Abstract
Background
Whereas accumulating studies on patients with coronavirus disease 2019 (COVID‐19) report high incidences of thrombotic complications, large studies on clinically relevant thrombosis in patients with other respiratory tract infections are lacking. How this high risk in COVID‐19 patients compares to those observed in hospitalized patients with other viral pneumonias such as influenza is unknown.
Objectives
To assess the incidence of venous and arterial thrombotic complications in hospitalized patients with influenza as opposed to that observed in hospitalized patients with COVID‐19.
Methods
This was a retrospective cohort study; we used data from Statistics Netherlands (study period: 2018) on thrombotic complications in hospitalized patients with influenza. In parallel, we assessed the cumulative incidence of thrombotic complications—adjusted for competing risk of death—in patients with COVID‐19 in three Dutch hospitals (February 24 to April 26, 2020).
Results
Of the 13 217 hospitalized patients with influenza, 437 (3.3%) were diagnosed with thrombotic complications, versus 66 (11%) of the 579 hospitalized patients with COVID‐19. The 30‐day cumulative incidence of any thrombotic complication in influenza was 11% (95% confidence interval [CI], 9.4–12) versus 25% (95% CI, 18–32) in COVID‐19. For venous thrombotic (VTC) complications and arterial thrombotic complications alone, these numbers were, respectively, 3.6% (95% CI, 2.7–4.6) and 7.5% (95% CI, 6.3–8.8) in influenza versus 23% (95% CI, 16–29) and 4.4% (95% CI, 1.9–8.8) in COVID‐19.
Conclusions
The incidence of thrombotic complications in hospitalized patients with influenza was lower than in hospitalized patients with COVID‐19. This difference was mainly driven by a high risk of VTC complications in the patients with COVID‐19 admitted to the Intensive Care Unit. Remarkably, patients with influenza were more often diagnosed with arterial thrombotic complications. 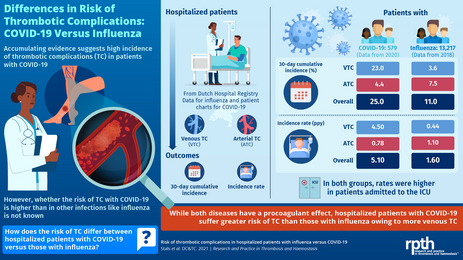
Keywords: COVID‐19, human, incidence, influenza, pneumonia, thrombosis, viral
Essentials.
Whether patients with coronavirus disease 2019 (COVID‐19) have more thrombosis compared to influenza is unclear.
Hospitalized patients with influenza and COVID‐19 were compared.
Thirty‐day cumulative incidence of thrombosis was lower in influenza (11%) than in COVID‐19 (25%).
The difference was mainly driven by a high risk of venous thrombotic complications in patients with COVID‐19 admitted to the Intensive Care Unit.
1. INTRODUCTION
The clinical course of acute infections may become complicated by venous and arterial thrombotic disease. 1 , 2 Precipitating factors for thrombotic complications in this setting include inflammation, activation of the coagulation system, immobilization, and diffuse intravascular coagulation. 1 , 2 In light of this, international guidelines recommend pharmacologic thromboprophylaxis in patients with infectious diseases if they are hospitalized. 3
Respiratory viruses, including influenza, were already known to lead to a procoagulant state. 4 , 5 , 6 Moreover, previous studies showed a transient increase in the incidence of thrombotic vascular complications after respiratory tract infections. 1 , 7 To date, it is largely unknown how often influenza infection leads to thrombotic complications, since only small case series yielding conflicting results have been published. 4 , 8 , 9 , 10 Contrarywise, the coronavirus disease 2019 (COVID‐19) pandemic has led to a large number of cohort studies on thrombotic complications in hospitalized patients with COVID‐19. These studies showed that COVID‐19 infections were characterized by a sometimes excessive activation of blood coagulation, 11 , 12 , 13 and high incidences of thrombotic complications were reported in patients with COVID‐19, aggravated by admittance to the Intensive Care Unit (ICU). 14 , 15 , 16 , 17 , 18 , 19 How this high incidence of thrombotic complications in patients with COVID‐19 compares to those observed in hospitalized patients with non–COVID‐19 viral infections such as influenza is largely unknown. 20 This is especially relevant to understand the etiology of COVID‐19–associated thrombosis. 21 , 22
We evaluated the incidence of thrombotic complications in hospitalized patients with influenza as opposed to that in hospitalized patients with COVID‐19, in an effort to better understand the epidemiology of COVID‐19–associated thrombotic complications.
