Abstract
Aims
To identify the main diagnostic features of SARS‐CoV‐2‐positive patients at the time of hospitalisation and their prevalence.
Background
Since the COVID‐19 outbreak in China in December of 2019, several studies attempted to identify the epidemiological, viral and clinical characteristics of SARS‐CoV‐2. Given the rapid widespread transmission of the COVID‐19 disease worldwide, a more comprehensive and up‐to‐date understanding of its features is needed to better inform nurses, clinicians and public health policy makers.
Methods
A rapid review and meta‐analysis were carried out to identify the main diagnostic features of SARS‐CoV‐2‐positive patients at the time of hospitalisation. All case series, cross‐sectional, case–control and cohort studies published from 01/01/2020 till 30/06/2020 in English and Chinese that stated all or at least two of the outcomes of interest (clinical features, laboratory and radiological findings) were included. We performed a random‐effects model meta‐analysis to calculate pooled prevalence and 95% confidence intervals. Conduction of the review adheres to the PRISMA checklist.
Results
21 studies involving 8837 patients were included in the quantitative synthesis. Fever, cough and fatigue were the most common clinical features, while the most relevant laboratory abnormalities at the time of hospitalisation were lymphopenia, elevated C‐reactive protein and lactate dehydrogenase. CT images showed a bilateral lung involvement, with ground glass infiltrates and patchy shadows on most patients.
Conclusion
This review provides an up‐to‐date synthesis of main diagnostic features of SARS‐CoV‐2‐positive patients at the time of hospitalisation.
Relevance to Clinical Practice
Our findings could provide guidance for nurses and clinicians to early identification of positive patients at the time of the hospitalisation through a complete definition of main clinical features, laboratory and CT findings.
Keywords: COVID‐19, diagnostic features, meta‐analysis, review, severe acute respiratory syndrome coronavirus 2
What does this paper contribute to the wider global clinical community?
This review provides an up‐to‐date summary of the main clinical characteristics of SARS‐COV‐2‐positive patients at the time of hospitalisation.
Among clinical symptoms, fever, cough and fatigue showed the highest pooled prevalence.
The most common alterations of blood routine reported at the time of hospitalisation were lymphopenia, increased infection‐related biomarkers and elevated liver functions values.
1. INTRODUCTION
In late December 2019, several cases of viral pneumonia of unknown aetiology were first reported in Wuhan, capital of Hubei, China, with epidemiological links to the Huanan Seafood Wholesale Market. The Chinese Centre for Disease Control and Prevention isolated the causative agent of the outbreak as a novel Coronavirus (nCoV), namely severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (Lu et al., 2020). Soon after, the number of cases increased exponentially, spreading across China and worldwide. On 11 March 2020, the World Health Organization (WHO) officially declared the COVID‐19 outbreak a pandemic (Mahase, 2020). At the time of writing, more than 51 million cases of SARS‐CoV‐2 have been confirmed and more than 1.270.000 people have died (WHO, 2020a).
Since then, several studies attempted to identify the epidemiological, viral and clinical characteristics of SARS‐CoV‐2 patients. The majority of those studies were case reports, case series and cross‐sectional studies, which described evolution and outcomes of the disease, as well as risk factors, clinical, laboratory and image findings (Long et al., 2020; Chu et al., 2020; Cheng et al., 2020; Ai et al., 2020). Two systematic reviews published between March and April 2020 (Rodriguez‐Morales et al., 2020; Fu et al., 2020) gave a preliminary characterisation of the disease, describing the most commonly reported clinical features, the laboratory abnormalities and CT images of SARS‐CoV‐2 patients, mostly from China.
However, the current literature on COVID‐19 is rapidly evolving, with new peer‐reviewed and preprint articles published every day across the world, and its main features remain unclear (Rodriguez‐Morales et al., 2020; Struyf et al., 2020). Given the rapid widespread transmission of the COVID‐19 disease worldwide, a more comprehensive and up‐to‐date understanding of its features is needed to better inform clinicians, nurses and public health policy makers.
2. AIMS
A rapid review was conducted to identify the main diagnostic features of SARS‐CoV‐2‐positive patients at the time of hospitalisation, aiming to help stakeholders and clinicians optimise the diagnostic process.
To achieve our aim, we:
-
‐
synthetised and described the diagnostic features of SARS‐CoV‐2‐positive patients at the time of hospitalisation;
-
‐
assessed the prevalence of the main diagnostic features in SARS‐CoV‐2‐confirmed cases.
3. METHODS
Conduction of the review adheres to PRISMA checklist for systematic reviews and meta‐analyses (Moher et al., 2009). See File S1.
3.1. Study selection
All the articles that provide a clear description of the patient with a confirmed diagnosis of SARS‐CoV‐2 at the time of hospitalisation were included. The confirmation of the diagnosis of SARS‐CoV‐2 was ascertained in agreement with the diagnostic criteria of the Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (2020b). Therefore, all the studies which clearly stated the baseline characteristics of patient with positive RNA assay were included.
The search was limited to articles in English or Chinese, published from 01/01/2020 till 30/06/2020. Case reports, diagnostic accuracy studies without a clear statement of the baseline‐positive SARS‐CoV‐2 characteristics and studies focused on pregnant women or paediatric patients were excluded. To speed up data extraction and synthesis, only studies with a sample size of at least 90 patients were included.
The outcomes of interest were the reported clinical and diagnostic features and the laboratory findings. We chose to include all the case series, cross‐sectional, case–control and cohort studies that stated all or at least two of the outcomes of interest (e.g. clinical and diagnostic features or clinical and laboratory findings). For the clinical features, all the reported signs and symptoms were collected. In the same way, all the image characteristics and the lesions region of the diagnostic imaging as well as the stated blood routine, coagulation function, infection‐related biomarkers and blood biochemistry were collected.
3.2. Search strategies
The Europe PMC, LitCovid Database, Medline, The Cochrane Library, Science Direct, Embase and The Cumulative Index to Nursing and Allied Health Literature (CINAHL) were screened independently by two experienced reviewers with the support of Zotero Reference Manager (V.5.0). In case of disagreement between the reviewers on eligibility, a senior author was consulted. Two different search strings were used (File S2), both combining different synonyms and mesh terms for SARS‐CoV‐2 and diagnosis or testing.
Since this is a rapid review, no specific searches of grey literature were done. After a discussion between the authors, we decided to include only peer‐reviewed published articles. All the articles published in Chinese were summarised by a team member with a certified knowledge of the Chinese language and then discussed with another team member.
3.3. Data extraction
One experienced review author‐extracted data from the included studies into a standardised table. A second review author checked the data extraction for completeness and correctness. Whether an article reported duplicate information from the same patient, the information of both reports was combined in order to obtain complete data, but only counted as a single case. Observational studies that stated the overall proportion of symptoms, laboratory characteristics and CT images or/and the mean values of clinical features or laboratory findings for SARS‐CoV‐2‐positive patients at baseline were included for quantitative synthesis (meta‐analysis). The data item included the following: author, country, year, study design, aim, characteristic of the study participants, diagnostic reference criteria, age, gender, comorbidities, clinical features (e.g. fever, cough), laboratory findings (e.g. lymphocytes count) and imaging (e.g. CT signs).
