Abstract
Objective: This study was designed to analyse the effect of combined application of iguratimod with methotrexate in the treatment of active rheumatoid arthritis (RA). Methods: A total of 115 patients with active RA admitted to our hospital were enrolled and divided into group A (n=58) and group B (n=57) according to the method of random number table. Patients in group B were treated with methotrexate alone, while patients in group A were treated with methotrexate combined with iguratimod. The curative efficacy was compared between the two groups. Results: At 6 months after treatment, the levels of CTX-1 and RANKL in group A were higher than those in group B, and the levels of OPG, IL-17 and TGF-α in group A were lower than those in group B (P<0.05). The level of Th17 cells in group A was higher than that in group B, and the level of Treg cells in group A was lower than that in group B at 6 months after treatment (P<0.05). Tender joints count, swollen joints count and DAS28 score in group A were less than those in group B at 6 months after treatment (P<0.05). The duration of morning stiffness of the joints and the score of joint pain degree in group A were less than those in group B at 1, 2, 3, 4, 5, and 6 months after treatment (P<0.05). Conclusion: The combined application of methotrexate and iguratimod in the treatment of active RA can effectively improve bone metabolism, regulate the levels of Th17 and Treg cells, play a prominent role in anti-inflammatory effect, and relieve symptoms, and thus achieve a more satisfactory curative effect.
Keywords: Rheumatoid arthritis, activity, treatment, iguratimod, bone metabolism, Th17 cells, Treg cells
Introduction
Rheumatoid arthritis (RA) is a type of inflammatory disease that occurs in synovial joints, with the incidence of about 1% in the population [1]. If patients with RA fail to receive timely treatment, the synovial joints will be more seriously affected with the slow progression of the disease, causing stiffness and pain of joints, which will affect physical function or eventually lead to disability [2].
Regarding the treatment of RA, the application of anti-rheumatic drugs can relieve symptoms, which mainly regulates the function of immune system to correct joint damage and joint inflammation caused by immune dysfunction [3]. Due to the lack of good specificity of target, anti-rheumatic drugs need relatively a longer time to take effects, but the effect can also last for a long time after they start working [4]. Methotrexate is a type of drug that has been widely used in the treatment of RA and is believed to have an obvious effect on patients with common RA [5]. However, due to the complicated pathogenesis of RA, and the heterogeneity of disease manifestations, some patients treated with methotrexate alone have low curative effect [6]. Therefore, with the extensive research and the emergence of more drug types, the current clinical practice is more focused on the treatment of RA in combination with different drugs so as to improve the effectiveness of drug treatment. Iguratimod is a small molecular anti-rheumatic drug, which has been proved safe and effective in the treatment of active RA, and is well tolerated when combined with methotrexate [7].
Previous studies have mostly focused on the monotherapy of RA with iguratimod or methotrexate, or the two drugs respectively combined with other drugs. The effectiveness and safety of combination therapy of iguratimod and methotrexate have not been extensively studied. In this study, 115 patients with active RA weres enrolled to analyse the value of the combination therapy of the two drugs.
Materials and methods
General information
A total of 115 patients with active RA admitted to our hospital were enrolled and divided into group A (n=58) and group B (n=57) according to the method of random number table. Inclusion criteria: patients met the diagnostic criteria for RA [8]; RA in active period according to the classification criteria of European League Against Rheumatism (EULAR) [9]; who aged over 18 years; no limit in gender; who voluntarily signed informed consent; this study was approval by the ethics society of First People’s Hospital, Fuyang District. Exclusion criteria: patients combined with cardiac and renal diseases, endocrine system disorders, immune system disorders, and active gastrointestinal disorders; those pregnant or lactating women; those allergy to the drugs used in this study; and those combined with mental disorders.
Methods
Patients in the group B were treated with methotrexate alone, and took methotrexate tablets once a week [specification: 2.5 mg*100 s, SFDA approval number: H31020644, manufacturer: Shanghai Shangyao Xinyi Pharmaceutical Co., Ltd. (Former Shanghai Xinyi Pharmaceutical Co., Ltd.)], 10 mg (4 tablets) each time.
Patients in the group A were treated with methotrexate combined with iguratimod. Methotrexate tablets were taken once a week, 10 mg each time. Iguratimod tablets (specifications: 25 mg*14 tablets, SFDA approval number: H20110084, manufacturer: Simcere Pharmaceutical Co., Ltd.) were taken 25 mg every morning and evening.