2. METHODS
2.1. Setting and study population
We used anonymous information on registered diagnoses during hospital admissions in Dutch hospitals from the National Basic Register of Hospital Care of Dutch Hospital Data (DHD), which includes all general and academic Dutch hospitals, provided via Statistics Netherlands (Centraal Bureau voor de Statistiek [CBS]), for information on hospitalized patients with influenza. Patients hospitalized with an admission date between January 1, 2018, and November 30, 2018, with a diagnosis of influenza were included for the primary analysis, and data from January 1, 2013, to December 31, 2018, were used to collect information on comorbidities. Information on the use of pharmacologic thromboprophylaxis was not available in DHD.
We also used data from adult patients with COVID‐19 admitted to the wards and ICUs of one university hospital (Leiden University Medical Center [LUMC], Leiden, the Netherlands) and two nonuniversity teaching hospitals (Amphia Hospital Breda and Alrijne Hospital Leiderdorp, both in the Netherlands) between February 24 and April 26, 2020. COVID‐19 was confirmed by a positive polymerase chain reaction (PCR) test or considered positive in patients with a negative PCR but highly suggestive symptoms and typical COVID‐19 abnormalities on computed tomography (CT) scan of the chest (COVID‐19 Reporting and Data System 4 or 5 following Dutch Radiology Society 23 ) with no alternative diagnosis. All patients with COVID‐19 had received pharmacologic thromboprophylaxis from admission on, according to local hospital protocols, which changed over time (Appendix S3). Informed consent was obtained by an opt‐out approach. This study was approved by the Institutional Review Boards of the LUMC for observational studies and was performed on behalf of the Dutch COVID & Thrombosis Coalition. 24
2.2. Objectives
The primary objective was to assess the incidence of venous and arterial thrombotic complications in hospitalized patients with influenza (general ward and ICU combined) and compare this with that observed in our cohort of hospitalized patients with COVID‐19.
2.3. Data collection
A diagnosis of influenza in the allocated time frame was identified in the DHD data based on International Classification of Diseases (ICD) codes (Appendix S1). For those patients who had more than one influenza‐related hospitalization, only the first hospitalization was included. In these data, a differentiation is made between admission to a normal ward only or (temporarily) admission to an ICU, but it was not possible to distinguish exact days of admittance on the general ward versus ICU. Therefore, patients with influenza in the DHD database were categorized as hospitalized at the ward or ICU by the following definition:
Ward: ‘hospitalizations that did not involve ICU admission” (includes patients that were only admitted to the wards)
ICU: “hospitalizations that did involve ICU admission” (includes patients who were admitted to the ICU at some time point during hospitalization).
Patient characteristics of included patients with influenza, such as age, sex, and comorbidities, were collected. A history of comorbidities, such as malignancy and prior history of venous thromboembolism (VTC) were screened for within 8 years before the influenza‐related hospitalization. Before 2018, categorization of the hospitalization as ward and/or ICU was not possible, as this information was not available in the CBS database. Therefore, we studied the incidence of thrombotic complications in influenza‐related hospitalizations during influenza seasons between 2013 and 2017 as a sensitivity analysis, rather than as a main analysis. Influenza seasons started from the 40th week of one year to the 20th week of the next year.
The patient charts of patients with COVID‐19 were retrospectively scrutinized for baseline characteristics and outcomes of interest. For COVID‐19 we were able to strictly distinguish general ward from ICU admission by exact days of admittance, but we used the influenza categorization of ward and ICU to be consistent.
2.4. Outcomes
Information on thrombotic complications in influenza was identified by ICD codes (Appendix S1). Any VTC (deep‐vein thrombosis [DVT] and/or pulmonary embolism [PE]) or arterial thrombotic complication (arterial thromboembolism [ATC]: ischemic stroke, myocardial infarction, and/or systemic arterial embolism) that occurred during the index hospitalization was regarded as an outcome event. The exact date of the thrombotic complication during admission was unavailable in DHD. Therefore, the date halfway into the hospitalization period was chosen as the date of the thrombotic complication in patients with influenza.