3.4. Quality assessment
The risk of bias was assessed by one author with the Joanna Briggs institute (JBI) ‘Checklist for case series’
and ‘Critical appraisal checklist for cohort’. A risk of bias judgement (Low; Moderate; High) was attributed to each included study following Melo et al. (2018) scoring system. Then, the 25% of the rating judgment was checked independently by another author, as suggested by the World Health Organization guidelines for rapid review (2017). Moreover, publication bias was assessed using a funnel plot and computing the Egger's test (Egger et al., 1997).
3.5. Statistical approach
All the different units of measure of the outcomes of interest were converted in the referred international unit (e.g. from mg/dl to mmol/L). All the data presented as median and interquartile range were converted to mean and standard deviation (SD) following the Cochrane handbook of systematic review of interventions (7.7.3.5). Percentages or mean and standard deviation (SDs) were calculated to describe the distributions of categorical and continuous variables. Pooled prevalence and its 95% confidence interval (CI) were used to summarise the weighed effect size for each study grouping variable in a random effect model. We chose a random effect model because we assumed high clinical, methodological and statistical heterogeneity. The proportion of cases (e.g. fever or lymphocytopenia) on the total number of cases for each outcome of interest was meta‐analysed using the R function metaprop. Whether computed with function metamean in R, raw weighted mean and their 95% confidence intervals were reported (e.g. mean age in years).
Measure of heterogeneity, as the I 2 index and the tau squared test were estimated and reported for both pooled prevalence and pooled mean. We expected high heterogeneity from the results (Ioannidis et al., 2007); therefore, we chose to keep the pooled overall estimates but to estimate also a prediction interval for all the outcomes of interest as it presents the expected range of true effects in similar studies (IntHout et al., 2016).
4. RESULTS
The literature search yielded 10727 references. After removal of duplicates, 7829 references were screened for title and abstract and 21 observational studies were identified, all included in the quantitative analysis (Figure 1). The main descriptive characteristics of the included studies are shown in Table 1.
Figure 1.
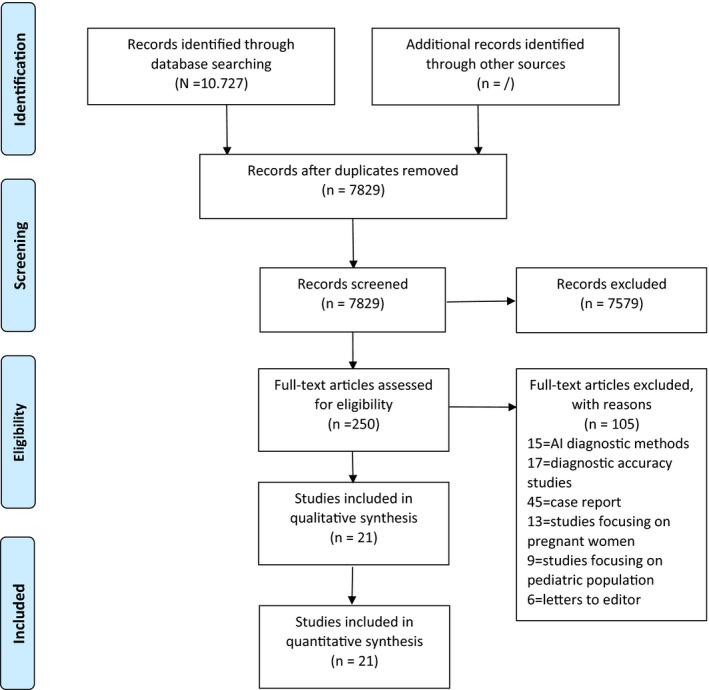
Search strategy [Colour figure can be viewed at wileyonlinelibrary.com]
Table 1.
overview of all the included studies.
| Authors | Country | Published | Study | N | Mean age | Data collected at | Clinical Features | Laboratory findings | Diagnostic imaging | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2020) | China | 15/2/2020 | CS | 99 | 55 | AH | ✓ | ✓ | ✓ | Low |
| Guan et al. (2020) | China | 28/2/2020 | CR | 1099 | 47 | AH | ✓ | ✓ | ✓ | Low |
| Cao et al. (2020) | China | 13/3/2020 | CS | 102 | 54 | AH | ✓ | ✓ | ✓ | Low |
| Qiu et al. (2020) | China | 5/5/2020 | CS | 104 | 43 | AH | ✓ | ✓ | ✓ | Moderate |
| Li et al. (2020) | China | 10/4/2020 | CS | 225 | 50 | AH | ✓ | ✓ | ✓ | High |
| Dai et al. (2020) | China | 6/4/2020 | CR | 234 | 44 | AH | ✓ | ✓ | ✓ | Low |
| Hong et al. (2020) | Korea | 24/4/2020 | CR | 98 | 55 | AH; ICU | ✓ | ✓ | ✓ | Moderate |
| Chen et al. (2020) | China | 11/4/2020 | CS | 203 | 54 | AH | ✓ | ✓ | ✓ | Moderate |
| Huang et al. (2020) | China | 8/5/2020 | CS | 202 | 44 | AH | ✓ | ✓ | ✓ | Low |
| Zhang et al. (2020) | China | 10/5/2020 | CS | 194 | 48 | AH | ✓ | ✓ | ✓ | Low |
| Lian et al. (2020) | China | 12/5/2020 | CS | 465 | 45 | AH | ✓ | ✓ | ✓ | Low |
| Israelsen et al. (2020) | Denmark | 15/5/2020 | CS | 175 | 71 | AH ICU | ✓ | ✓ | ✓ | Low |
| Li et al. (2020) | China | 15/5/2020 | CR | 93 | 51 | AH | ✓ | ✓ | ✓ | Moderate |
| Song et al. (2020) | China | 16/5/2020 | CS | 111 | 55 | AH | ✓ | ✓ | ✓ | Low |
| Argenziano et al. (2020) | USA | 29/5/2020 | CS | 764 | 59 | AH; ICU; ED | ✓ | ✓ | Low | |
| Buckner et al. (2020) | USA | 22/5/2020 | CS | 105 | 69 | AH | ✓ | ✓ | Low | |
| Imam et al. (2020) | USA | 4/6/2020 | Co | 1305 | 61 | AH | ✓ | ✓ | ✓ | Low |
| Zhao et al. (2020) | China | 4/6/2020 | CR | 1000 | 61 | AH | ✓ | ✓ | ✓ | High |
| Li et al. (2020) | China | 11/6/2020 | CS | 1449 | 57 | AH | ✓ | ✓ | ✓ | Low |
| Xu et al. (2020) | China | 15/5/2020 | CR | 703 | 46 | AH | ✓ | ✓ | ✓ | Low |
| Wang et al. (2020) | China | 30/4/2020 | CS | 107 | 51 | AH | ✓ | ✓ | ✓ | High |
Abbreviations: AH, Admission at hospital; Co, Cohort studies; CR, Cross‐sectional studies; CS, Case series studies; ED, Emergency department; ICU, Intensive unit care admission.
The total sample of participants included 8837 adults with a laboratory‐confirmed diagnosis of SARS‐CoV‐2 by real‐time RT‐PCR, accordingly to the World Health Organization or the National Health Commission diagnostic guidelines (Table 1). The reasons for excluding the other records are listed in the PRISMA flow chart case (Figure 1). All the included studies published before May 2020 were issued in Asia, while the latter were issued in USA and Europe too (Table 1). The most common type of studies included was retrospective case series or cross‐sectional design (Table 1). Data were primarily collected with the support of electronic records or by retrospective manual review of the clinical record at the time of hospitalisation; therefore, only one study provided baseline data for patients admitted at the emergency department (Figure 1). All the included studies reported SARS‐CoV‐2 patients’ clinical features and laboratory findings, while diagnostic imaging was less commonly and clearly stated especially in the recently issued articles (Table 1).