Both groups of patients received additional glucocorticoid therapy, with the dose controlled below 10 mg/d, and vitamin D, calcium, folic acid, and gastric drugs could be reasonably used for basic symptomatic treatment. Patients in both groups were treated continuously for 6 months.
Outcome measurement
Bone metabolism (BM): The related markers to be measured included collagen special sequence-1 (CTX-1), total procollagen type 1 amino-terminal propeptide (P1NP), receptor activator of nuclear factor κB ligand (RANKL), osteoprotegerin (OPG), parathyroid hormone (PTH), and 25-hydroxy vitamin D3 (25(OH)D3). Before treatment and at 6 months after treatment, 5 ml of fasting venous blood sample was taken as test specimen in the two groups of patients in the early morning and centrifuged at 3000 rpm for 10 min to separate the serum. The levels of RANKL and OPG were measured by enzyme-linked immunosorbent assay (ELISA). The levels of CTX-1, P1NP, PTH, and 25(OH)D3 were measured by electrochemiluminescence immunoassay. The detection kits were purchased from Roche, and the operation was carried out strictly in accordance with the instructions.
Inflammatory level: Inflammatory indicators included interleukin-10 (IL-10), interleukin-10 (IL-17), transforming growth factor β (TGF-β), and TGF-α. Before treatment and at 6 months after treatment, 5 ml of fasting venous blood sample was taken as test specimen in the two groups of patients in the early morning and centrifuged at 3000 rpm for 10 min. The levels of serum IL-10, IL-17, TGF-β, and TGF-α were measured by ELISA. The detection kits were purchased from Roche, and the operation was carried out strictly in accordance with the instructions.
Th17 and Treg cells: Before treatment and at 6 months after treatment, 5 ml of fasting venous blood sample was taken as test specimen in the two groups of patients in the early morning. Peripheral blood mononuclear cells were isolated. Th17 cells labelled with anti-human IL-17-PE and anti-human CD4-FITC monoclonal antibody and Treg cells labelled with anti-human CD25-PE, anti-human CD4-FITC and anti-human forkhead/winged spiral transcription factor 3-PE-Cy5 monoclonal antibody were detected by Beckman ASTRIOS EQ flow cytometer. (Antibodies were purchased from e Bioscience Inc.).
Arthral condition: Tender joints count, swollen joints count and the scores of joint disease activity (DAS28) before treatment and at 6 months after treatment were recorded [10]. DAS28=0.56*sqrt (TJC28) + 0.28*sqrt (SW28) + 0.70*Ln (ESP/CRP) + 0.014*VAS.
Note: ESR indicates erythrocyte sedimentation rate; SJC indicates swollen joint count; TJC indicates tender joints count; VAS indicates the score of visual analogue scale; CRP indicates C-reactive protein; Ln or In means taking the natural logarithm, and sqrt means taking the square root.
Morning stiffness of joints: The duration of the morning stiffness of the diseased joints at rest was measured before treatment and at 1, 2, 3, 4, 5 and 6 months after treatment.
Pain degree of joint: The degree of pain was evaluated by numerical rating scale (NRS) [11] before treatment and at 1, 2, 3, 4, 5 and 6 months after treatment. Patients were guided to choose a number that could represent their experience of pain in 11 numbers from 0 to 10, among which 0 means no pain, and 10 means the most severe pain.
Statistical analysis
SPSS23.0 was used to process the data. The count data were expressed by [n (%)] and analysed by X2 test. The measurement data were expressed by (χ ± s) and analysed by t test. The multi-factor comparison was analysed by ANVOA/F test. The figures were made by Graphpad Prism 8. P<0.05 was considered statistically significant.
Results
General information
There was no statistical difference in the ratio of gender, the ratio of patients in moderately active period to patients in severely active period, average age, average body mass index (BMI) and average disease course between the two groups (P>0.05) (Table 1).
Table 1.
