Abstract
Acute pancreatitis (AP) is commonly accompanied by intense pain and is associated with high mortality rates. However, the effectiveness of existing therapeutic approaches remains unsatisfactory. Stem cell therapy, which can promote the regeneration of damaged tissue and alleviate systemic inflammatory responses, has brought new possibility for patients suffering from AP. In particular, hair follicle-derived mesenchymal stem cells (HF-MSCs) are proposed as a suitable cell source for treating pancreatic diseases, but further research on their effectiveness, safety, and underlying mechanisms is warranted for clinical implementation. In this work, the therapeutic potential of HF-MSC transplantation was studied in an L-arginine-induced AP rat model. HF-MSCs were extracted from infant Sprague-Dawley (SD) rats, expanded in vitro, and detected by flow cytometry. HF-MSCs were labeled by PKH67 and transplanted into rats with AP via tail vein injection. Serum specimens were collected at 24 h, 48 h, and 72 h after transplantation, and the levels of amylase, lipase, and anti-inflammatory factors, namely interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), were analyzed. Pancreas samples were collected and assayed by immunofluorescence and immunohistochemistry 1 week after transplantation to monitor the differentiation of HF-MSCs and the functional recovery of the damaged pancreas. Intravenously delivered rat HF-MSCs spontaneously homed to the damaged pancreas and expressed pancreatic progenitor cell markers, relieved inflammation, and boosted pancreatic regeneration. These findings indicate that HF-MSC transplantation is a potentially effective treatment for AP.
Keywords: Hair follicle mesenchymal stem cells, acute pancreatitis, cell transplantation, homing, differentiation
Introduction
Acute pancreatitis (AP) is an autodigestive pancreatic disease with a high mortality rate and a complex pathogenic etiology. The incidence of AP is approximately 4/10,000 worldwide, and the associated morbidity has been substantially increasing annually [1]. Among these patients, 90-95% has mild or moderate-to-severe AP characterized by relatively minor symptoms and a favorable prognosis. Severe AP in the remaining 5-10% of patients is typically accompanied by severe complications such as shock, heart failure, pancreatic encephalopathy, and even systemic inflammatory response syndrome (SIRS). AP often co-occurs with islet dysfunction, which leads to hyperglycemia [2]. Severe hyperglycemia can exacerbate secondary infections, which eventually leads to insulin resistance, resulting in an adverse feedback loop that fuels AP progression. More importantly, AP associated islet dysfunction can progress to pancreatic diabetes. Pancreatic progenitor cells, which play a significant role in islet functional recovery and damaged pancreatic regeneration [3], are important prognostic factors for patients with AP. The main therapeutic strategies for AP currently involve the resolution of symptoms by anti-inflammatory, anti-shock, pancreatic secretion inhibition, and other treatments, but these are not particularly effective [4].
Stem cells have recently attracted attention as treatment of many refractory diseases. Characterized by self-renewal ability and pluripotent differentiation, mesenchymal stem cells (MSC) are multipotent stem cells with widespread clinical applications [5]. These cells lack major histocompatibility complex II antigen [6], making them ideal seed cells for transplantation therapies. The therapeutic effects of MSC in several autoimmune, traumatic, ischemic, and inflammatory diseases are excellent [7]. Similarly, MSC have been considered as a prospective therapy for AP because of their homing capacity, and anti-inflammatory, anti-apoptotic, and immunomodulatory effects [8-11].
Hair follicles (HF) are mammalian skin appendages with a stem cell-abundant, archetypal neuroectodermal-mesodermal interaction system [12]. Mammals have ample HF reserves, which undergo cycles of anagen, catagen, and telogen phases, throughout their lifespan [13]. Unlike other stem cell sources, HF collection is noninvasive for donors. Stem cells derived from HF are primarily located in the hair bulb and external root sheath, and are characterized by pluripotential differentiation and powerful proliferation ability [14,15]. Transplanted HF-MSC participate in angiogenesis and accelerate the regeneration and repair of damaged tissues [16,17], indicating that they might stimulate pancreatic cell proliferation and thus promote post-injury pancreatic regeneration like other MSC [18,19]. However, the potential of HF-MSC as a therapeutic tool for AP has not yet been directly investigated.
According to the above advantages, transplanted HF-MSC might spontaneously home to the damaged pancreas and differentiate into pancreatic progenitor cells, then significantly contributing to regeneration and reprogramming of the damaged pancreas. To validate this hypothesis, we established a rat model of AP induced by L-arginine, in which rats were injected with HF-MSC or saline (sham control). The homing ability of fluorescently labeled HF-MSC to the pancreas and their differentiation was monitored, along with pathological changes to the damaged pancreas. Our findings provide a theoretical reference for the development of HF-MSC as a novel treatment strategy for AP.