The methodological quality of included studies was assessed according to the study designs with the Joanna Briggs Institute (JBI) checklists. As shown in Table 1, nearly half of included studies were rated as ‘Low’ risk of bias. Only four studies were rated as ‘Moderate’ and even fewer as ‘High’ (Table 1). The lack of consecutive inclusion or a clear description of participants, as well as many imprecisely reported outcomes, elevated the risk of bias in nearly half of the included studies. None of the cross‐sectional designs provide any measure or adjustment for potential confounding variables. Publication bias was assessed with a funnel plot for the standard error, with no evidence of bias (File S3). Additionally, the Egger's test (p =.61) performed by the gender variable suggested no notable evidence of publication bias.
4.1. Demographical characteristics and comorbidities
The pooled mean age of the patients across the 21 studies was 51.43 years old (95%CI 48.35;54.52), with a prediction interval ranging from 41.54 to 61.32 mean years as in shown in Table 2. However, the pooled mean age of the sample was fairly lowered by the pooled mean age of the studies issued in Asia, in which the mean age was 50 years old versus 65 years old of the studies issued in Western regions (e.g. America or Europe). Male was only slightly more prevalent than female in the total sample (Table 2). Common comorbidities were hypertension (22.49%, 95%CI 15.49;31.49) and diabetes (13%, 95%CI 8.82;18.88), both with prediction intervals ranging from less than 5% to more than 50% (Table 2). The rate of obesity was reported only by two studies (Table 2) and, despite only including estimates, it appears to be the most prevalent comorbidity of hospitalised SARS‐CoV‐2‐positive patients (40.19%, 95%CI 29.23;52.22). Surprisingly, both chronic obstructive pulmonary disease (3.66%, 95%CI 2.41;5.52) and malignancies (3.19%, 95%CI 2.07;4.87) were not very prevalent comorbidities in SARS‐CoV‐2‐positive patients (Table 2).
Table 2.
Meta‐analysis outcomes (random‐effects model)
| Variable | K | Mean or Prevalence (%) | 95%CI | Prediction Interval | n | I 2 | t 2 | p | |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | Mean age | 21 | 51.43 | 48.35;54.52 | 41.54;61.32 | 8837 | 46% | 19.85 | .01 |
| Male | 20 | 52.91 | 50.44;55.37 | 43.10;62.50 | 8821 | 77% | 0.03 | .001 | |
| Female | 20 | 46.91 | 44.50;49.34 | 37.5;56.48 | 8821 | 76% | 0.03 | .01 | |
| Comorbidities | CD | 17 | 9.32 | 5.89;14.44 | 1.13;47.87 | 6944 | 97% | 0.99 | .01 |
| Diabetes | 16 | 13 | 8.82;18.88 | 2.17;50.38 | 6845 | 97% | 0.74 | .01 | |
| COPD | 14 | 3.66 | 2.41;5.52 | 0.75;15.92 | 6632 | 88% | 0.49 | .01 | |
| Hypertension | 16 | 22.49 | 15.49;31.49 | 3.71;68.60 | 5765 | 98% | 0.83 | .01 | |
| Malignancies | 14 | 3.19 | 2.07;4.87 | 0.61;14.89 | 6585 | 88% | 0.53 | .01 | |
| Obesity | 2 | 40.19 | 29.23;52.22 | Ni | 1093 | 81% | 0.32 | .02 | |
| Clinical Features | |||||||||
| Signs | Temperature >38° | 6 | 38.96 | 25.65;54.14 | 6.19;86.05 | 3483 | 98% | 0.56 | .01 |
| Mean temperature | 5 | 36.96 | 36.53;37.39 | 35.75;38.18 | 2898 | 47% | 0.09 | .1 | |
| Mean RR | 5 | 20.01 | 19.06;20.95 | 18.47;21.54 | 2353 | 0% | 0 | .99 | |
| Mean HR | 4 | 83.91 | 75.40;92.42 | 65.23;102.59 | 1353 | 5% | 0 | .97 | |
| Symptoms | Fatigue | 16 | 34.10 | 29.11;39.46 | 16.24;58.00 | 7465 | 95% | 0.19 | .01 |
| Cough | 19 | 64.05 | 60.33;67.60 | 47.16;78.04 | 7525 | 89% | 0.10 | .01 | |
| Fever | 20 | 77.50 | 71.45;82.58 | 43.45;93.92 | 7636 | 97% | 0.48 | .01 | |
| Diarrhoea | 19 | 9.42 | 7.34;12.01 | 2.84;27.51 | 8823 | 93% | 0.34 | .01 | |
| Myalgia | 19 | 20.25 | 16.44;23.76 | 8.04;41.20 | 8719 | 93% | 0.22 | .01 | |
| Dyspnoea | 20 | 22.19 | 15.38;30.92 | 3.19;71.17 | 8944 | 99% | 1.00 | .01 | |
| Headache | 16 | 8.69 | 6.94;10.82 | 3.47;20.10 | 8294 | 88% | 0.18 | .01 | |
| Rhinorrhoea | 10 | 5.44 | 3.02;9.63 | 0.59;35.68 | 5939 | 97% | 0.86 | .01 | |
| Sore throat | 9 | 12.66 | 7.58;20.40 | 1.74;54.28 | 6417 | 97% | 0.70 | .01 | |
| Anorexia | 6 | 19.40 | 12.70;28.48 | 3.86;59.03 | 2813 | 94% | 0.34 | .01 | |
| CT Finding | Bilateral Involvement | 15 | 65.22 | 49.45;77.62 | 10.37;96.70 | 7291 | 99% | 1.53 | .01 |
| Pleural effusion | 3 | 3.06 | 0.78;11.26 | 0.00;99.99 | 2159 | 96% | 1.44 | .01 | |
| GGO | 9 | 38.25 | 24.80;53.78 | 5.45;86.93 | 5041 | 99% | 1.30 | .01 | |
| Consolidation | 3 | 7.20 | 2.62;16.51 | 0;99.99 | 3100 | 98% | 1.14 | .01 | |
| Patchy shadows | 5 | 58.34 | 36.80;76.52 | 4.45;97.60 | 2543 | 98% | 1.33 | .01 | |
| Laboratory findings | WBC ↑ | 9 | 10.23 | 5.70;17.67 | 1.07;54.35 | 3783 | 96% | 1.28 | .01 |
| WBC ↓ | 10 | 21.50 | 16.08;28.13 | 6.79;50.73 | 3985 | 95% | 0.29 | .01 | |
| Lymphocytes ↓ | 10 | 50.08 | 33.81;65.62 | 7.19;92.63 | 2918 | 98% | 1.05 | .01 | |
| CRP ↑ | 11 | 58.34 | 49.45;66.72 | 25.83;84.91 | 5022 | 97% | 0.34 | .01 | |
| LDH ↑ | 9 | 47.38 | 39.59;55.29 | 21.85;74.35 | 4422 | 96% | 0.21 | .01 | |
| ALT ↑ | 11 | 18.30 | 8.95;33.78 | 0.85;85.38 | 4804 | 99% | 2.30 | .01 | |
| AST ↑ | 11 | 26.03 | 14.82;40.56 | 2.25;83.75 | 5593 | 99% | 1.30 | .01 | |
| Al ↓ | 7 | 59.20 | 32.68;81.25 | 2.60;98.74 | 2945 | 99% | 2.10 | .01 | |
| Cr ↑ | 8 | 22.36 | 5.79;56.27 | 0.09;98.78 | 3601 | 99% | 5.16 | .01 | |
| Tbil ↑ | 6 | 16.10 | 4.30;45.03 | 0.08;97.72 | 3110 | 99% | 3.24 | .01 | |
| D‐Dimer ↑ | 7 | 27.04 | 19.53;35.22 | 7.99;60.29 | 2840 | 95% | 0.26 | .01 | |
Abbreviations: Al, Albumin; ALT, Alanine transaminase; AST, Aspartate transaminase; CD, Cardiac disease; COPD, Chronic obstructive pulmonary disease; Cr, Serum creatinine; CRP, C‐reactive protein; GGO, Ground glass opacities; LDH, Lactate dehydrogenase; Ni, not imputable; Tbil, Total bilirubin; WBC, White blood count.