Comparison of general information between the two groups (x̅ ± s)/[n (%)]
| General information | Group A (n=58) | Group B (n=57) | t/X2 | P | |
|---|---|---|---|---|---|
| Gender | Male | 30 (51.72) | 32 (56.14) | 0.226 | 0.635 |
| Female | 28 (48.28) | 25 (43.86) | |||
| Age (years) | 46.86±21.43 | 45.81±20.19 | 0.270 | 0.787 | |
| BMI (kg/m2) | 22.12±1.96 | 23.07±1.57 | 2.866 | 0.005 | |
| Average disease course (months) | 65.72±15.95 | 66.29±16.24 | 0.190 | 0.850 | |
| Activity rating | Moderately active period | 32 (55.17) | 31 (54.39) | 0.007 | 0.932 |
| Severely active period | 26 (44.83) | 26 (45.61) | |||
BM
At 6 months after treatment, the levels of CTX-1 and RANKL were decreased, while the levels of P1NP, OPG, PTH, and 25(OH)D3 were all increased. The levels of CTX-1, RANKL and OPG at 6 months after treatment were significantly different from those before treatment within group (P<0.05). The levels of CTX-1 and RANKL in group A were higher than those in group B, and the level of OPG in group A was lower than those in group B at 6 months after treatment (P<0.05) (Figure 1).
Figure 1.
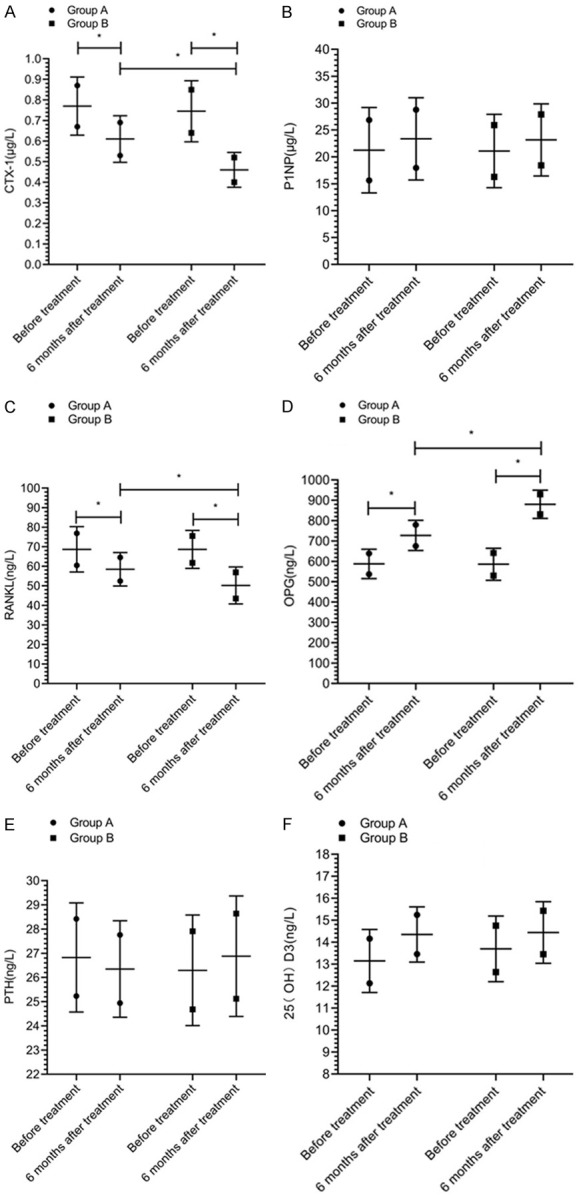
Bone metabolic markers. There was no significant difference in the levels of CTX-1 (A), P1NP (B), RANKL (C), OPG (D), PTH (E), 25(OH)D3 (F) between the two groups before treatment (P>0.05). The levels of CTX-1 and RANKL in group A were higher than those in group B, and the level of OPG in group A was lower than that in group B at 6 months after treatment (P<0.05). There was no significant difference in the levels of P1NP, PTH, and 25(OH)D3 between the two groups at 6 months after treatment (P>0.05). * indicates that the difference between the two groups is statistically significant.
Inflammatory level
At 6 months after treatment, the levels of IL-10, IL-17 and TGF-α were decreased, while the level of TGF-β was increased. The levels of IL-17 and TGF-α at 6 months after treatment were significantly different from those before treatment within group (P<0.05). The levels of IL-17 and TGF-α in group A were lower than those in group B at 6 months after treatment (P<0.05) (Figure 2).
Figure 2.