Materials and methods
Experimental animals
Forty-eight 7-9-week-old and four 7-10-day-old male Sprague-Dawley (SD) rats weighing 170-250 and 21-27 g, respectively, were obtained from the Animal Facility of the Second Affiliated Hospital of Harbin Medical University, Harbin, China. All rats were housed in single cages in an animal room at 24°C with a 12/12 h light/dark cycle and fed with standard laboratory rodent food and water. All animal experiments were conducted in compliance with the relevant ethical standards and regulations approved by the Ethics Committee at the Experimental Center of the Second Affiliated Hospital of Harbin Medical University.
Extraction and culture of HF-MSC
Primary HF-MSC were derived from the four 7-9 day-old SD rats. The skin of the vibrissa was cut and digested with 0.1% collagenase, then HF were gently peeled from the skin under observation using a binocular microscope (Nikon, Japan). The isolated HF were incubated for 40 min at 37°C under a humidified 5% CO2 atmosphere in 24-well plates that were coated with collagen IV. Complete medium comprised 90% DMEM/F12 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin (all from Gibco Laboratories, Gaithersburg, MD, USA). The cell culture medium was refreshed every 15 h and non-adherent cells were discarded with the spent culture medium. When cells with a paving stone appearance adhered around the HF, the culture medium was refreshed every 48 h. Extracted primary cells were passaged after approximately 2 weeks, and third-generation HF-MSC with good growth characteristics were harvested. All procedures were conducted in a sterile environment.
The cells were labeled with the green fluorescent cell membrane dye PKH67 (Sigma-Aldrich Corp., St. Louis, MO, USA) as described by the manufacturer and detected using a BX51 fluorescence microscope (Olympus Corporation, Tokyo, Japan). When cell viability measured by trypan blue staining reached 95%, the cell suspension was adjusted to approximately 1 × 106 cells/mL before transplantation.
Flow cytometry of HF-MSC
The extracted HF-MSC was assessed by fluorescence-activated cell sorting. The cells were detected by flow cytometry (Becton Dickinson and Co., Franklin Lakes, NJ, USA). Approximately 1 × 106 cells were incubated with 1.0 µL of rat monoclonal antibodies against CD31, CD45, CD29, and CD90 (Becton Dickinson and Co.), and the isotype control antibody served as the negative control. Fluorescence emission was evaluated using CellQuest software (Becton Dickinson and Co).
Rat model of acute pancreatitis
Rats (n = 48) weighing 270 ± 10 g were randomly allocated (n = 16/group) to the following groups. The AP group was injected with 2.0 g/kg L-arginine in 0.9% normal saline twice daily at 1.5 h intervals for 3 days, and once intravenously (i.v.) with 0.9% normal saline into the tail vein 24 h later [20]. The sham group injected was intraperitoneally (i.p.) with of 0.9% normal saline. The AP+HF-MSC group was injected i.p. with 2.0 g/kg L-arginine in 0.9% normal saline, followed by an i.v. injection of 5 × 106 cells/kg body weight of PKH67-labeled HF-MSC into the tail vein 24 h later. Rats from each group were sacrificed at 24, 48, and 72 h after HF-MSC transplantation for serological and histopathological analyses. The remaining rats were sacrificed 1 week after transplantation for histological analysis of the pancreas.
Hematoxylin-eosin (HE) staining and immunohistochemistry
Pancreases were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and cut into 5-mm thick sections for hematoxylin and eosin (HE) staining. Morphological changes in the pancreas in each group were compared using an optical microscope (Nikon Corp., Tokyo, Japan). Pathological damage induced by pancreatitis was scored as described [21,22]. The sections were submerged in 3% H2O2 for 10 min, then non-specific binding was blocked with 10% normal donkey serum for 30 min. The sections were incubated with rabbit anti-pancreatic and duodenal homeobox 1 (PDX-1; Abcam, UK; 1:200), mouse anti-neurogenin-3 (Ngn3; Santa Cruz Biotechnology, USA; 1:100), and mouse anti-pancreas specific transcription factor 1a (PTF-1a; Santa Cruz Biotechnology; 1:200) at 4°C overnight, followed by 1:200-diluted TRITC-conjugated AffiniPure goat anti-rabbit or anti-mouse IgG antibody (Zhongshan Golden Bridge Biotechnology, Beijing, China) for 30 min, followed by DAB chromogenic substrate (Zhongshan Golden Bridge Biotechnology Co.), then counterstained with hematoxylin, dehydrated, and mounted. The stained sections of each group were assessed using an optical microscope (Nikon, Japan), and positive areas were semi-quantitatively scored and statistically analyzed using Image-Pro Plus 7.0 software.
Immunofluorescence
Immunofluorescence assays of the pancreas were conducted as described [20]. In brief, pancreatic sections were incubated with 5% normal donkey serum and 1:200-diluted rabbit anti-PDX-1 (Abcam, Cambridge, UK, 1:400), mouse anti-Ngn3 (Santa Cruz Biotechnology, USA, 1:200), and mouse anti-PTF-1a (Santa Cruz Biotechnology Inc., Dallas, TX, USA) primary antibodies at 4°C overnight. After rinsing with PBS, the sections were reacted with anti-rabbit/mouse IgG (1:200) for 30 min. Nuclei were stained with 4’-6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA, USA) for 5 min, then the sections were mounted with an anti-fluorescence quenching agent. Representative images were captured using a laser confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany). All experimental procedures described above were conducted in a dark room.