4.2. Clinical manifestations of COVID‐19
Symptoms were commonly reported in the included articles, while signs were often missing or described approximately as shown in Table 3. Fever and cough were the only clinical features reported by the totality of the included studies as commonly associated to SARS‐CoV‐2 patients (Table 2). However, cough, which was stated in all of the included studies, showed an overall pooled prevalence of only 64% (95%CI 60.33;67.60) and a large prediction interval ranging from less than 50% to more than 80% (Table 2). Fever, as a self‐reported symptom, showed a pooled prevalence of 77.5% with a pretty narrow confidence interval (95%CI 71.45;82.58) but a fairly larger prediction interval (Table 2). Only 6 studies reported the rate of SARS‐CoV‐2‐positive patients with a temperature >38° Celsius, and the overall pooled prevalence of the sign was 38.96% (95%CI 25.65;54.14), while 5 studies clearly stated the mean temperature of the sample with thresholds ranging from 36.7° to 38.5° and with a non‐statistically significant pooled mean of 36.96° Celsius (Table 2). Other signs mean values (e.g. respiratory or heart rate) were reported only by four included studies (Table 3), and despite pooled, they did not result statistically significant in the quantitative synthesis.
Table 3.
Qualitative summary of the findings
| Variables | K | Inter‐study frequency (%) | Variables | K | Inter‐study frequency (%) | ||
|---|---|---|---|---|---|---|---|
| Signs | Temperature | 8 | 38 | Blood Routine | WBC | 13 | 61 |
| Respiratory Rate | 4 | 19 | Lymphocyte count | 18 | 85 | ||
| Hearth rate | 4 | 19 | Lymphocyte ratio | 7 | 33 | ||
| Systolic blood pressure | 4 | 19 | Neutrophil count | 9 | 42 | ||
| Neutrophil ratio | 6 | 28 | |||||
| Symptoms | Fever | 20 | 95 | Monocyte count | 2 | 9 | |
| Chills | 9 | 42 | Basophil count | 1 | 4 | ||
| Cough | 21 | 100 | Eosinophil count | 1 | 4 | ||
| Diarrhoea | 19 | 90 | Platelet count | 11 | 52 | ||
| Fatigue | 16 | 76 | Haemoglobin level | 11 | 52 | ||
| Chest distress | 14 | 66 | Haematocrit | 3 | 14 | ||
| Pharyngalgia | 12 | 57 | |||||
| Dyspnoea | 13 | 61 | Coagulation function | APTT | 8 | 38 | |
| Rhinorrhoea | 8 | 38 | D‐dimer | 8 | 38 | ||
| Anorexia | 7 | 33 | PT | 3 | 14 | ||
| Expectoration | 11 | 52 | |||||
| Nausea | 13 | 61 | Infection‐related biomarkers | CRP | 15 | 71 | |
| Vomit | 10 | 48 | ESR | 10 | 48 | ||
| Headache | 14 | 66 | IIL−6 | 3 | 14 | ||
| Myalgia | 19 | 90 | PCT | 12 | 57 | ||
| Image characteristics | GGO | 12 | 57 | Blood biochemistry | Al | 9 | 42 |
| Patchy shadows | 7 | 33 | ALT | 17 | 80 | ||
| Consolidation | 4 | 19 | AST | 16 | 76 | ||
| Pleural effusion | 3 | 14 | Tbil | 13 | 61 | ||
| Interstitial thickening | 2 | 9 | BUN | 10 | 48 | ||
| Crazy‐paving pattern | 2 | 9 | Cr | 8 | 38 | ||
| LDH | 10 | 48 | |||||
| Lesion region | Bilateral pulmonary | 19 | 90 | Hs‐cTnT | 4 | 19 | |
| Subpleura | 4 | 19 | CK | 9 | 42 | ||
| Peripheral zone | 3 | 14 | Ferratin | 3 | 14 | ||
| Troponin I | 3 | 14 |
Abbreviations: Al, Albumin; ALT, Alanine transaminase; APTT, Activated partial thromboplastin time; AST, Aspartate transaminase; BUN, blood urea nitrogen; CK, Creatine kinase; Cr, Serum creatinine; CRP, C‐reactive protein; GGO, Ground glass opacities; Hs‐cTnT, High‐sensitive cardiac troponin T; IL‐6, Interleukin; LDH, Lactate dehydrogenase; PCT, Procalcitonin; PT, Prothrombin time; Tbil, Total bilirubin; WBC, White blood count.
Diarrhoea was the most commonly reported gastrointestinal symptom (Table 3) and showed a pooled prevalence of 9.42% (95%CI 7.34;12.01). Instead, anorexia was recorded only by 7 included studies, but showed a pooled prevalence of 19.40% (95%CI 12.70;28.48) and a higher prediction interval than diarrhoea (Table 2). Respiratory symptoms, like dyspnoea, pharyngalgia and rhinorrhoea, were not commonly reported in the included studies (Table 2), and when pooled, did not show a high pooled prevalence (Table 2). However, some studies were not clear in the reporting of symptoms, often using terms such ‘sore throats’, ‘shortness of breath’ or ‘runny nose’ as synonymous for pharyngalgia, dyspnoea and rhinorrhoea. Neuromotor symptoms, such as myalgia and headache, were frequently reported in the included studies (Table 3), but both showed low prevalence with prediction interval narrow than 50% (Table 2). Other possible relevant symptoms of SARS‐CoV‐2, such as loss of smell or taste and conjunctivitis, were stated only by one included article.
4.3. Laboratory findings
Among the 21 studies which reported the laboratory findings of SARS‐CoV‐2 patients (Table 3), 18 studies stated a widespread decrease of the lymphocyte count. Other commonly reported alterations of the blood routine in the included articles involved a general decrease of the leucocyte count, while the haemoglobin level did not seem to vary much (Table 3). Indeed, a decrease in lymphocytes counts was present in the 50% of the total sample (95%CI 33.81;65.62), while leucocyte count showed a pooled prevalence of only 21.5% (Table 2).