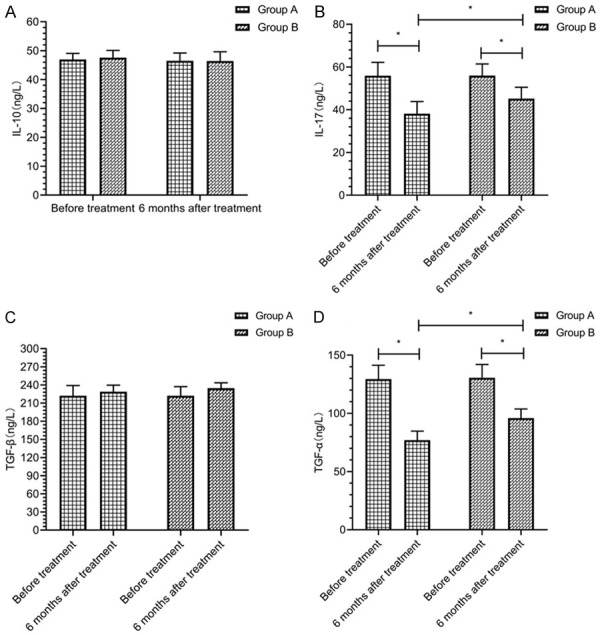
Inflammatory level. There was no significant difference in the levels of IL-10 (A), IL-17 (B), TGF-β (C), and TGF-α (D) between the two groups before treatment (P>0.05). There was no statistical difference in the levels of IL-10 (A) and TGF-β (C) between the two groups at 6 months after treatment (P>0.05). The levels of IL-17 (B) and TGF-α (D) in group A were lower than those in group B at 6 months after treatment (P<0.05). * indicates that the difference between the two groups is statistically significant.
Th17 and Treg cells
At 6 months after treatment, the level of Th17 cells was decreased in both groups, while the level of Treg cells was increased, which were statistically significant compared with those before treatment within group (P<0.05). The level of Th17 cells in group A was higher than that in group B, and the level of Treg cells in group A was lower than that in group B at 6 months after treatment (P<0.05) (Figure 3).
Figure 3.
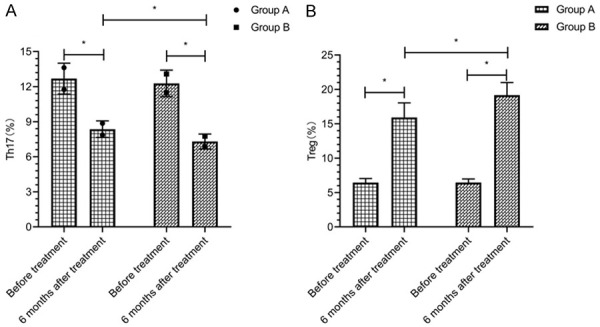
Th17 and Treg cells. There was no significant difference in the levels of Th17 cells (A) and Treg cells (B) between the two groups before treatment (P>0.05). The level of Th17 cells (A) in group A was higher than that in group B, and the level of Treg cells (B) in group A was lower than that in group B at 6 months after treatment (P<0.05). * indicates that the difference between the two groups is statistically significant.
Arthral condition
At 6 months after treatment, tender joints count, swollen joints count and DAS28 scores were decreased in both groups, which were statistically significant compared with those before treatment within group (P<0.05). Tender joints count, swollen joints count and DAS28 score in group A were less than those in group B at 6 months after treatment (P<0.05) (Figure 4).
Figure 4.
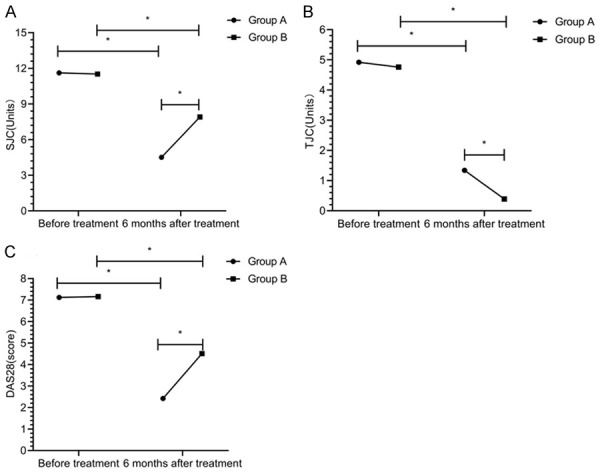
Arthral condition. There was no significant difference in swollen joints count (A), tender joints count (B), and DAS28 scores (C) between the two groups before treatment (P>0.05). Tender joints count, swollen joints count and DAS28 score in group A were less than those in group B at 6 months after treatment (P<0.05). * indicates that the difference between the two groups is statistically significant.
Morning stiffness of joints
The duration of morning stiffness was gradually shortened at 1, 2, 3, 4, 5 and 6 months after treatment and there was a statistical difference between different times (P<0.05). The duration of morning stiffness in group A was shorter than that in group B at 1, 2, 3, 4, 5 and 6 months after treatment (P<0.05) (Figure 5).