In patients with COVID‐19, information on thrombotic complications was identified on the basis of scrutinizing patient charts. Thrombotic complications consisted of acute PE, DVT, ischemic stroke, myocardial infarction, and systemic arterial embolism. No VTC screening strategies were applied during this study in patients with COVID‐19. Upon clinical suspicion of one of the thrombotic complications, appropriate diagnostic tests were applied, that is, computed tomography pulmonary angiography for suspected PE and compression ultrasonography for suspected DVT; cardiac enzymes, including troponin; electrocardiography and echocardiography for suspected acute coronary syndrome; and CT scan of the brain and CT angiography of the carotid and intracerebral arteries for suspected ischemic stroke.
2.5. Statistical analysis
Patient characteristics were described using standard descriptive statistics. The analysis on the comparison between patients with influenza and patients with COVID‐19 comprised the full time of hospitalization (ward and ICU combined). Hospitalized patients with influenza were followed from the day of hospital admission (ie, the starting date) until day of discharge, day of death, or end of data collection (December 31, 2018), whichever came first. Patients with COVID‐19 were followed from the day of hospital admission until discharge, transfer to another hospital, death, or end of data collection (between April 17 and 26, 2020), whichever came first.
The incidence of thrombotic complications was estimated for all ICU and non‐ICU patients combined, patients admitted to general wards only and for patients who had been admitted to the ICU at some time point during hospitalization (according to the DHD categorization of ward and ICU), and for all thrombotic complications as well as for venous and arterial complications separately. Cumulative incidences were estimated using the Kaplan‐Meier method and the cumulative incidence competing risk method, to adjust for the competing risk of death. In addition, incidence rates were calculated over the total follow‐up period.
SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA) and R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) served for data analysis.
3. RESULTS
3.1. Patients
A total of 13 217 hospitalized patients with influenza and 579 hospitalized patients with COVID‐19 were included. Characteristics of both patient cohorts are summarized in Table 1. Patients with influenza and COVID‐19 were comparable with regard to age, history of cancer, and length of hospital stay (median, 5 days [IQR, 3–10] in influenza and 7 days [IQR, 4–11] in COVID‐19). There were more male patients with COVID‐19 than with influenza.
TABLE 1.
Characteristics of hospitalized influenza patients and hospitalized COVID‐19 patients
|
Influenza (N = 13,217) a |
COVID‐19 (N = 579) a |
|
|---|---|---|
| Age, y, mean (SD) | 69 (19) | 67 (13) |
| Male sex, n (%) | 6306 (48) | 380 (66) |
| Body weight, kg, median (IQR) | NA | 84 (73–95) |
| Cancer history, n (%) b | 2343 (18) | 86 (15) |
| Prior history VTC, n (%) b | 404 (3.1) | NA |
| Therapeutic anticoagulation at admission, n (%) | NA | 77 (13) |
| Length of hospital stay, days, median (IQR) | 5 (3–10) | 7 (4–11) |
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range; NA, not available; SD, standard deviation; VTC, venous thromboembolism.
Ward and Intensive Care Unit patients combined.
For influenza, this could only be determined within 8 years before date of admission.
3.2. Thrombotic complications in patients with influenza
Of the influenza patients, 437 of 13 217 patients (3.3%) were diagnosed with thrombotic complications, 126 with venous and 319 with arterial complications. The 30‐day cumulative incidence of all thrombotic complications, adjusted for competing risk of death, was 11% (95% CI, 9.4–12) (Table 2). The 30‐day adjusted cumulative incidence for venous thrombotic complications was 3.6% (95% CI, 2.7–4.6) and for arterial thrombotic complications was 7.5% (95% CI, 6.3–8.8). The incidence rates in hospitalized influenza patients were 1.6/patient‐year (95% CI, 1.4–1.7) for any thrombotic complication, 0.44/patient‐year (95% CI, 0.37–0.53) for venous thrombotic complications, and 1.1/patient‐year (95% CI, 1.0–1.3) for arterial thrombotic complications. The incidences calculated for the year 2018 were comparable with previous years (2013–2017; Appendix S2).
TABLE 2.