Serological analysis
Rat blood specimens were collected from the abdominal aorta at 24, 48, and 72 h after HF-MSC transplantation. The contents of serum amylase and lipase (both Sigma-Aldrich), TNF-α and IL-6 (both USCN Business Co., Ltd., Wuhan, China) were measured as described by the manufacturers of the respective ELISA kits. Absorbance at 450 nm was measured using a microplate reader (BioTek U.S., Winooski, VT, USA). Values for each factor were calculated by comparisons with standard curves.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA), Chi-square tests and Student t-tests using SPSS 17.0 statistical software, and are expressed as means ± SEM. Statistical charts were prepared using GraphPad Prism 8.0 software. Values with P < 0.05 were defined as being statistically significant.
Results
Isolation, culture, and identification of HF-MSC
The primary HF-MSC cultured in vitro were characterized by adherent growth with a largely paving-stone appearance (Figure 1A). The morphology of HF-MSC gradually transitioned to a spindle-like appearance in generations 2-3 (Figure 1B and 1C). The cell membrane and nuclei were detected by microscopy as bright green under fluorescence emitted by PKH67, and as blue DAPI stain, respectively (Figure 1D).
Figure 1.
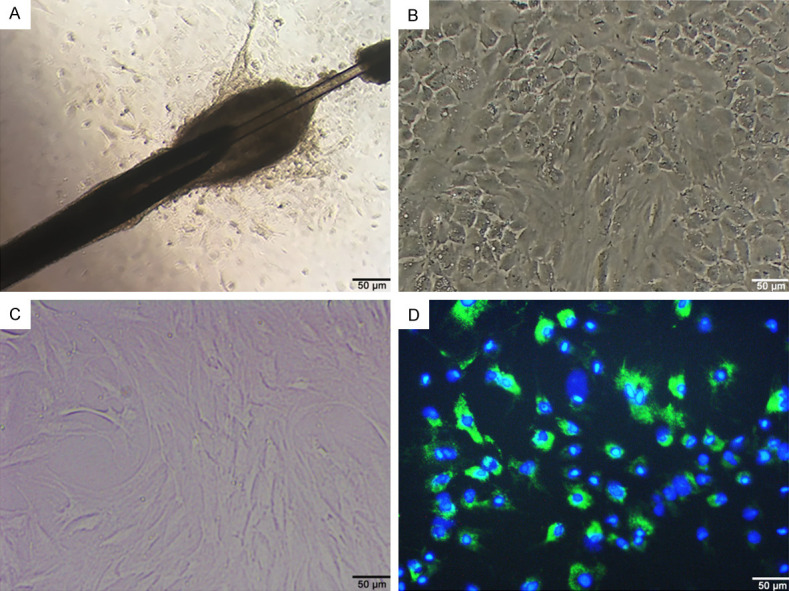
Culture and PKH67 labeling of HF-MSC. Primary (A), second-generation (B), third-generation (C), and PKH67-labeled (D) HF-MSC. Scale bar, 100 μm. HF-MSC, hair follicle mesenchymal stem cells; PKH67, green, fluorescent stain for cell membranes.
Flow cytometry showed that 97.79% and 97.50% of the cells expressed the MSC markers CD29 and CD90, respectively (Figure 2A and 2B). In contrast, few cells expressed hematopoietic stem cell markers; 1.60% and 2.30% of the cells expressed CD45 and CD31, respectively. Therefore, the cells extracted from HF were mainly HF-MSC (Figure 2C, 2D).
Figure 2.
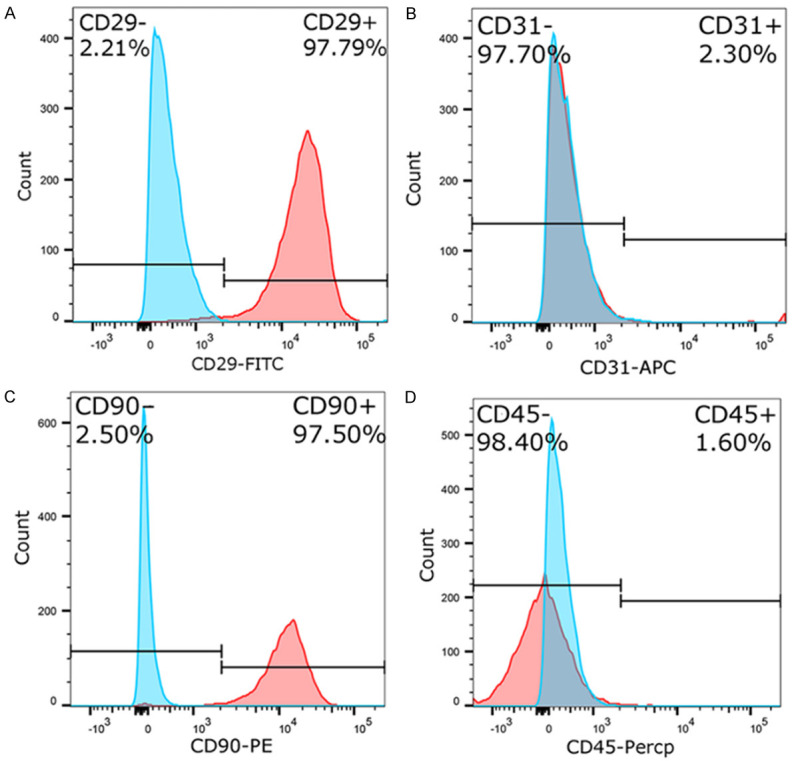
Fluorescence-activated cell sorting analysis. A. CD29 labeled with FITC. B. CD31 labeled with APC. C. CD90 labeled with PE. D. CD45 labeled with PerCP. APC, allophycocyanin; CD, cluster of differentiation; PE, phycoerythrin; PerCP, peridinin-chlorophyll-protein.