Between the infection‐related biomarkers, an increased level of C‐reactive protein level (CRP) was stated in all the articles except six (Table 3). The pooled prevalence of the increased level of CRP was 58.3% (95%CI 49.45;66.72); however, it showed a pretty wide prediction interval (Table 2). All the other increased infection‐related biomarkers (e.g. erythrocyte sedimentation rate, interleukin and procalcitonin) were less commonly reported and referred predominantly as normal (Table 3). Eight studies also reported normal values of coagulation function (Table 3), except for D‐dimer, which resulted increased in seven studies with a pooled prevalence of 27% (Table 2).
Liver function values were reported by 17 of the included studies, mostly showing normal or increased levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (Table 2). Both ALT and AST were increased in 11 included studies with an overall pooled prevalence of 18.3% (95%CI 8.95;33.78) and 26% (95%CI 14.82;40.56), respectively, and wide prediction intervals (Table 2). Increase of total bilirubin was slightly less prevalent than ALT and AST, and it has an even wider prediction interval (Table 2). Renal function (e.g. blood urea nitrogen, serum creatinine and glomerular filtration rate) was scarcely reported and referred predominantly as normal (Table 3). Other increased blood biochemistry values reported in the included studies (Table 3) were lactate dehydrogenase (48%), serum high‐hypersensitivity troponin T (19%), creatinine kinase CK (42%) and ferratin (14%). Increased values of lactate dehydrogenase (LDH) showed a pooled prevalence of 47.38% (95%CI 21.85;55.29), while increased value of serum creatinine of only 22.36% (Table 2).
4.4. Radiologic findings
The processing of computed tomography (CT) images is often reported in the included studies as the fastest method to confirm a diagnosis of SARS‐CoV‐2 at the time of hospitalisation. Characteristic images of the SARS‐CoV‐2 patients in the CT scans were ground glass opacities (GGO) and consolidations (Table 3). However, consolidation rates were reported only by 3 studies with an overall pooled prevalence of 7.2% (Table 2). Instead, GGO were estimated in 38.2% (95%CI 24.89;53.78) of the total sample (Table 2). Moreover, only a few included studies provided a clear classification of how many patients have GGO, consolidation or both. Five articles stated the rate of observed patchy shadow without linking them to any possible causes such as pleural effusion or pneumonia (Table 3), and despite pooled they were the most prevalent CT images reported in SARS‐CoV‐2 patients at hospitalisation (Table 2).
The pulmonary opacifications or patchy shadows were stated in the included studies as located in the peripheral zones of both lungs (Table 3), with the lower lobe of the left lung as the more involved one. However, while bilateral involvement is a prevalent clinical gauge of SAR‐CoV‐2‐positive patients in the included studies, pleural effusion rates were reported only by 3 included articles and showed a pooled prevalence of only 3% (95%CI 0.78;11.26) with a wide prediction interval (Table 2).
5. DISCUSSION
A rapid diagnosis is essential to ensure SARS‐CoV‐2 patients receive proper care and to reduce the risks of contagion, and this rapid review updates the diagnostic features of SARS‐CoV‐2 patients at the time of hospitalisation. Indeed, the actual golden standard tests for SARS‐CoV‐2 diagnosis are real‐time RT‐PCR by swab, which has low sensitivity (Carver & Jones, 2020), and often it must be repeated one or more days apart. SARS‐CoV‐2‐positive patients at the time of hospitalisation predominantly presented fever, cough, fatigue, dyspnoea, myalgia and anorexia. At least 38% (95%CI 25.65;54.14) of SARS‐CoV‐2‐positive patients at hospitalisation show a temperature higher than 38° Celsius and 65.22% (95%CI 49.45;77.62) of them show bilateral pulmonary involvement at the CT scan. Both lymphocytes count and albumin are decreased in at least 50% of the cases, while CRP, LDH and liver function values are significantly increased. This rapid review provides an updated summary of the most commonly reported symptoms, signs, diagnostic features and laboratory findings of SARS‐CoV‐2 patients, to speed up the triage process and help clinician in their daily decision‐making process.
More than half of the patients included in the overall sample are male, with a mean age of 51 years and comorbidities such as diabetes and hypertension being prevalent respectively in 9.32% and 22.49% of SARS‐CoV‐2 patients at the time of hospitalisation. These findings are coherent with the previous literature (Rodriguez‐Morales et al., 2020), but are still biased by the high rate of included articles published in China. Indeed, in our meta‐analysis the most prevalent comorbidity appears to be obesity, which was stated only by two included articles both issued in America with a pooled prevalence of 40.19% (95%CI 29.23;52.22). More articles from America, Africa and Europe with a structured research design are needed to fully comprehend how age, comorbidities and healthcare system disparities impact on positive SARS‐COV‐2 diagnosis.
At the timing of hospitalisation, SARS‐CoV‐2 patients present predominately fever and cough, coherently with previous findings (Rodriguez‐Morales et al., 2020) and actual SARS‐CoV‐2 screening policy (WHO, 2020c). Cough has a 64.05% (95%CI 60.33;67.60) prevalence, which is a higher rate compared to those declared in a previous meta‐analysis (Rodriguez‐Morales et al., 2020), but a wide prediction interval (Table 2). Indeed, a recent Cochrane review (Struyf et al., 2020) highlighted how cough has a too poor specificity index to be considered a prominent diagnostic feature of SARS‐CoV‐2. Other respiratory symptoms, such as dyspnoea or pharyngalgia, are often reported in the included studies since ACE2 receptors appear to be the entry point for SARS‐CoV‐2 to into human cells, but both have an overall pooled prevalence lower than 30% with wide prediction intervals. Our estimated pooled prevalence for both dyspnoea and fatigue is lower than the one reported in Rodriguez‐Morales et al. (2020) meta‐analysis. However, we only included baseline data of SARS‐CoV‐2‐positive patients at the time of hospitalisation in the pooled estimates and we considered articles from America and Europe, which Rodriguez‐Morales et al. (2020) did not include. Further studies should clearly define the characteristic of SARS‐CoV‐2 cough (e.g. dry or wet) and whether it is more prevalent in mild or severe cases, older patients or associated to particular comorbidities. In Struyf et al work (2020), fever higher than 37.8° Celsius is one of the symptoms that increased the probability of a SARS‐CoV‐2‐positive diagnosis when present. However, in our review we found out then only 38.9% (95%CI 25.65;54.14) of the total sample had a temperature higher than 38° Celsius, and the temperature rate was only reported by six studies while its threshold by even fewer. Indeed, while diagnostic symptoms are commonly reported in the articles, diagnostic signs are often not stated, and this is an important evidence gap. Mean temperature, respiratory rate, heart rate and blood pressure were only reported by 4 included studies, and more evidences are needed to fully define SARS‐CoV‐2‐positive patients. Further research should improve the reporting of SARS‐CoV‐2‐positive patient signs at the time of hospitalisation and investigate whether mean age or disease severity (e.g. mild or severe patients) changes the prevalence or the mean value of diagnostic signs and reported symptoms. As previously suggested by Rodriguez‐Morales et al. (2020), we confirm that even if diarrhoea is still a commonly reported gastrointestinal symptom of SARS‐CoV‐2‐positive patients at the time of hospitalisation, it has a pretty low pooled prevalence. Instead, anorexia which is another uncommonly reported symptom showed a pooled prevalence almost as high as myalgia and higher than headache, both of which are considered to be possible red flags for a SARS‐CoV‐2‐positive diagnosis (Struyf et al., 2020). SARS‐CoV‐2 diagnostic features are still unclear and may be slightly different in different populations. Further studies should improve the completeness and clearness of their reported symptoms, stating clear definitions of them in the methods sessions and reporting even unusual symptoms such loss of taste or smell or anorexia. Moreover, further studies need to assess multiple variable for their possible confounding effect on the summary estimates such as respiratory pathogens (seasonality), time since infection, disease severity or age.