Cumulative incidences of thrombotic complications in hospitalized influenza patients versus hospitalized COVID‐19 patients
|
Influenza a Crude % (95% CI) |
Influenza a Adjusted c % (95% CI) |
COVID‐19 b Crude % (95% CI) |
COVID‐19 b Adjusted c % (95% CI) |
|
|---|---|---|---|---|
| All thrombotic complications | ||||
| 10 days | 4.8 (4.3–5.4) | 4.6 (4.1–5.2) | 13 (9.3–17) | 12 (8.9–15) |
| 20 days | 8.9 (7.8–10) | 8.2 (7.3–9.2) | 30 (21–38) | 24 (18–30) |
| 30 days | 12 (10–14) | 11 (9.4–12) | 32 (22–41) | 25 (18–32) |
| VTC | ||||
| 10 days | 1.3 (0.97–1.6) | 1.2 (0.95–1.5) | 12 (8.2–15) | 11 (7.7–14) |
| 20 days | 2.8 (2.2–3.5) | 2.6 (2.1–3.2) | 27 (19–36) | 21 (16–28) |
| 30 days | 4.1 (2.9–5.2) | 3.6 (2.7–4.6) | 29 (20–38) | 23 (16–29) |
| ATC | ||||
| 10 days | 3.6 (3.2–4.1) | 3.5 (3.1–4.0) | 2.4 (0.83–4.0) | 2.2 (1.1–4.1) |
| 20 days | 6.3 (5.4–7.2) | 5.8 (5.0–6.7) | 3.9 (0.57–7.2) | 3.2 (1.4–6.4) |
| 30 days | 8.4 (6.9–10) | 7.5 (6.3–8.8) | 5.7 (0.8–11) | 4.4 (1.9–8.8) |
All thrombotic complications: VTC and ATC together.
Abbreviations: ATC, arterial thrombotic complications; CI, confidence interval; COVID‐19, coronavirus disease 2019; VTC, venous thrombotic complications.
13 217 patients with influenza (ward and Intensive Care Unit combined).
579 patients with COVID‐19 (ward and Intensive Care Unit combined).
Adjusted: cumulative incidence adjusted for competing risk of death.
For patients with influenza who had only been admitted to the ward, the adjusted 30‐day cumulative incidences for all thrombotic complications, venous thrombotic complications, and arterial thrombotic complications were 8.9% (95% CI, 7.5–10), 2.4% (95% CI, 1.8–3.2), and 6.6% (95% CI, 5.4–8.0), respectively. Of these ward‐admitted patients, 766 of 12 412 (6.2%) died during hospitalization. In patients with influenza who had been admitted to the ICU, the adjusted 30‐day cumulative incidences for all thrombotic complications, venous thrombotic complications, and arterial thrombotic complications were 18% (95% CI, 14–23), 7.7% (95% CI, 5.0–11), and 11% (95% CI, 7.9–15), respectively (Table 3). In this group of patients, 171 out of 805 (21%) died during hospital admission.
TABLE 3.
Adjusted cumulative incidences of thrombotic complications in ward and ICU influenza and COVID‐19 patients a
|
Influenza ward N = 12 412 % (95% CI) |
COVID−19 ward N = 401 % (95% CI) |
Influenza ICU N = 805 % (95% CI) |
COVID−19 ICU N = 178 % (95% CI) |
|
|---|---|---|---|---|
| All thrombotic complications | ||||
| 10 days | 4.3 (3.8–4.9) | 4.7 (2.6–7.9) | 6.8 (5.1–8.9) | 21 (15–28) |
| 20 days | 7.2 (6.2–8.2) | 6.1 (3.1–11) | 13 (10–17) | 35 (27–44) |
| 30 days | 8.9 (7.5–10) | 6.1 (3.1–11) | 18 (14–23) | 36 (28–45) |
| VTC | ||||
| 10 days | 1.1 (0.80–1.4) | 3.1 (1.4–5.9) | 2.5 (1.5–3.9) | 21 (15–27) |
| 20 days | 2.2 (1.7–2.8) | 4.4 (1.8–8.8) | 4.9 (3.2–7.1) | 33 (25–42) |
| 30 days | 2.4 (1.8–3.2) | 4.4 (1.8–8.8) | 7.7 (5.0–11) | 34 (26–43) |
| ATC | ||||
| 10 days | 3.4 (2.9–3.9) | 1.7 (0.61–3.9) | 4.4 (3.0–6.1) | 2.5 (0.83–5.9) |
| 20 days | 5.2 (4.4–6.1) | 1.7 (0.61–3.9) | 8.7 (6.4–11) | 3.8 (1.3–8.5) |
| 30 days | 6.6 (5.4–8.0) | 1.7 (0.61–3.9) | 11 (7.9–15) | 5.3 (2.0–11) |
All thrombotic complications: VTC and ATC together; adjusted: cumulative incidence adjusted for competing risk of death.