Transplanted HF-MSC alleviated pancreatic injury
Establishment of the AP rat model and restoration of the pancreas after HF-MSC transplantation were verified by HE staining (Figure 3). Optical microscopy revealed no obvious edema or inflammatory infiltration in pancreatic tissues from the sham group (Figure 3A). In contrast, pancreatic tissues from the AP group showed cell edema, necrosis, hemorrhage, and inflammatory infiltration. Scores for pathological pancreatic injury were obviously lower in the AP+HF-MSC, than in the AP group, and gradually decreased over time (P < 0.05, Figure 3D).
Figure 3.
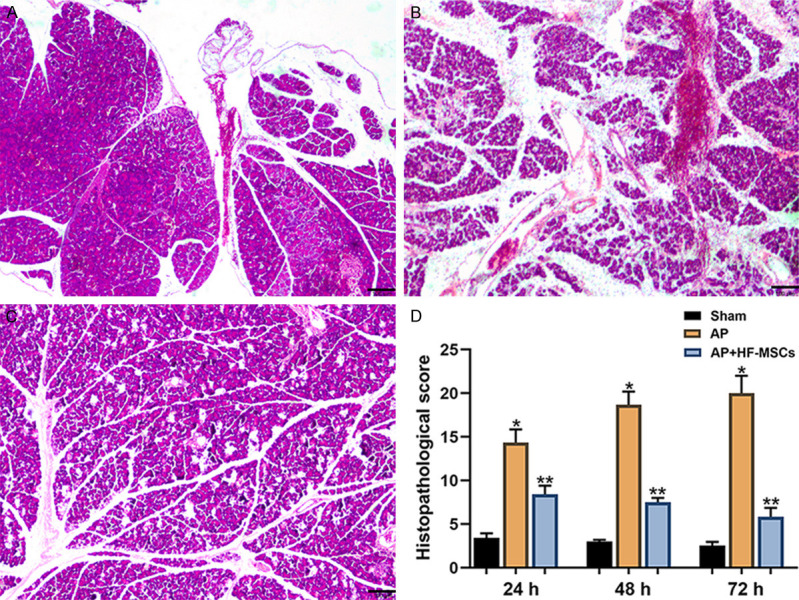
Pathological changes in the pancreas. Representative images of hematoxylin-eosin staining of (A) sham (B) rats injected with L-arginine (AP group) and (C) rats transplanted 3 days after L-arginine injection (AP+HF-MSC group). Scale bars = 100 μm. (D) Histopathological scores of groups at different time points (*P < 0.05; †P < 0.001). AP, acute pancreatitis; HE, hematoxylin-eosin; HF-MSC, hair follicle-mesenchymal stem cells.
HF-MSC transplantation reduced mortality of rats with AP
At 1, 2, and 7 days post-transplantation, all rats in the sham group survived, 1, 3, and 4 rats, in the AP group died, respectively, and 1 each in the AP+HF-MSC group died at these time points, respectively. Mortality did not significantly differ between the AP and AP+HF-MSC group at 24 h (P > 0.05), but became obviously reduced over time in the AP+HF-MSC (P < 0.05).
HF-MSC inhibited systemic inflammatory responses in rats with AP
Serum amylase, lipase, TNF-α, and IL-6 levels were monitored at daily intervals (24, 48, and 72 h) in the three animal groups after transplantation. Serum levels of amylase, lipase, and inflammatory factors (TNF-α, IL-6) in the AP group obviously increased, then gradually decreased over time in the AP, compared with the sham group (P < 0.05). Levels of serum amylase, TNF-α, and IL-6 significantly decreased in the AP+HF-MSC, compared with that in the AP group (P < 0.05, Figure 4).
Figure 4.