Figure 5.
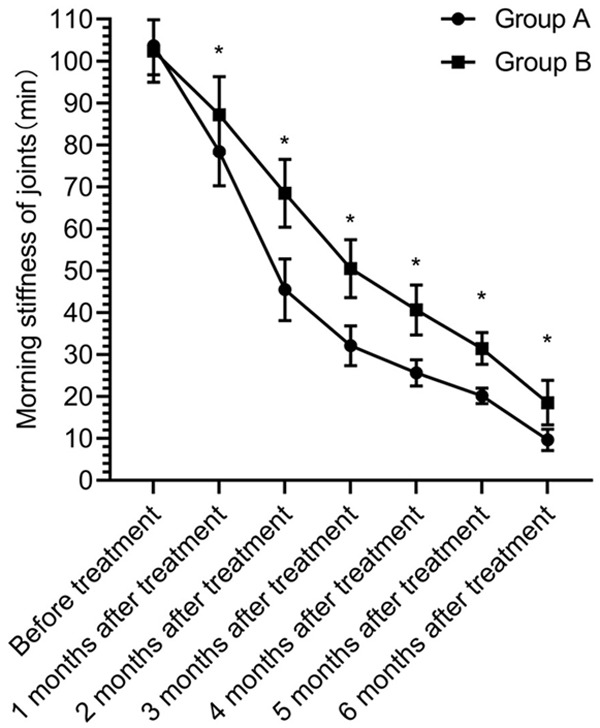
Duration of morning stiffness of joints. There was no significant difference in the duration of morning stiffness of joints between the two groups before treatment (P>0.05). The duration of morning stiffness in group A was shorter than that in group B at 1, 2, 3, 4, 5 and 6 months after treatment (P<0.05). * indicates that the difference between the two groups is statistically significant.
Pain degree of joint
The scores of pain degree were gradually decreased at 1, 2, 3, 4, 5 and 6 months after treatment and there was a statistical difference between different times (P<0.05). The score of pain degree in group A was lower than that in group B at 1, 2, 3, 4, 5 and 6 months after treatment (P<0.05) (Figure 6).
Figure 6.
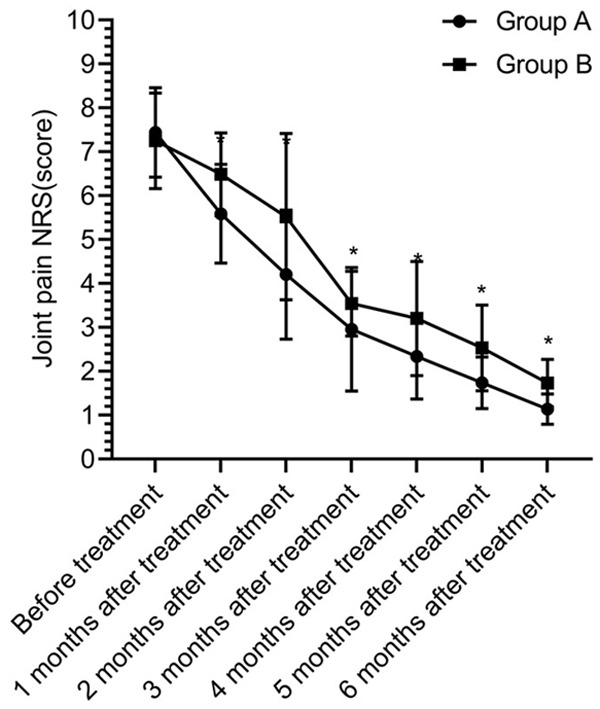
Pain degree of joint. There was no significant difference in the scores of pain degree between the two groups before treatment (P>0.05). The score of pain degree in group A was lower than that in group B at 1, 2, 3, 4, 5 and 6 months after treatment (P<0.05). * indicates that the difference between the two groups is statistically significant.
Discussion
RA is systemically inflammatory arthritis, which can cause permanent damage to the joints. It is a type of autoimmune joint disease with high clinical incidence [12]. Mild symptoms manifest as swelling and pain of joints. In severe cases, joint deformities and rigidity may occur, with extensive inflammation of connective tissue throughout the body [13]. In addition to joint-related symptoms, there are symptoms of other related systems, such as arteritis, subcutaneous nodules, fatigue, fever, peripheral neuropathy, etc. [14]. Therefore, effective treatment should be implemented after the diagnosis of RA to quickly control the symptoms and improve the prognosis of patients.