Abbreviations: ATC, arterial thrombotic complications; CI, confidence interval; COVID‐19, coronavirus disease 2019; VTC, venous thrombotic complications.
Following the classification of ward (hospitalizations that did not involve ICU) and ICU (hospitalizations that did involve ICU) as is done in the CBS hospitalization data.
3.3. Thrombotic complications in patients with COVID‐19
Of the 579 patients with COVID‐19, 66 patients (11%) were diagnosed with 70 thrombotic complications. PE was the most frequent thrombotic complication of all (n = 54; 77%). The 30‐day cumulative adjusted incidence of all thrombotic complications was 25% (95% CI, 18–32) (Table 2). The 30‐day adjusted cumulative incidence for venous thrombotic complications was 23% (95% CI, 16–29) and for arterial thrombotic complications 4.4% (95% CI, 1.9–8.8). Incidence rates in patients with COVID‐19 were 5.1/patient‐year (95% CI, 3.9–6.4) for any thrombotic complication, 4.5/patient‐year (95% CI, 3.4–5.8) for venous thrombotic complications, and 0.78/patient‐year (95% CI, 0.39–1.4) for arterial thrombotic complications.
For the patients with COVID‐19 who had only been admitted to the ward, the adjusted 30‐day cumulative incidences for all thrombotic complications, VTC alone, and ATC alone were 6.1% (95% CI, 3.1–11), 4.4% (95% CI, 1.8–8.8), and 1.7% (95% CI, 0.61–3.9), respectively. Of these ward‐admitted patients, 66 out of 401 died during hospitalization (17%). In patients with COVID‐19 who had been admitted to the ICU at any moment during the in‐hospital stay, the adjusted 30‐day cumulative incidences for all thrombotic complications, VTC alone, and ATC alone were 36% (95% CI, 28–45), 34% (95% CI, 26–43), and 5.3% (95% CI, 2.0–11), respectively (Table 3). In this group of patients, 45 out of 178 died during hospital admission (25%).
4. DISCUSSION
In this study, we observed that, compared to hospitalized patients with influenza, patients admitted with COVID‐19 had a distinctly increased risk for thrombotic complications by day 30. This risk was driven by a difference in venous thrombotic complications (3.6% in influenza and 23% in COVID‐19) and was particularly observed in patients admitted to the ICU.
How often influenza infection led to clinically relevant thrombotic disease was hitherto underreported, since only small case series have been published, yielding conflicting results. 4 , 8 , 9 , 10 Nevertheless, respiratory viruses, including influenza, were already known to lead to a procoagulant state. 4 , 5 , 6 Previous studies, for instance, showed a transient increase in the risk of vascular complications after respiratory tract infections, with a twofold increase for venous thrombotic disease and an even fivefold increase for ischemic heart disease. 1 , 7 Of note, in our study, arterial thrombotic complications, and in particular myocardial infarction, occurred more in patients with influenza than in patients with COVID‐19. A recent study showed stroke rates quite similar to our study. 25 Strokes occurred in 0.2% in the patients with influenza and in 1.6% in the patients with COVID‐19 25 ; in our study, this was 0.8% in the patients with influenza and 1.7% in the patients with COVID‐19. Furthermore, another Chinese study was recently published, which compared the incidence of VTC in patients hospitalized with COVID‐19 versus those hospitalized with community‐acquired pneumonia (CAP). 20 Surprisingly, this study found no difference in VTC rates between CAP and COVID‐19. However, patients with COVID‐19 in this study were overall younger and had fewer comorbidities. 20 In addition, arterial thrombotic complications were not included in this study, and VTC risk is known to be lower in East Asians than in White Caucasians, which limits generalizing these findings to other populations. A recent large cross‐sectional study of 80.261 patients with influenza illustrated that the high risk of arterial thrombotic complications coincides with influenza virus infection. 26 Therefore, it is reasonable to believe that viral pneumonias such as influenza virus and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) both interact with the coagulation system. 4
Virus‐specific features could play an important role and maybe explain the observed difference in thrombotic complications between patients with influenza and patients with COVID‐19. 4 , 27 , 28 Recent studies suggest the possibility of in situ immunothrombosis in patients with COVID‐19 due to a SARS‐CoV‐2 specific effect. Following this hypothesis, COVID‐19–associated alveolar injury and an extreme inflammatory response contribute to small‐vessel thrombus formation in the lungs. Autopsy studies support this hypothesis by reporting the presence of platelet‐fibrin thrombi in small arterial vessels of the lungs. 22 , 27 , 29 On the other hand, other studies observed a high incidence of DVT upon screening as well, which is compatible with the conventional thromboembolic origin of PE. 30 , 31 Therefore, both mechanisms likely play a role in the pathophysiology of COVID‐19–associated thrombosis, and further research in this field is needed.