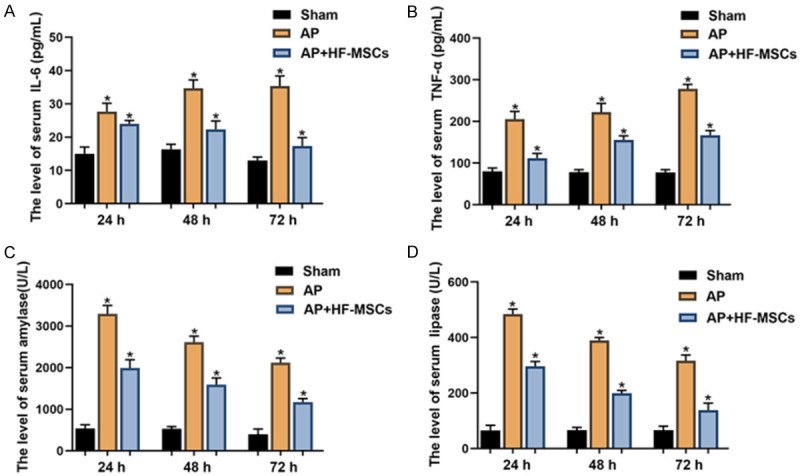
Serological analysis. Serum levels of (A) IL-6, (B) TNF-α, (C) amylase, and (D) lipase in three groups at 24, 48, and 72 h post-transplantation (n = 4 per group).
HF-MSC homed to damaged pancreas and expressed pancreatic progenitor cell markers
Immunofluorescence staining and confocal laser scanning microscopy showed co-localized PKH67-labeled transplanted cells, pancreatic progenitor cell markers (PDX-1, Ngn3, PTF-1a), and nuclei in the AP+HF-MSC group (Figure 5). The cell membrane stained with PKH67 emitted bright green fluorescence, and cell proliferation capacity was unimpaired. The HF-MSC clustered in the damaged pancreas, particularly in the islets, and rarely in the liver, spleen, and lung. Neurogenin 3, PDX-1, and PTF-1a are specific markers of pancreatic progenitor cells with an important influence on pancreas restoration and regeneration. Preliminary experiments revealed that HF-MSC localized in the damaged pancreas at 24 h after transplantation, but only a few cells expressed markers of pancreatic progenitor cells. However, most transplanted cells expressed pancreatic progenitor cell markers in pancreatic tissues at one week after transplantation. PKH67-positive cells (green) expressed markers of pancreatic progenitor cells (PDX-1, Ngn3, PTF-1a) visualized as red fluorescence. The cytoplasm appeared yellow or white, and nuclei were stained blue with DAPI (Figure 5).
Figure 5.
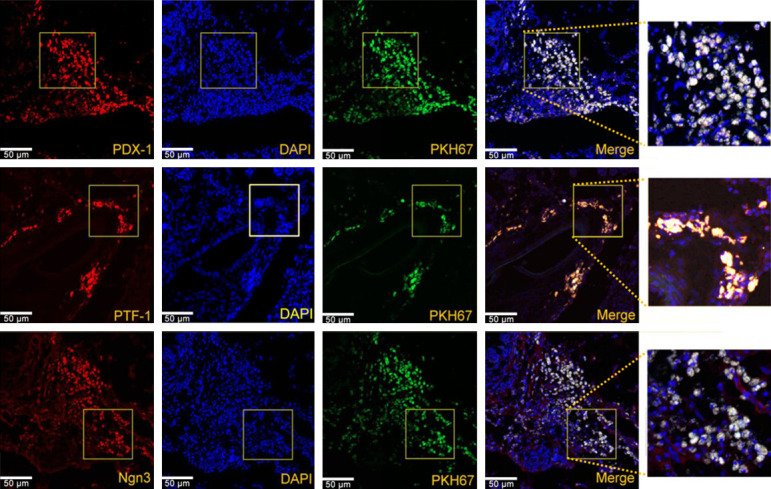
Immunofluorescence emission of PDX-1, Ngn3, and PTF-1a after HF-MSC transplantation. Co-localized, transplanted HF-MSC and pancreatic progenitor cell markers in pancreas. PKH67-labeled cells (green) are co-localized with pancreatic progenitor cell markers PDX-1, PTF-1a, and Ngn3. Nuclei are labeled with DAPI (blue). PKH67-positive cells are scant in rat spleen, lung, and liver. Scale bar, 50 μm.
HF-MSC transplantation promoted expression of pancreas-associated proteins
Immunohistochemical analysis showed that PDX-1-, Ngn3-, and PTF-1a-positive cells were more prevalent in the pancreas of rats in the AP+HF-MSC, compared with the other two groups. The sham group had the least PDX-1-, Ngn3-, or PTF-1a-positive cells (P < 0.05 for all; Figure 6). These findings indicated that pancreatic cell regeneration significantly improved after HF-MSC transplantation.
Figure 6.
