for the first time suggested that spinal Nox2 was involved in the development of CIBP. Consistent with our western blot data, our immunofluorescence results confirmed that Nox2 was upregulated in CIBP + DMSO group when compared with naïve or sham + DMSO group. APO could significantly reduce the development of hyperalgesia and inhibit the upregulation of Nox2. Previously, it has been reported that treatment with 100 mg/kg APO completely prevented the development of hyperalgesia [25]. However, in the present study, APO (200 mg/kg, i.p.) could delay or slow the development of CIBP, but could not inhibit the development of CIBP (Figure 3C). The reasons remain to be further explored. We further conducted double immunofluorescence to examine the cellular localization of Nox2 in the spinal cord of CIBP rats. In the present study, we provided the first evidence that Nox2 was mainly colocalized with microglia and neurons in the L4-5 spinal cord of CIBP models (Figure 5A-C). Moreover, we have observed that APO obviously alleviated the activation of spinal microglia (Figures 5C and 6D). Thus, we concluded that APO may attenuate the expression of Nox2 via inhibiting the activation of spinal microglia (Figure 6). Previous reports have showed that microglia may be responsible for the initiation of pain hypersensitivity of neuropathic pain [36]. However, our previous study and others illustrated that microglia was not activated until day 14 (advanced phase) in CIBP rats [11]. Thus, it is possible that APO attenuated bone cancer pain by inhibiting the activation of microglia in the spinal cord in the advanced and late phase (day 14 to day 21). Further, it has been reported that APO could enhance osteogenesis and increase bone mass [37]. However, the underlying mechanisms remain elusive.
Previously, we have provided the first evidence that ROS are involved in the maintenance of CIBP [11]. However, the exact source of ROS under CIBP models has not been reported. Multiple resources have been reported to produce ROS in the CNS. Nox are the predominant source of ROS production [18,38] and it appeared to be the only enzyme whose sole function is to generate ROS [39]. In the present study, we observed that tumor cells implantation induced ROS production in the superficial dorsal horn of the spinal cord, and chronic treatment with 200 mg/kg APO could significantly decreased the production of ROS (Figure 7). As abovementioned, we suggested that NOX2 and ROS production was involved in the development of CIBP, and peripheral ROS generation could also be reduced by APO, suggesting that Nox2 in the spinal cord may be an important target for the development of therapeutic agents in CIBP. The precise mechanisms of tumor-induced ROS generation need to be investigated in future studies.
Collectively, these findings suggest that Nox2 inhibitors play an important role in the downregulation of the ROS production in the spinal cord, and demonstrates an extended critical window of efficacious CIBP treatment in clinic.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of P.R. China 81873732, 81371250 and 81571053.
Disclosure of conflict of interest
None.
References
- 1.Smith TJ, Saiki CB. Cancer pain management. Mayo Clin Proc. 2015;90:1428–1439. doi: 10.1016/j.mayocp.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J. Clin. Oncol. 2014;32:1647–1654. doi: 10.1200/JCO.2013.51.7219. [DOI] [PubMed] [Google Scholar]
- 3.Chen SP, Zhou YQ, Liu DQ, Zhang W, Manyande A, Guan XH, Tian YK, Ye DW, Omar DM. PI3K/Akt pathway: a potential therapeutic target for chronic pain. Curr Pharm Des. 2017;23:1860–1868. doi: 10.2174/1381612823666170210150147. [DOI] [PubMed] [Google Scholar]
- 4.WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Geneva: 2018. [PubMed] [Google Scholar]
- 5.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 6.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13:141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassler SN, Johnson KM, Hulsebosch CE. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J Neurochem. 2014;131:413–7. doi: 10.1111/jnc.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Jin MC. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152:844–852. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Chen F, Braun C, Zhou YQ, Rittner H, Tian YK, Cai XY, Ye DW. Role of curcumin in the management of pathological pain. Phytomedicine. 2018;48:129–140. doi: 10.1016/j.phymed.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Rittner H, Mei W, Tian YK, Zhang HX, Chen F, Ye DW. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol. 2018;14:391–397. doi: 10.1016/j.redox.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibi M, Matsuno K, Shiba D, Katsuyama M, Iwata K, Kakehi T, Nakagawa T, Sango K, Shirai Y, Yokoyama T, Kaneko S, Saito N, Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth KA, Maione S, de Novellis V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55:158–166. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 15.Choi SR, Roh DH, Yoon SY, Kang SY, Moon JY, Kwon SG, Choi HS, Han HJ, Beitz AJ, Oh SB, Lee JH. Spinal sigma-1 receptors activate NADPH oxidase 2 leading to the induction of pain hypersensitivity in mice and mechanical allodynia in neuropathic rats. Pharmacol Res. 2013;74:56–67. doi: 10.1016/j.phrs.