Methodological strengths of our study include the large sample size and multicenter study design. In addition, VTC screening strategies were not applied during the course of this study, and diagnostic tests were performed only upon clinical suspicion. Limitations of our study regarding influenza data are that not all baseline patient characteristics were available, since these could be determined only during a specific time period before hospital admission, and that exact dates of thrombotic complications were not available. Furthermore, we do not know whether thrombosis prophylaxis was given to the hospitalized patients with influenza and, if so, which dose was given. National guidelines in the Netherlands recommend pharmacologic thromboprophylaxis with low‐molecular‐weight heparin (LMWH) in ward admitted patients in the case of a PADUA Prediction Score ≥4 and in the absence of contraindications. All patients admitted to the ICU receive pharmacologic thromboprophylaxis, irrespective of the PADUA Prediction Score. 32 Standard practice in the LUMC, Amphia Hospital, and Alrijne Hospital is the administration of nadroparin 2850 IU subcutaneously per day. It is likely that these recommendations were followed for the hospitalized patients with influenza, but still we do not know for sure. In addition, outcome classification differed between patients with influenza and patients with COVID‐19, as information on thrombotic complications was identified in influenza based on ICD codes and in COVID‐19 by patient chart review. Besides, it is important to recall that thrombotic risks in influenza may differ between the seasonal influenza virus—from which patients were included in this study—and the H1 N1 influenza virus pandemic from the year 2009‐2010. For COVID‐19 data, limitations include the different doses of thrombosis prophylaxis being prescribed over time and the lowering threshold to suspect VTC over the course of time in patients with COVID‐19. In addition, thrombosis in COVID‐19 due to in situ immunothrombosis could have been underestimated, since this diagnosis is often established only after death in pathology reports. Still, we were interested in clinically relevant confirmed thrombosis and believe that this underestimation will likely be minimal, especially given this lowering threshold to suspect VTC in COVID‐19. Furthermore, compared to patients with influenza, we observed a higher mortality rate in the patients with COVID‐19 admitted to the ward, which indicates that the patients with COVID‐19 admitted to the ward had more severe illness than ward‐admitted patients with influenza. Although this could (partially) explain the observed difference in thrombotic risk between the ward‐admitted patients in both groups, this cannot explain differences found in thrombotic complications between patients who were admitted to the ICU, as mortality rates in these patients were quite comparable, but a large difference was found in (especially venous) thrombotic complications. Altogether, the comparison between patients with influenza and patients with COVID‐19 could have been biased because of the difference in outcome classification and the lowered threshold for diagnostic testing upon suspicion of VTC in patients with COVID‐19 was probably lower than in patients with influenza after the high incidence of thrombotic complications in COVID‐19 was elucidated.
In conclusion, this study suggests that the incidence of thrombotic complications in hospitalized patients with influenza is lower than in hospitalized patients with COVID‐19. The difference in thrombotic complications in our study was particularly driven by an exceptionally high risk for VTC in the patients with COVID‐19 that had been admitted to the ICU. Remarkably, patients with influenza were more often diagnosed with arterial thrombotic complications than with VTC. Further studies are needed to substantiate our findings and to explore explanations for this difference.