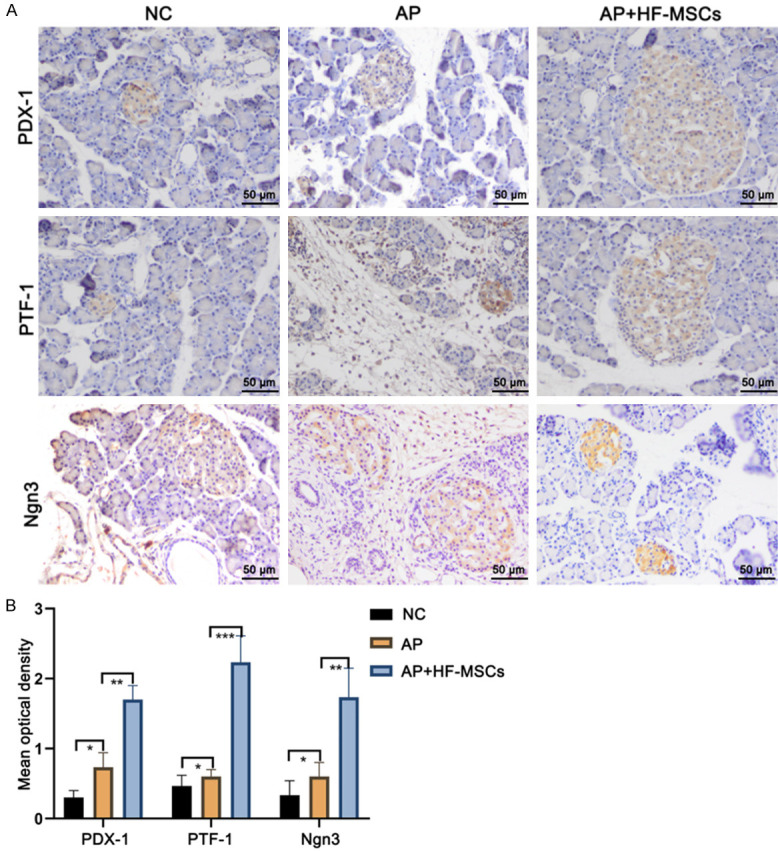
Immunohistochemical detection of proteins in pancreas. A. Sharp increase in PDX-1-, Ngn3-, and PTF-1a-positive cells in AP+HF-MSC group. B. Quantitative findings of pancreatic progenitor marker proteins in three groups. *P < 0.05; Scale bar, 50 μm.
Discussion
To date, therapeutic options for AP are limited to symptom management. In addition to critical clinical manifestations, severe AP is accompanied by various complications that severely endanger the lives of patients [23]. Therefore, MSC transplantation, which promotes regeneration of the damaged pancreas while simultaneously relieving inflammation, is considered to have significant therapeutic potential for treating AP. Saito et al. [24] first proposed the homing ability of transplanted MSC to spontaneously move to target organs or tissues under specific conditions. Damaged tissues or organs release various cytokines, chemokines, and adhesion factors that guide MSC to damaged sites, where they play key roles in regeneration and restoration.
Bone marrow mesenchymal stem cells (BMSC) have been transplanted in most studies of AP [18]. However, invasive BMSC collection can cause serious trauma and infections, and age restrictions limit donor selection. Therefore, determining an alternative optimal cell source for transplantation has remained crucial for clinical applications. The present study expanded and characterized HF-MSC in vitro, then applied them to treat rats with AP in vivo. The therapeutic outcomes were undeniably promising. The HF-MSC offer important and substantial advantages over other MSC sources. Their multi-directional differentiation potential, abundant reserves, non-invasive collection and convenient separation renders HF-MSC ideal therapeutic candidates.
To the best of our knowledge, this is the first empirical demonstration of MSC from hair follicles differentiating into pancreatic progenitor cells in a rat model of AP. It has been demonstrated that, in a rat model of chronic pancreatitis, MSC differentiate into acinar-like cells at 2 weeks after transplantation [25]. We harvested and assessed differentiation into pancreatic cells at 1 week after transplantation because the focus of the present study was AP. PKH67 is a stable cell membrane marker with low cytotoxicity that has minimal effects on cell proliferation or differentiation [26]. Therefore, labeling cells with PKH67 enabled fluorescence-based tracking of transplanted HF-MSC homing and expansion in vivo. The transplanted HF-MSC thrived in the recipient pancreas, and spontaneously homed to the damaged pancreas without causing tumorigenesis. Moreover, few transplanted HF-MSC were detected in the liver, lung, kidney, and other important organs, indicating that transplantation is unlikely to cause embolization in rats. Most importantly, co-localized PKH67, DAPI staining, and specific markers of pancreatic progenitor cells (Ngn3, PDX-1, and PTF-1a) indicated that the transplanted HF-MSC retained their capacity for proliferation and differentiation in vivo after implanting in the damaged pancreas.