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Kallenborn-Gerhardt W, Hohmann SW, Syhr KMJ, Schröder K, Sisignano M, Weigert A, Lorenz JE, Lu R, Brüne B, Brandes RP, Geisslinger G, Schmidtko A. Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain. 2014;155:2161–2170. doi: 10.1016/j.pain.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Doyle T, Esposito E, Bryant L, Cuzzocrea S, Salvemini D. NADPH-oxidase 2 activation promotes opioid-induced antinociceptive tolerance in mice. Neuroscience. 2013;241:1–9. doi: 10.1016/j.neuroscience.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle T, Bryant L, Muscoli C, Cuzzocrea S, Esposito E, Chen Z, Salvemini D. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett. 2010;483:85–89. doi: 10.1016/j.neulet.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cifuentes-Pagano E, Csanyi G, Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci. 2012;69:2315–2325. doi: 10.1007/s00018-012-1009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.J David L. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 21.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 22.Hou L, Sun F, Huang R, Sun W, Zhang D, Wang Q. Inhibition of NADPH oxidase by apocynin prevents learning and memory deficits in a mouse Parkinson’s disease model. Redox Biol. 2019;22:101134. doi: 10.1016/j.redox.2019.101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone F, Teixeira PC, Braunersreuther V, Mach F, Vuilleumier N, Montecucco F. Pathophysiology and treatments of oxidative injury in ischemic stroke: focus on the phagocytic NADPH oxidase 2. Antioxid Redox Signal. 2015;23:460–89. doi: 10.1089/ars.2013.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallenborn-Gerhardt W, Schroder K, Geisslinger G, Schmidtko A. NOXious signaling in pain processing. Pharmacol Ther. 2013;137:309–317. doi: 10.1016/j.pharmthera.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Olukman M, Onal A, Celenk FG, Uyanikgil Y, Cavusoglu T, Duzenli N, Ulker S. Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats. Neural Regen Res. 2018;13:1657–1664. doi: 10.4103/1673-5374.232530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao WC, Zhang B, Liao MJ, Zhang WX, He WY, Wang HB, Yang CX. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett. 2014;560:81–85. doi: 10.1016/j.neulet.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhou YQ, Chen SP, Liu DQ, Manyande A, Zhang W, Yang SB, Xiong BR, Fu QC, Song ZP, Rittner H, Ye DW, Tian YK. The role of spinal GABAB receptors in cancer-induced bone pain in rats. J Pain. 2017;18:933–946. doi: 10.1016/j.jpain.2017.02.438. [DOI] [PubMed] [Google Scholar]
- 29.Ge MM, Chen SP, Zhou YQ, Li Z, Tian XB, Gao F, Manyande A, Tian YK, Yang H. The therapeutic potential of GABA in neuron-glia interactions of cancer-induced bone pain. Eur J Pharmacol. 2019;858:172475. doi: 10.1016/j.ejphar.2019.172475. [DOI] [PubMed] [Google Scholar]
- 30.Chen SP, Sun J, Zhou YQ, Cao F, Braun C, Luo F, Ye DW, Tian YK. Sinomenine attenuates cancer-induced bone pain via suppressing microglial JAK2/STAT3 and neuronal CAMKII/CREB cascades in rat models. Mol Pain. 2018;14:1744806918793232. doi: 10.1177/1744806918793232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan X, Fu Q, Xiong B, Song Z, Shu B, Bu H, Xu B, Manyande A, Cao F, Tian Y. Activation of PI3Kgamma/Akt pathway mediates bone cancer pain in rats. J Neurochem. 2015;134:590–600. doi: 10.1111/jnc.13139. [DOI] [PubMed] [Google Scholar]
- 32.Song Z, Xiong B, Zheng H, Manyande A, Guan X, Cao F, Ren L, Zhou Y, Ye D, Tian Y. STAT1 as a downstream mediator of ERK signaling contributes to bone cancer pain by regulating MHC II expression in spinal microglia. Brain Behav Immun. 2017;60:161–173. doi: 10.1016/j.bbi.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Chen SP, Zhou YQ, Wang XM, Sun J, Cao F, HaiSam S, Ye DW, Tian YK. Pharmacological inhibition of the NLRP3 in fl ammasome as a potential target for cancer-induced bone pain. Pharmacol Res. 2019;147:104339. doi: 10.1016/j.phrs.2019.104339. [DOI] [PubMed] [Google Scholar]
- 34.Fu Q, Shi D, Zhou Y, Zheng H, Xiang H, Tian X, Gao F, Manyande A, Cao F, Tian Y, Ye D. MHC-I promotes apoptosis of GABAergic interneurons in the spinal dorsal horn and contributes to cancer induced bone pain. Exp Neurol. 2016;286:12–20. doi: 10.1016/j.expneurol.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Fiorella K, Eik H, Sebastian A, Ariel S. Reactive oxygen species production in the phagosome: impact on antigen presentation in dendritic cells. Antioxid Redox Signal. 2013;18:714–729. doi: 10.1089/ars.2012.4557. [DOI] [PubMed] [Google Scholar]
- 36.Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32:972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Ming L, Shang F, Shen L, Chen J, Jin Y. Apocynin suppression of NADPH oxidase reverses the aging process in mesenchymal stem cells to promote osteogenesis and increase bone mass. Sci Rep. 2015;5:18572. doi: 10.1038/srep18572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stavros S, Sobey CG, Kirstin W, Schmidt HHHW, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]