RELATIONSHIP DISCLOSURE
F.K. reports research grants from Bayer, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Daiichi‐Sankyo, MSD, Actelion, the Dutch Heart Foundation, ZonMW Dutch Healthcare Fund, and the Dutch Thrombosis Association, all outside the submitted work. M.H. reports grants from ZonMW Dutch Healthcare Fund, and grants and personal fees from Boehringer‐Ingelheim, Pfizer‐BMS, Bayer Health Care, Aspen, Daiichi‐Sankyo, and Leo Pharma, all outside the submitted work. The other authors having nothing to disclose.
AUTHOR CONTRIBUTIONS
See Online Appendix.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Statistics Netherlands for providing data from the Dutch Hospital Data registry.
Contributors:
CONSORTIUM MEMBERS DUTCH COVID & THROMBOSIS COALITION
Amsterdam University Medical Center
Location AMC
Dr M. Coppens
Prof Dr N.P. Juffermans
Prof Dr S. Middeldorp
Location VUMC
Prof Dr C.M.P.M. Hertogh
J.G. Hugtenburg
Dr E.J. Nossent
Erasmus Medical Center
Drs J. van den Akker
Dr R. Bierings
Dr H. Endeman
Dr M. Goeijenbier
Prof Dr D.A.M.P.J. Gommers
Prof Dr M.P.G. Koopmans
Prof Dr T. Kuiken
T. Langerak
Dr M.N. Lauw
Prof Dr M.P.M. de Maat
D. Noack
M.S. Paats
M.P. Raadsen
Dr B. Rockx
Dr C. Rokx
Dr C.A.M. Schurink
K. Tong‐Minh
Dr L van den Toorn
Dr C.A. den Uil
C. Visser
Farmadam pharmacy
Tineke Roest
Institute of Research of Hospital de la Santa Creu i Sant Pau, Barcelona
Dr J. Manuel Soria
Leiden University Medical Center
M.L. Antoni
Dr M. Bos
Drs Burggraaf
Prof S.C. Cannegieter
Prof Dr H.C.J. Eikenboom
Dr P.L. den Exter
Dr J.J.M. Geelhoed
Prof Dr M.V. Huisman
Prof E. de Jonge
Dr F.H.J. Kaptein
Dr F.A. Klok
Dr L.J.M. Kroft
Dr L. Nab
Dr M.K. Ninaber
Prof Dr H. Putter
Dr. A.M. da Rocha Rondon
Dr A.H.E. Roukens
Dr M.A.M. Stals
Prof Dr H.H. Versteeg
Dr. H.W. Vliegen
Dr B.J.M. van Vlijmen
Maastricht University Medical Center
Dr B.C.T. van Bussel
Prof Dr T.M. Hackeng
Dr T. van de Berg
Prof Dr H. ten Cate
Dr ir. Y. Henskens
Dr H. Spronk
Prof Dr L. Schurgers
Dr R. Bruggemann
Dr B. Spaetgens
Dr K. Winckers
Dr R. Olie
Dr M. Mulder
Dr A. Hulshof
Prof Dr M.A. Spruit
Radboud University Medical Center
Dr J. Leentjens
Dr Q. de Mast
Sanquin Research, Amsterdam
Dr M. van den Biggelaar
Prof. dr. J.C.M. Meijers
Prof Dr J. Voorberg
Synapse Research Institute
Dr B. de Laat, biochemicus
Thrombosis services Maastricht
Dr A. Ten Cate‐Hoek
University Medical Center Groningen
Prof Dr T. Lisman
Prof Dr K. Meijer
University Medical Center Utrecht
O.L. Cremer
Dr G. Geersing
Prof Dr H.A.H. Kaasjager
N. Kusadasi
A. Huisman
Dr M. Nijkeuter
Prof Dr R.E.G. Schutgens
Dr R.T. Urbanus
J. Westerink
Handling Editor: Dr Susan Kahn.
Funding information
This study was funded by unrestricted grants of the participating hospitals. The steering committee, consisting of the authors, had final responsibility for the study design, oversight, and data verification and analyses. The sponsor was not involved in the study. All members of the steering committee contributed to the interpretation of the results, approved the final version of the manuscript, and vouch for the accuracy and completeness of the data reported. The corresponding author (M.V.H.) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The final decision to submit the manuscript was made by the corresponding author on behalf of all co‐authors.