Neurogenin 3, PDX-1, and PTF-1a are pivotal factors that regulate pancreatic proliferation and differentiation. Insulin synthesis and secretion are regulated by PDX-1 that is expressed mostly in islets, where it directly interacts with the insulin promoter [27]. Lee et al. [28] found that mesenchymal cells derived from human adipose tissue differentiate into insulin-producing cell aggregates via PDX-1 overexpression in the pancreas and duodenum. Morphogenesis of the pancreatic ductal epithelium is controlled by Ngn3, a crucial marker of pancreatic progenitor cells [29]. Pancreas specific transcription factor 1a regulates the fate of pancreatic epithelial cells during early development and determines whether cells in the embryo will continue to home toward the pancreas or the duodenum [30]. Acute pancreatitis is commonly accompanied by the islet structural damage and islet cell necrosis [31]. The transplanted HF-MSC homed to the damaged pancreas and specifically implanted in islets while expressing PDX-1, Ngn3, and PTF-1a. These activities indicate their potential to protect islet function, and consequently improve the prognosis of AP, which will be investigated in-depth in the near future.
Improving the homing rate of transplanted MSC is an important but complex issue for stem cell transplantation, which is at the early research stage. The specific mechanism underlying HF-MSC homing also requires further investigation. Stromal cell-derived factor-1 (SDF-1) and its receptor CXCR4 reportedly affect the homing process of MSC [32], which might also apply to HF-MSC.
One limitation of the current study is the lack of an objective index with which to measure pain in the rat models of AP. A change in physical activity might serve as a proxy, but is not a direct measurement of the degree of pain. Furthermore, the experimental period was not long enough to evaluate the potential long-term complications of HF-MSC transplantation, such as tumorigenesis and graft versus host disease. This study was limited to assessing the capacity of HF-MSC to differentiate in vivo. Therefore, HF-MSC and pancreatic acinar cells should be co-cultured to verify HF-MSC differentiation in vitro.
Despite these limitations, the present study confirmed the therapeutic potential of HF-MSC transplantation in a rat model of AP. The extracted HF-MSC largely retained the proliferation and differentiation potential of primary cells after multi-generation culture. Moreover, transplanted HF-MSC homed, differentiated, and exerted potent anti-inflammatory effects in the rat model of AP, providing an essential basis that will guide future clinical applications of HF-MSC transplantation to patients with AP.
Conclusion
Transplanted HF-MSC can alleviate pancreatic damage by homing toward injured sites and expressing pancreatic progenitor cell markers that lead to the continuous inhibition of inflammatory factor release while promoting the regeneration of damaged pancreatic tissues. These findings provide evidence of a novel strategy for treating AP.
Disclosure of conflict of interest
None.
References
- 1.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 2.Deng WH, Chen C, Wang WX, Yu J, Li JY, Liu L. Effects of ORP150 on appearance and function of pancreatic beta cells following acute necrotizing pancreatitis. Pathol Res Pract. 2011;207:370–376. doi: 10.1016/j.prp.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011;11:279–294. doi: 10.1159/000329188. [DOI] [PubMed] [Google Scholar]
- 4.Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A, Bonner-Weir S. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci. 2010;123:2792–802. doi: 10.1242/jcs.065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479–496. doi: 10.1038/s41575-019-0158-2. [DOI] [PubMed] [Google Scholar]
- 6.Kim N, Cho SG. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol. 2016;103:129–37. doi: 10.1007/s12185-015-1918-6. [DOI] [PubMed] [Google Scholar]
- 7.Kholodenko IV, Kurbatov LK, Kholodenko RV, Manukyan GV, Yarygin KN. Mesenchymal stem cells in the adult human liver: hype or hope? Cells. 2019;8:1127. doi: 10.3390/cells8101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras-Kallens P, Terraza C, Oyarce K, Gajardo T, Campos-Mora M, Barroilhet MT, Álvarez C, Fuentes R, Figueroa F, Khoury M, Pino-Lagos K. Mesenchymal stem cells and their immunosuppressive role in transplantation tolerance. Ann N Y Acad Sci. 2018;1417:35–56. doi: 10.1111/nyas.13364. [DOI] [PubMed] [Google Scholar]