At present, the satisfaction of monotherapy of RA is gradually decreasing, and more and more clinical trials of combination drug therapy are conducted. It has been shown clinically that the combined application of methotrexate and other slow-acting anti-rheumatic drugs can help improve the compliance of treatment, thus ensuring efficacy [15]. Iguratimod is a new type of slow-acting anti-rheumatic drug. A study has confirmed that the combination of methotrexate and iguratimod in the treatment of RA can achieve a more effective control of inflammatory level, significantly relieve symptoms and reduce disease activity [16]. Another study has found that iguratimod has a certain effect on BM, which can regulate the RANKL/RANK/OPG signalling pathway, participate in BM and delay bone destruction [17]. RANKL is a member of tumor necrosis factors, which can accelerate the activation and differentiation of osteoclasts after binding to the RANK receptors on the surface of osteoclasts [18]. OPG is the decoy receptor of RANKL, which can hinder the connection between RANKL and RANK and avoid bone resorption [19]. CTX-1 is a sensitive and specific marker of bone destruction. The levels of CTX-1 and P1NP regulated by the RANKL/RANK/OPG signalling pathway can reflect the activation state of bone resorption and bone formation [20]. In this study, the levels of CTX-1 and RANKL in group A at 6 months after treatment were higher than those in group B, and the level of OPG in group A was lower than that in group B (P<0.05). The results showed that iguratimod can effectively control the levels of bone metabolic markers, inhibit bone destruction, and play a protective role in bone metabolism. In the experiment of murine osteoclast precursor cell (RAW264, 7) induced by the soluble receptor activator NF-κB ligand (s RANKL), iguratimod can resist the increase of tartrate-resistant acid phosphatase and inhibit the formation of TRACP-positive multinucleated cells [21].
In normal immune status, Th17 cells and Treg cells are in a balanced state. Th17 cells have a prominent pro-inflammatory effect, which can accelerate the formation of osteoclasts and cause more severe bone destruction [22]. If the expression of Treg cells is inhibited, the inflammation reaction of joint will be aggravated. Therefore, it is necessary to reduce inflammation and liver damage by promoting the expression of Treg cells [23]. In the unbalanced immune status of patients with RA, Treg cells are significantly reduced, while the expression of Th17 is significantly increased [24]. In this study, group A exhibited higher level of Th17 cells and lower level of Treg cells than group B after treatment (P<0.05), indicating that iguratimod has a regulatory effect on the levels of Th17 and Treg cells, and can down-regulate the expression of Th17 and promote the level of Treg cells, thus controlling inflammatory level accordingly. IL-17 and TGF-α in group A were lower than those in group B at 6 months after treatment (P<0.05). Th17 cells function by producing IL-17, TNF-α and other cytokines. IL-17 can also be used as a pro-inflammatory mediator and has a direct impact on the appearance of inflammatory response. Therefore, it is very important to control the level of IL-17. A study showed that after treatment, the level of IL-17 in patients treated with iguratimod was significantly lower than that in patients treated without iguratimod (P<0.05) [25]. At 6 months after treatment, the tender joints count, swollen joints count and DAS28 score in group A were less than those in group B. The duration of morning stiffness and the score of pain degree in group A were less than those in group B at 1, 2, 3, 4, 5, and 6 months after treatment (P<0.05). A study showed that the VAS score in the group of application of iguratimod combined with methotrexate was lower than that in the group of application of methotrexate at 1, 3, and 6 months after treatment (P<0.05) [26]. It suggested that the application of methotrexate combined with iguratimod in the treatment of RA can more quickly and effectively control the clinical symptoms, reduce morning stiffness, and reduce swelling and pain of joints than the monotherapy of methotrexate. It is speculated that the mechanism is related to the inhibition of the production of bradykinin. By inhibiting COX-2, swelling and pain of joints can be relieved. The above research results all confirmed that the combination of methotrexate and iguratimod has a more obvious effect than the monotherapy of methotrexate. Due to the complementary mechanisms of the two drugs, iguratimod can inhibit IL-17 signal and methotrexate can inhibit TGF signal [27]. Iguratimod can inhibit B lymphocytes and methotrexate can mainly inhibit T lymphocytes [28]. Both drugs can inhibit the proliferation of osteoclasts. At the same time, iguratimod can also accelerate the differentiation of osteoblasts, but this effect cannot be obtained with a single application of methotrexate, so the combination of the two drugs can be more efficient [29].