The WHO firstly called SARS‐CoV‐2 a ‘novel coronavirus‐infected pneumonia’, since many image characteristics at first appeared to be consistent with viral pneumonia. The use of CT as a primary screening tool is discouraged, since it has a very low specificity (Kim et al., 2020). However, in resource‐constrained environments, imaging is still indicated for triage of patient with suspected SARS‐CoV‐2 and we chose to report the main commonly reported image characteristic or lesion regions provided by the included studies in this rapid review. GGO (38.25%) and ‘generic’ patchy shadows (58.34%) are the most prevalent images characteristics in CT of positive SARS‐CoV‐2 patients at the time of hospitalisation, and our findings are consistent with Bao et al. (2020) meta‐analysis. However, in our meta‐analysis consolidation has a consistently lower pooled proportion than the one stated by Bao et al. (2020), and this could be probably due to the overall low reporting accuracy of the included articles. Most of the included articles were not accurate in the reporting of CT findings, providing more a generic description rather than a clear definition, especially the ones recently published or issued in America (Table 3). Bilateral lung is the prevalent lesion distribution region with a pooled estimate of 65.22% (95%CI 49.45;77.62), while we chose not to estimate peripheral distribution since it was only stated in 3 included articles. Future studies should deepen the relationship between clinical features and CT scan image characteristics of the SARS‐CoV‐2 patients at the time of hospitalisation to speed up the diagnostic process.
The predominantly altered laboratory findings are lymphocytes, prothrombin time, LDH, inflammatory markers and the indices of liver function (Table 2). Instead, the indices of kidney function are not frequently reported as altered in SARS‐CoV‐2‐positive patients at the time of hospitalisation. Lymphopenia is present in 50% of the SARS‐CoV‐2‐positive patients across 10 studies with an overall sample of 2918 patients, and this finding suggests SARS‐CoV‐2 affects lymphocytes with a higher prevalence than that implied in a Rodriguez‐Morales et al. (2020) meta‐analysis. Previous studies suggested that inflammatory markers can be strong predictors of the progression of SARS‐CoV‐2 infection (Kermalia et al., 2020; Zeng et al., 2020), and CRP is increased in nearly 50% of our sample at the time of hospitalisation. However, other inflammatory markers such interleukin or procalcitonin were not reported as altered in the included studies. Liver function values, such as ALT, AST and total bilirubin, are increased in at least the 15% of the overall samples. These results are coherent with other recent studies showing liver as the most frequently damaged organ outside the respiratory system (Samidoust et al., 2020; Xu et al., 2020). Future studies should investigate whether high liver function values in SARS‐CoV‐2 patients at the time of hospitalisation could help define a diagnosis of severe SARS‐CoV‐2. Both D‐Dimer and LDH were significantly increased in SARS‐CoV‐2 patients at the time of hospitalisation, with the LDH pooled prevalence second only to the one of CRP (Table 2). Necrosis of the cell membrane and lung damage is a trigger of LDH secretion and previous studies found abnormal levels of LDH in ICU patients, suggesting that LDH could be a predictive biomarker of disease severity (Kermalia et al., 2020; Zeng et al., 2020). However, if the laboratory finding changes, given different stages of illness, comorbidities or age at the time of hospitalisation are still not stated in the included studies and should investigate it further. Moreover, often the laboratory findings were reported in the included studies without a threshold reference or not in the adequate international unit of measure.
Limits of the review are due to the type and quality of the included articles, which are often case series. Even if updated, our review still includes few articles issued in Europe or America compared to those issued in China, and this could have led to an overestimation or an underestimation of some SARS‐CoV‐2 diagnostic features at the time of hospitalisation. Moreover, only articles in English or Chinese were included and this could have led to the loss of some meaningful articles. More detailed patient information, particularly regarding diagnostic signs, comorbidities and possible confounding variables are needed.
6. CONCLUSION
This review provides an up‐to‐date synthesis of main diagnostic features of SARS‐CoV‐2‐positive patients at the time of hospitalisation. The prevalence of obesity in SARS‐CoV‐2‐hospitalised patients and its implications should be investigated further. Future research should improve the completeness and clearness of their reported SARS‐CoV‐2 patients’ signs and symptoms, reporting their characteristic signs at the time of hospitalisation and unusual symptoms such loss of taste or smell or anorexia. Moreover, future research should investigate whether abnormal laboratory findings such as lymphopenia, increased inflammatory markers and higher liver function values, could be associated with disease severity. Additionally, more studies from European and American Countries are needed to provide more comprehensive and generalisable results.
7. RELEVANCE TO CLINICAL PRACTICE
As the number of reported COVID‐19 cases keeps rising globally, especially in the United States, nurses and clinicians’ knowledge of the disease is also gradually increasing thanks to ongoing research and clinical practice experience. Our findings could provide guidance for nurses and clinicians to early identification of positive patients at the time of the hospitalisation through a complete updated definition of main clinical features, laboratory and CT findings.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of these articles.
AUTHOR CONTRIBUTIONS
GA, DC and MLR involved in conceptualization. GD, MZ, FD and AC involved in data curation. AC, GA, MLR and DC involved in formal analysis. AG, DC, AC, FD and MLR involved in investigation. MLR, GA, DC, GD and AC involved in methodology. MLR, GA, DC and FD involved in project administration. GA, MLR and GD involved in supervision. GA, DC, AC, MZ, MLR and MZ involved in visualization. GA, DC, MLR and FD wrote the original draft. MLR, GA, GD and MZ involved in writing, reviewing and editing.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
We thank Miss Annalisa Dorbolò and Dr Barbara Pala for the advices.
Funding information
The authors declare that they receive no specific funding for this work.
Contributor Information
Gloria Anderson, Email: andersongloria0@gmail.com.
Daniela Casasanta, Email: danielacasa92@hotmail.it.