- 9.Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider G, Saur D. Mesenchymal stem cells: therapeutic potential for acute pancreatitis. Gastroenterology. 2011;140:779–82. doi: 10.1053/j.gastro.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Qian D, Song G, Ma Z, Wang G, Jin L, Hu M, Song Z, Wang X. MicroRNA-9 modified bone marrow-derived mesenchymal stem cells (BMSCs) repair severe acute pancreatitis (SAP) via inducing angiogenesis in rats. Stem Cell Res Ther. 2018;9:282. doi: 10.1186/s13287-018-1022-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Yi R. Concise Review: mechanisms of quiescent hair follicle stem cell regulation. Stem Cells. 2017;35:2323–2330. doi: 10.1002/stem.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Pang L, Zhao H, Song L, Wang Y, Sun Q, Guo C, Wang B, Qin X, Pan A. mir-128 regulates differentiation of hair follicle mesenchymal stem cells into smooth muscle cells by targeting SMAD2. Acta Histochem. 2016;118:393–400. doi: 10.1016/j.acthis.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Mapar MA, Safarpour M, Mapar M, Haghighizadeh MH. A comparative study of the mini-punch grafting and hair follicle transplantation in the treatment of refractory and stable vitiligo. J Am Acad Dermato. 2014;70:743–747. doi: 10.1016/j.jaad.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y. From hair to heart: hair follicle stem cells differentiate to beating cardiac muscle cells. Cell Cycle. 2015;14:2362–66. doi: 10.1080/15384101.2015.1042633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle. 2008;7:1865–69. doi: 10.4161/cc.7.12.6056. [DOI] [PubMed] [Google Scholar]
- 18.Cai J, Zhou X, Yu H, Xue H, Li D. Effect of bone marrow mesenchymal stem cells on RhoA/ROCK signal pathway in severe acute pancreatitis. Am J Transl Res. 2019;11:4809–4816. [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Su J, Xi SS, Ke XF, Zhu Y, Lin HP, Zeng XK, Liu BW, Zhu ML, Dai WY, Hu W. Human umbilical cord mesenchymal stem cells pretreated with Angiotensin-II attenuate pancreas injury of rats with severe acute pancreatitis. Biomed Pharmacother. 2019;11:4809–4816. doi: 10.1016/j.biopha.2019.109052. [DOI] [PubMed] [Google Scholar]
- 20.Qu B, Chu Y, Zhu F, Wang B, Liu T, Yu B, Jin S. Granulocyte colony-stimulating factor enhances the therapeutic efficacy of bone marrow mesenchymal stem cell transplantation in rats with experimental acute pancreatitis. Oncotarget. 2017;8:21305–21314. doi: 10.18632/oncotarget.15515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt J, Rattner DW, Lewandrowski K. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grewal HP, Mohey el Din A, Gaber L, Kotb M, Gaber AO. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody. Am J Surg. 1994;167:214–218. doi: 10.1016/0002-9610(94)90076-0. discussion 218-219. [DOI] [PubMed] [Google Scholar]
- 23.Horibe M, Sasaki M, Sanui M, Sugiyama D, Iwasaki E, Yamagishi Y, Sawano H, Goto T, Ikeura T, Hamada T, Oda T, Yasuda H, Shinomiya W, Miyazaki D, Hirose K, Kitamura K, Chiba N, Ozaki T, Yamashita T, Koinuma T, Oshima T, Yamamoto T, Hirota M, Moriya T, Shirai K, Mayumi T, Kanai T. Continuous regional arterial infusion of protease inhibitors has no efficacy in the treatment of severe acute pancreatitis: a retrospective multicenter cohort study. Pancreas. 2017;46:510–517. doi: 10.1097/MPA.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002;74:19–24. doi: 10.1016/s0003-4975(02)03591-9. discussion 24. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Gou W, Kim DS, Dong X, Strange C, Tan Y, Adams DB, Wang H. Adipose stem cell therapy mitigates chronic pancreatitis via differentiation into acinar-like cells in mice. Mol Ther. 2017;25:2490–2501. doi: 10.1016/j.ymthe.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagyova M, Slovinska L, Blasko J, Grulova I, Kuricova M, Cigankova V, Harvanova D, Cizkova D. A comparative study of PKH67, DiI, and BrdU labeling techniques for tracing rat mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2014;50:656–663. doi: 10.1007/s11626-014-9750-5. [DOI] [PubMed] [Google Scholar]
- 27.McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia. 2001;44:1203–1214. doi: 10.1007/s001250100628. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Kim SC, Kim SJ, Lee H, Jung EJ, Jung SH, Han DJ. Differentiation of human adipose tissue-derived stem cells into aggregates of insulin-producing cells through the overexpression of pancreatic and duodenal homeobox gene-1. Cell Transplant. 2013;22:1053–1060. doi: 10.3727/096368912X657215. [DOI] [PubMed] [Google Scholar]
- 29.Magenheim J, Klein AM, Stanger BZ, Ashery-Padan R, Sosa-Pineda B, Gu G, Dor Y. Ngn3+ endocrine progenitor cells control the fate and morphogenesis of pancreatic ductal epithelium. Dev Biol. 2011;359:26–36. doi: 10.1016/j.ydbio.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang YJ, Park JT, Parsons MJ, Leach SD. Fate mapping of ptf1a-expressing cells during pancreatic organogenesis and regeneration in zebrafish. Dev Dyn. 2015;244:724–35. doi: 10.1002/dvdy.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Kuroda Y. Long-term outcome of severe acute pancreatitis. J Hepatobiliary Pancreat Surg. 2008;15:397–402. doi: 10.1007/s00534-007-1266-x. [DOI] [PubMed] [Google Scholar]
- 32.Yu Q, Liu L, Lin J, Wang Y, Xuan X, Guo Y, Hu S. SDF-1α/CXCR4 axis mediates the migration of mesenchymal stem cells to the hypoxic-ischemic brain lesion in a rat model. Cell J. 2015;16:440–7. doi: 10.22074/cellj.2015.504. [DOI] [PMC free article] [PubMed] [Google Scholar]