In summary, the combined application of methotrexate and iguratimod can effectively improve BM, regulate the levels of Th17 cells and Treg cells, play a prominent role in anti- inflammatory effect, and relieve symptoms in the treatment of active RA, thus achieving a more satisfactory curative effect. However, there were only two groups of subjects included in this study, and the mechanism of influence of BM, Th17 cells, and Treg cells was not studied in depth. In the future, a more comprehensive study with larger sample size should be conducted to elaborate the application value of iguratimod in RA.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure of conflict of interest
None.
References
- 1.Lora V, Cerroni L, Cota C. Skin manifestations of rheumatoid arthritis. G Ital Dermatol Venereol. 2018;153:243–255. doi: 10.23736/S0392-0488.18.05872-8. [DOI] [PubMed] [Google Scholar]
- 2.Littlejohn EA, Monrad SU. Early diagnosis and treatment of rheumatoid arthritis. Prim Care. 2018;45:237–255. doi: 10.1016/j.pop.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Nam JL, Takase-Minegishi K, Ramiro S, Chatzidionysiou K, Smolen JS, van der Heijde D, Bijlsma JW, Burmester GR, Dougados M, Scholte-Voshaar M, van Vollenhoven R, Landewé R. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2017;76:1113–1136. doi: 10.1136/annrheumdis-2016-210713. [DOI] [PubMed] [Google Scholar]
- 4.Subesinghe S, Bechman K, Rutherford AI, Goldblatt D, Galloway JB. A systematic review and metaanalysis of antirheumatic drugs and vaccine immunogenicity in rheumatoid arthritis. J Rheumatol. 2018;45:733–744. doi: 10.3899/jrheum.170710. [DOI] [PubMed] [Google Scholar]
- 5.Friedman B, Cronstein B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine. 2019;86:301–307. doi: 10.1016/j.jbspin.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway R, Carey JJ. Methotrexate and lung disease in rheumatoid arthritis. Panminerva Med. 2017;59:33–46. doi: 10.23736/S0031-0808.16.03260-2. [DOI] [PubMed] [Google Scholar]
- 7.Xia Z, Lyu J, Hou N, Song L, Li X, Liu H. Iguratimod in combination with methotrexate in active rheumatoid arthritis: therapeutic effects. Z Rheumatol. 2016;75:828–833. doi: 10.1007/s00393-015-1641-y. [DOI] [PubMed] [Google Scholar]
- 8.Aletaha D, Smolen J. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320:1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 9.Shiboski C, Shiboski S, Seror R, Criswell L, Labetoulle M, Lietman T, Rasmussen A, Scofield H, Vitali C, Bowman S, Mariette X. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2016;69:35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongin D, Lauper K, Turesson C, Hetland M, Kristianslund E, Kvien T, Santos M, Pavelka K, Iannone F, Finckh A, Courvoisier D. Imputing missing data of function and disease activity in rheumatoid arthritis registers: what is the best technique? RMD Open. 2019;5:e000994. doi: 10.1136/rmdopen-2019-000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosas S, Paço M, Lemos C, Pinho T. Comparison between the Visual Analog Scale and the Numerical Rating Scale in the perception of esthetics and pain. Int Orthod. 2017;15:543–560. doi: 10.1016/j.ortho.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170:Itc1–Itc16. doi: 10.7326/AITC201901010. [DOI] [PubMed] [Google Scholar]
- 13.Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46:183–196. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 15.Jekic B, Maksimovic N, Damnjanovic T. Methotrexate pharmacogenetics in the treatment of rheumatoid arthritis. Pharmacogenomics. 2019;20:1235–1245. doi: 10.2217/pgs-2019-0121. [DOI] [PubMed] [Google Scholar]
- 16.Zheng N, Guo C, Wu R. Iguratimod is effective in refractory rheumatoid arthritis patients with inadequate response to methotrexate-cyclosporin A-hydroxychloroquine-prednisone. Scand J Rheumatol. 2018;47:422–424. doi: 10.1080/03009742.2017.1376109. [DOI] [PubMed] [Google Scholar]