REFERENCES
- Ai, J. W. , Wang, Y. , Liu, X. , Qu, G. , Zhang, M. , Pei, S. P. , Tang, B. , Yuan, S. , Li, Y. , Wang, L. , Huang, G. , & Pei, B. (2020). The cross‐sectional study of hospitalized coronavirus disease 2010 patients in Xiangyyang, Hubei province. MedRxiv. 10.1101/2020.02.19.20025023. [DOI] [Google Scholar]
- Argenziano, M. , Bruce, S. , Slater, C. , Tiao, J. , Baldwin, M. , Barr, R. , Chang, B. , Chau, K. , Choi, J. , Gavin, N. , Goyal, P. , Mills, A. , Patel, A. , Romney, M. , Safford, M. , Schluger, N. , Sengupta, S. , Sobieszczyk, M. , Zucker, J. , … Chen, R. (2020). Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ, 369, m1996 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, C. , Xuehuang, L. , Zhang, H. , Li, Y. , & Liu, J. (2020). Coronavirus Disease 2019 (COVID‐19) CT Findings: A Systematic Review and Meta‐analysis. JACR, 17(6), 701–709. 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, F. , McCulloch, D. , Atluri, V. , Blain, M. , McGuffin, S. , Nalla, A. , Huang, M. , Greninger, A. , Jerome, K. , Cohen, S. , Neme, S. , Green, M. , Chu, H. , & Kim, H. (2020). Clinical features and outcomes of 105 hospitalized patients with COVID‐19 in Seattle, Washington. Clinical Infectious Diseases. 71(16), 2167–2173. 10.1093/cid/ciaa632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J. , Tu, W. , Cheng, W. , Yu, L. , Liu, Y. , Hu, X. , & Liu, Q. (2020). Clinical features and short‐term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clinical Infectious Diseases, 71(15), 748–755. 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver, K. , & Jones, N. (2020). Comparative accuracy of oropharyngeal and nasopharyngeal swabs for diagnosis of COVID‐19. Retrieved from www.cebm.net/covid‐19/comparative‐accuracy‐of‐oropharyngeal‐and‐nasopharyngeal‐swabs‐for‐diagnosis‐of‐covid‐19/ [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , & Han, Y. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet, 395(10223), 507–513. 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Dai, Z. , Mo, P. , Li, X. , Ma, Z. , Song, S. , Chen, X. , Luo, M. , Liang, K. , Gao, S. , Zhang, Y. , Deng, L. , & Xiong, Y. (2020). Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China: A single‐centered, retrospective study. The Journals of Gerontology: Series A, 75(9), 1788–1795, 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. , Lu, Y. , Cao, Q. , Qin, L. , Pan, L. , Yan, F. , & Yang, W. (2020). Clinical features and chest CT manifestation of coronavirus disease 2019 (COVID‐19) in a Single‐Center study in Shanghai, China. American Journal of Roentgenology, 215(1), 121‐126. 10.2214/ajr.20.22959 [DOI] [PubMed] [Google Scholar]
- Chu, J. , Yang, N. , Wei, Y. , Yue, H. , Zhang, F. , Zhao, J. , He, L. I. , Sheng, G. , Chen, P. , Li, G. , Wu, S. , Zhang, B. O. , Zhang, S. , Wang, C. , Miao, X. , Li, J. , Liu, W. , & Zhang, H. (2020). Clinical characteristics of 54 medical staff with COVID‐19: a retrospective study in a single center in Wuhan, China. Journal of Medical Virology, 92(7), 807–813. 10.1002/jmv.25793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, H. , Zhang, X. , Xia, J. , Zhang, T. , Shang, Y. , Huang, R. , Liu, R. , Wang, D. , Li, M. , Wu, J. , Xu, Q. , & Li, Y. (2020). High‐resolution Chest CT features and clinical characteristics of patients infected with COVID‐19 in Jiangsu, China. International Journal of Infectious Diseases, 95, 106–112. 10.1016/j.ijid.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger, M. , Davey Smith, G. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315, 629–634. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, L. , Wang, B. , Yuan, T. , Chen, X. , Ao, Y. , Fitzpatrick, T. , Li, P. , Zhou, Y. , Lin, Y.‐F. , Duan, Q. , Luo, G. , Fan, S. , Lu, Y. , Feng, A. , Zhan, Y. , Liang, B. , Cai, W. , Zhang, L. , Du, X. , … Zou, H. (2020). Clinical characteristics of coronavirus disease 2019 (COVID‐19) in China: a systematic review and meta‐analysis. Journal of Infection, 80(6), 656–665. 10.1016/j.jinf.2020.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. , Ni, Z. , Hu, Y. , Liang, W. , Ou, C. , He, J. , Liu, L. , Shan, H. , Lei, C. , Hui, S. C. , Du, B. , Li, L. , Zeng, G. , Yuen, K. , Chen, R. , Tang, C. , Wang, T. , Chen, P. , Xiang, J. …, Zhong, N. (2020). Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, K. , Lee, K. , Chung, J. , Shin, K. , Choi, E. , Jin, H. , Jang, J. , Lee, W. , & Ahn, J. (2020). Clinical features and outcomes of 98 patients hospitalized with SARS‐CoV‐2 infection in Daegu, South Korea: A Brief Descriptive Study. Yonsei Medical Journal, 61(5), 431–437. 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R. , Zhu, L. , Xue, L. , Liu, L. , Yan, X. , Wang, J. , Zhang, B. , Xu, T. , Ji, F. , Zhao, Y. , Cheng, J. , Wang, Y. , Shao, H. , Hong, S. , Cao, Q. , Li, C. , Zhao, X. , Zou, L. , Sang, D. , … Wu, C. (2020). Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multi‐center study. PLoS Neglected Tropical Diseases, 14(5), 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam, Z. , Odish, F. , Gill, I. , O’Connor, D. , Armstrong, J. , Vanood, A. , Ibironke, O. , Hanna, A. , Ranski, A. , & Halalau, A. (2020). Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID‐19 patients in Michigan, United States. Journal of Internal Medicine, 288(4), 469–476 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IntHout, J. , Ioannidis, J. P. , Rovers, M. M. , & Goeman, J. J. (2016). Plea for routinely presenting prediction intervals in meta‐analysis. British Medical Journal Open, 6, e010247. 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis, J. P. , Patsopoulos, N. A. , & Evangelou, E. (2007). Uncertainty in heterogeneity estimates in meta‐analyses. BMJ (Clinical Research ed.), 335(7626), 914–916. 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelsen, S. , Kristiansen, K. , Hindsberge, B. , Ulrik, C. , Andersen, O. , Jensen, M. , Andersen, S. , Rasmussen, C. , Jørgensen, H. , Østergaard, C. , Lindhardt, B. , Kronborg, G. , & Benfield, T. (2020). Characteristics of patients with COVID‐19 pneumonia at Hvidovre Hospital, March‐April 2020. Dan Med J., 67(6), A05200313. [PubMed] [Google Scholar]