Contributor Information
Suzanne C. Cannegieter, @s_cannegieter.
Frederikus A. Klok, @Erik_Klok_MD.
Menno V. Huisman, Email: m.v.huisman@lumc.nl.
DATA AVAILABILITY STATEMENT
Anonymous data on hospitalized patients with COVID‐19 as collected in this study can be made available to others, on request to the corresponding author of this manuscript (M.V.H.). The request will be granted after approval of a methodologically sound proposal, and a signed data‐sharing agreement is required. Data will be available after publication of the article and until 5 years following article publication. Unfortunately, data on hospitalized patients with influenza cannot be shared with third parties, as Statistics Netherlands does not permit this. No other relevant documents are available.
REFERENCES
- 1. Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075‐1079. [DOI] [PubMed] [Google Scholar]
- 2. Tichelaar YI, Kluin‐Nelemans HJ, Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis: a systematic review. Thromb Haemost. 2012;107:827‐837. [DOI] [PubMed] [Google Scholar]
- 3. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTC in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e195S‐e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goeijenbier M, van Wissen M, van de Weg C, et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong RS, Wu A, To KF, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. J Clin Virol. 2020;127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611‐2618. [DOI] [PubMed] [Google Scholar]
- 8. van Wissen M, Keller TT, Ronkes B, et al. Influenza infection and risk of acute pulmonary embolism. Thromb J. 2007;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Obi AT, Tignanelli CJ, Jacobs BN, et al. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord. 2019;7:317‐324. [DOI] [PubMed] [Google Scholar]
- 10. Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD. Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis. 2011;52:e14‐e17. [DOI] [PubMed] [Google Scholar]
- 11. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poissy J, Goutay J, Caplan M, et al. Pulmonary Embolism in COVID‐19 Patients: Awareness of an Increased Prevalence. Circulation. 2020;142:184‐186. [DOI] [PubMed] [Google Scholar]
- 16. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klok FA, Kruip M, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;191:148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas W, Varley J, Johnston A, et al. Thrombotic complications of patients admitted to intensive care with COVID‐19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mei F, Fan J, Yuan J, et al. Comparison of venous thromboembolism risks between COVID‐19 pneumonia and community‐acquired pneumonia patients. Arterioscler Thromb Vasc Biol. 2020;40:2332‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prokop M, Everdingen WV, Vellinga TVR, et al. CO‐RADS – a categorical CT assessment scheme for patients with suspected COVID‐19: definition and evaluation. Radiology. 2020;296(2):E97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dutch COVID & Thrombosis Coalition . Translational approach to unravel and prevent COVID‐19 associated thrombosis: caging the dragon. Res Pract Thromb Haemost. 2020;5(2):278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merkler AE, Parikh NS, Mir S, et al. (COVID‐19) vs patients with influenza. JAMA Neurol. 2020;77(11):1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chow EJ, Rolfes MA, O'Halloran A, et al. Acute cardiovascular events associated with influenza in hospitalized adults. Ann Intern Med. 2020;173(8):605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: a two‐centre descriptive study. Lancet Infect Dis. 2020;20(10):1135‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020;18:1517‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Llitjos J‐F, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18:1743‐1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santoliquido A, Porfidia A, Nesci A, et al. Incidence of deep vein thrombosis among non‐ICU patients hospitalized for COVID‐19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 2020;18(9):2358‐2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. NIV . Richtlijn Antitrombotisch Beleid. Nederlandse Internisten Vereniging; [updated December 2015]. Available from: https://richtlijnendatabase.nl/richtlijn/antitrombotisch_beleid/antitrombotisch_beleid_‐_korte_beschrijving.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Anonymous data on hospitalized patients with COVID‐19 as collected in this study can be made available to others, on request to the corresponding author of this manuscript (M.V.H.). The request will be granted after approval of a methodologically sound proposal, and a signed data‐sharing agreement is required. Data will be available after publication of the article and until 5 years following article publication. Unfortunately, data on hospitalized patients with influenza cannot be shared with third parties, as Statistics Netherlands does not permit this. No other relevant documents are available.


