Dear Editor
The COVID‐19 pandemic has led to a major increase in acute kidney injury (AKI) [1] amid a major constraint in the healthcare resources, including renal replacement treatment, and placed the healthcare providers' safety at a greater risk [2]. New protocols were developed for the treatment of positive COVID‐19 patients with AKI in the pandemic era to limit the potential of staff exposure while treating patients effectively, safely, and timely [3].
Herein, we share our experience during the pandemic. In our institution, Detroit Medical Center, Wayne State University, as an inner‐city tertiary center, we faced an overwhelming volume of reported cases of COVID‐19 associated with AKI requiring renal replacement therapy. During the peak of the surge in Detroit in April 2020, the demand for renal replacement therapy in our intensive care units doubled. Among the currently available renal replacement modalities, no data support the use of a specific one over the others. A common bedside observation noticed was an overwhelming hypercatabolic state, a major oxygenation deficit, and hypercoagulability.
Our inpatient intermittent hemodialysis (iHD) service typically performs 3 h of dialysis per day, three times weekly. However, due to the increased volume of iHD during the pandemic time, and shortage of well‐trained dialysis nurses (in some cases due to quarantine), we were obligated to reduce the treatment session to 2 h instead of the standard 3 h . This lead to suboptimal volume control, dialysis inadequacy especially in hypercatabolic state, and solute clearance.
Simultaneously, patients who were critically ill with multisystem organ failure were admitted to the critical care unit. The majority of these patients were not candidates for iHD due to hemodynamic instability. Based on this, patients who were hemodynamically stable and less hypercatabolic were put on iHD. Critically ill patients with the severe hypercatabolic state were deemed candidates for continuous renal replacement therapy (CRRT). This was selected by the nephrology team who was running the service at that time.
As the surge of cases continued, it became apparent that our infrastructure was overwhelmed due to the limited number of CRRT machines and the expert intensive care nurses who could perform CRRT (Figure 1).
FIGURE 1.
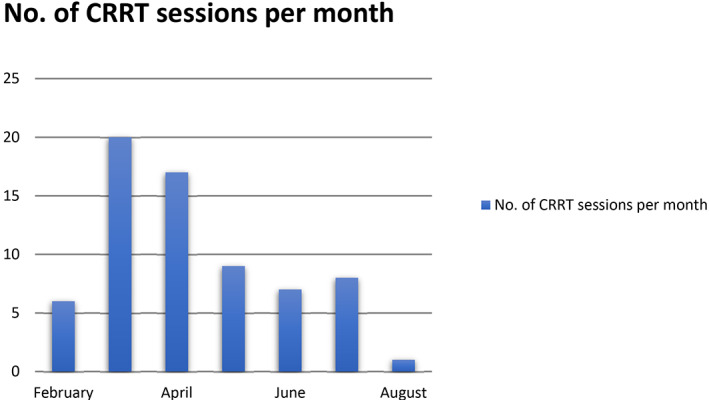
Total number of continuous renal replacement therapy (CRRT) sessions during the peak of the COVID‐19 pandemic in Detroit Medical Center, Michigan, USA [Color figure can be viewed at wileyonlinelibrary.com]
Following this scenario, a multidisciplinary meeting including nephrology, intensive care unit, and pharmacy departments in addition to nursing administration was held. A consensus plan was reached regarding a protocol to handle this situation. It was decided that a new modality be introduced to meet the overwhelming demand named high volume hemofiltration (HVHF) [4, 5]. We started patients on 8 h of daily dialysis with blood flow 300 mL/min, with 6 L replacement solution per hour, and ultrafiltration tailored per patient requirement. The fluid removal rate was ranging between running patients even to remove 50 mL/h net removal. This permitted a temporary solution to dialyze at least two patients per day utilizing one CRRT machine. Moreover, HVHF allowed nurses to supervise patients on CRRT with more flexibility, less exposure (8 h instead of 24 h), and more time to exchange the replacement solution (6 L per bag).
As we started the new protocol, some challenges were encountered. For instance, adjusting medications was a major problem in patients with HVHF in which medications need to be adjusted more frequently as normal glomerular filtration rate (GFR) in contrast to estimated GFR (eGFR) after holding off dialysis where medications should be readjusted to eGFR of the patient. This needed a highly coordinated pharmacy involvement by an already overwhelmed pharmacy service during the pandemic surge; however, it was implemented successfully.
In summary, despite the limited fixed resources and the high volume of presenting patients, we could manage to ration renal support based on the logistics and available infrastructure by establishing a new protocol using HVHF to reduce the incidence of mortality and morbidity during the pandemic. This letter highlights the importance of getting sufficient dialysis machines, staff, and protocols to face the demands of this evolving pandemic.
REFERENCES
- 1. Hirsch JS. Acute kidney injury in patients hospitalized with COVID‐19. Kidney international (0085–2538). 2020;98(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/.
- 4. Rimmelé T, Kellum JA. High‐volume hemofiltration in the intensive care unit: a blood purification therapy. Anesthesiology. 2012;116(6):1377–87. [DOI] [PubMed] [Google Scholar]
- 5. Clark E, Molnar AO, Joannes‐Boyau O, Honoré PM, Sikora L, Bagshaw SM. High‐volume hemofiltration for septic acute kidney injury: a systematic review and meta‐analysis. Critical care (London, England). 2014;18(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]