- Kermalia, M. , Khalsaa, R. K. , Pillaia, K. , Ismaila, Z. , & Harkyb, A. (2020). The role of biomarkers in diagnosis of COVID‐19 – A systematic review. Life Sciences, 254(1), 117788 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. , Hong, H. , & Yoon, S. H. (2020). Diagnostic performance of CT and reverse transcriptase‐polymerase chain reaction for coronavirus disease 2019: a meta‐analysis. Radiology, 296(3), E145–E155 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. O. , Wu, H. , Wang, W. , Song, H. , Huang, B. , Zhu, N. A. , Bi, Y. , Ma, X. , Zhan, F. , Wang, L. , Hu, T. , Zhou, H. , Hu, Z. , Zhou, W. , Zhao, L. I. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 395, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Yang, L. , Gui, S. , Pan, F. , Ye, T. , Liang, B. , Hu, Y. , & Zheng, C. (2020). Association of clinical and radiographic findings with the outcomes of 93 patients with COVID‐19 in Wuhan, China. Theranostics, 10(14), 6113–6121. 10.7150/thno.46569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Cao, Y. , Chen, L. , Wu, D. , Yu, J. , Wang, H. , He, W. , Chen, L. , Dong, F. , Chen, W. , Chen, W. , Li, L. , Ran, Q. , Liu, Q. , Ren, W. , Gao, F. , Chen, Z. , Gale, R. , & Hu, Y. (2020). Hematological features of persons with COVID‐19. Leukemia, 34(8), 2163–2172. 10.1038/s41375-020-0910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Tian, J. , Yang, F. , Lv, L. , Yu, J. , Sun, G. , Ma, Y. , Yang, X. , & Ding, J. (2020). Clinical characteristics of 225 patients with COVID‐19 in a tertiary Hospital near Wuhan, China. Journal of Clinical Virology, 127, 104363 10.1016/j.jcv.2020.104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian, J. , Jin, X. , Hao, S. , Jia, H. , Cai, H. , Zhang, X. , Hu, J. , Zheng, L. , Wang, X. , Zhang, S. , Ye, C. , Jin, C. , Yu, G. , Gu, J. , Lu, Y. , Yu, X. , Xiang, D. , Li, L. , Liang, T. , … Yang, Y. (2020). Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID‐19) from Zhejiang province in China. Influenza and Other Respiratory Viruses, 14(5), 564–574 10.1111/irv.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C. , Xu, H. , Shen, Q. , Zhang, X. , Fan, B. , Wang, C. , Zeng, B. , Li, Z. , Li, X. , & Li, H. (2020). Diagnosis of the Coronavirus disease (COVID‐19): rRT‐PCR or CT? European Journal of Radiology, 126, 108961. 10.1016/j.ejrad.2020.108961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase, E. (2020). Covid‐19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. British Medical Journal., 368(m1036), 10.1136/bmj.m1036 [DOI] [PubMed] [Google Scholar]
- Melo, G. , Dutra, K. L. , Rodrigues Filho, R. , Ortega, A. , Porporatti, A. L. , Dick, B. , Flores‐Mir, C. , & De Luca Canto, G. (2018). Association between psychotropic medications and presence of sleep bruxism: A systematic review. Journal of Oral Rehabilitation, 45(7), 545–554. 10.1111/joor.12633 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med, 6(7), e1000097. 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, C. , Deng, Z. , Xiao, Q. , Shu, Y. , Deng, Y. , Wang, H. , Liao, X. , Liu, H. , Zhou, D. , Zhao, X. , Zhou, J. , Wang, J. , Shi, Z. , & Long, D. , (2020). Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside‐Wuhan patients, China. Journal of Medical Virology, 92(10), 2027–2035, 10.1002/jmv.25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Morales, A. J. , Cardona‐Ospina, J. A. , Gutiérrez‐Ocampo, J. , Villamizar‐Peña, R. , Holguin‐Rivera, Y. , Escalera‐Antezana, J. P. , Alvarado‐Arnez, L. E. , Bonilla‐Aldana, D. K. , Franco‐Paredes, C. , Henao‐Martinez, A. F. , Paniz‐Mondolfi, A. , Lagos‐Grisales, G. A. , Ramírez‐Vallejo, E. , Suárez, J. A. , Zambrano, L. I. , Villamil‐Gómez, W. E. , Balbin‐Ramon, G. J. , Rabaan, A. A. , Harapan, H. , … Sah, R. (2020). Clinical, laboratory and imaging features of COVID‐19: A systematic review and meta‐analysis. Travel Medicine and Infectious Disease, 34, 101623 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samidoust, P. , Samidoust, A. , Samadani, A. A. , & Khoshdoz, S. (2020). Risk of hepatic failure in COVID‐19 patients: A systematic review and meta‐analysis. Le Infezioni in Medicina, 28, 96–103.PMID: 32532945. [PubMed] [Google Scholar]
- Song, S. , Wu, F. , Liu, Y. , Jiang, H. , Xiong, F. , Guo, X. , Zhang, H. , Zheng, C. , & Yang, F. (2020). Correlation between chest CT findings and clinical features of 211 COVID‐19 suspected patients in Wuhan, China. Open Forum Infectious Diseases, 7(6), ofaa171 10.1093/ofid/ofaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf, T. , Deeks, J. J. , Dinnes, J. , Takwoingi, Y. , Davenport, C. , Leeflang, M. M. G. , Spijker, R. , Hooft, L. , Emperador, D. , Dittrich, S. , Dome, J. , Horn, S. R. A. , & den Bruel, A. V. (2020). Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19 disease. Cochrane Database of Systematic Reviews, 7, CD013665 10.1002/14651858.CD013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Joanna Briggs Institute Critical Appraisal tools for use in JBI systematic reviews . Checklist for case series. Retrieved from: https://nursing.lsuhsc.edu/jbi/resources.aspx. [Google Scholar]
- Wang, D. , Yin, Y. , Hu, C. , Liu, X. , Zhang, X. , Zhou, S. , Jian, M. , Xu, H. , Prowle, J. , Hu, B. , Li, Y. , & Peng, Z. (2020). Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS‐CoV‐2, discharged from two hospitals in Wuhan, China. Critical Care, 24, 188 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2017). Rapid reviews to strengthen policy and system: a practical guide. Retrieved from: https://apps.who.int/iris/bitstream/handle/10665/258698/9789241512763‐eng.pdf;jsessionid=6B17522E55D0BA78BFCC32A9180DD245?sequence=1. [Google Scholar]
- World Health Organization (2020a). Coronavirus disease (COVID‐19) Dashboard. Retrieved from https://covid19.who.int [Google Scholar]
- World Health Organization (2020b). Report of the WHO‐China Joint Mission on Coronavirus disease 2019 (COVID‐19). Retrieved from https://www.who.int/docs/default‐source/coronaviruse/who‐china‐joint‐mission‐on‐covid‐19‐final‐report.pdf. [Google Scholar]
- World Health Organization (2020c). Modes of transmission of virus causing COVID‐19: implications for IPC precaution recommendations. Retrieved from https://www.who.int/news‐room/commentaries/detail/modes‐of‐transmission‐of‐virus‐causing‐covid‐19‐implications‐for‐ipc‐precaution‐recommendations [Google Scholar]
- Xu, P. , Tian, R. , Luo, S. , Zu, Z. , Fan, B. , Wang, X. , Xu, K. , Wang, J. , Zhu, J. , Shi, J. , Chen, F. , Wan, B. , Yan, Z. , Wang, R. , Chen, W. , Fan, W. , Zhang, C. , Lu, M. , Sun, Z. , … Zhang, L. (2020). Risk factors for adverse clinical outcomes with COVID‐19 in China: a multicenter, retrospective, observational study. Theranostics, 10(14), 6372–6383. 10.7150/thno.46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, F. , Huang, F. , Guoa, Y. , Yina, M. , Chena, X. , Xiao, L. , & Denga, G. (2020). Association of inflammatory markers with the severity of COVID‐19: A meta‐analysis. International Journal of Infectious Diseases, 96, 467–474. 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Shang, W. , Liu, Q. , Zhang, X. , Zheng, M. , & Yue, M. (2020). Clinical characteristics of 194 cases of COVID‐19 in Huanggang and Taian, China. Infection, 48(5), 687–694. 10.1007/s15010-020-01440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, M. , Wang, M. , Zhang, J. , Gu, J. , Zhang, P. , Xu, Y. , Ye, J. , Wang, Z. , Ye, D. , Pan, W. , Shen, B. , He, H. , Liu, M. , Lui, M. , Luo, Z. , Li, D. , Liu, J. , & Wan, J. (2020). Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging, 12(11), 10070–10086. 10.18632/aging.103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material