- 17.Tokai N, Yoshida S, Kotani T, Yoshikawa A, Kimura Y, Fujiki Y, Matsumura Y, Takeuchi T, Makino S, Arawaka S. Serum matrix metalloproteinase 3 levels are associated with an effect of iguratimod as add-on therapy to biological DMARDs in patients with rheumatoid arthritis. PLoS One. 2018;13:e0202601. doi: 10.1371/journal.pone.0202601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son A, Kang N, Oh SY, Kim KW, Muallem S, Yang YM, Shin DM. Homer2 and Homer3 modulate RANKL-induced NFATc1 signaling in osteoclastogenesis and bone metabolism. J Endocrinol. 2019;242:241–249. doi: 10.1530/JOE-19-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Infante M, Fabi A, Cognetti F, Gorini S, Caprio M, Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J Exp Clin Cancer Res. 2019;38:12. doi: 10.1186/s13046-018-1001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takada J, Dinavahi R, Miyauchi A, Hamaya E, Hirama T, Libanati C, Nakamura Y, Milmont CE, Grauer A. Relationship between P1NP, a biochemical marker of bone turnover, and bone mineral density in patients transitioned from alendronate to romosozumab or teriparatide: a post hoc analysis of the STRUCTURE trial. J Bone Miner Metab. 2020;38:310–315. doi: 10.1007/s00774-019-01057-1. [DOI] [PubMed] [Google Scholar]
- 21.Wu YX, Sun Y, Ye YP, Zhang P, Guo JC, Huang JM, Jing XZ, Xiang W, Yu SY, Guo FJ. Iguratimod prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis via inhibition of peroxisome proliferator-activated receptor-γ. Mol Med Rep. 2017;16:8200–8208. doi: 10.3892/mmr.2017.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin S, Chen H, Li Y, Zhong H, Sun W, Wang J, Zhang T, Ma J, Yan S, Zhang J, Tian Q, Yang X, Wang J. Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann Rheum Dis. 2018;77:1644–1652. doi: 10.1136/annrheumdis-2018-213511. [DOI] [PubMed] [Google Scholar]
- 23.Vasilev G, Ivanova M, Ivanova-Todorova E, Tumangelova-Yuzeir K, Krasimirova E, Stoilov R, Kyurkchiev D. Secretory factors produced by adipose mesenchymal stem cells downregulate Th17 and increase Treg cells in peripheral blood mononuclear cells from rheumatoid arthritis patients. Rheumatol Int. 2019;39:819–826. doi: 10.1007/s00296-019-04296-7. [DOI] [PubMed] [Google Scholar]
- 24.Kotake S, Yago T, Kobashigawa T, Nanke Y. The plasticity of Th17 cells in the pathogenesis of rheumatoid arthritis. J Clin Med. 2017;6:67. doi: 10.3390/jcm6070067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Ma C, Li P, Zhao F, Bi L. Effects of iguratimod on the levels of circulating regulators of bone remodeling and bone remodeling markers in patients with rheumatoid arthritis. Clin Rheumatol. 2017;36:1369–1377. doi: 10.1007/s10067-017-3668-8. [DOI] [PubMed] [Google Scholar]
- 26.Hagihara M, Mese T, Ohara S, Hua J, Ide S, Inoue M. Methotrexate-associated intravascular large B-cell lymphoma in a patient with rheumatoid arthritis: a very rare case. Intern Med. 2018;57:3001–3005. doi: 10.2169/internalmedicine.0875-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebina K, Miyama A, Tsuboi H, Kaneshiro S, Nishikawa M, Owaki H, Tsuji S, Hirao M, Etani Y, Goshima A, Hashimoto J, Yoshikawa H. The add-on effectiveness and safety of iguratimod in patients with rheumatoid arthritis who showed an inadequate response to tocilizumab. Mod Rheumatol. 2019;29:581–588. doi: 10.1080/14397595.2018.1486939. [DOI] [PubMed] [Google Scholar]
- 28.Arita Y, Taguchi H, Kobayashi M, Tono T, Ohsone Y, Okano Y. Pneumocystis jirovecii pneumonia developed in a patient with rheumatoid arthritis after 14 weeks of iguratimod add-on to treatment with methotrexate and etanercept. Mod Rheumatol. 2018;28:1041–1043. doi: 10.1080/14397595.2016.1181026. [DOI] [PubMed] [Google Scholar]
- 29.Wang XT, Li P, Xu TS, Ding R, Zhang X, Bi LQ. Effect of iguratimod and methotrexate on RANKL and OPG expression in serum and IL-1β-induced fibroblast-like synoviocytes from patients with rheumatoid arthritis. Cell Mol Biol (Noisy-le-grand) 2016;62:44–50. doi: 10.14715/cmb/2016.62.12.8. [DOI] [PubMed] [Google Scholar]


