This editorial refers to ‘Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial’†, by H.J.L. Heerspink et al., on page 1216.
In the general population, both decreasing estimated glomerular filtration rate (eGFR) and increasing albuminuria are well-established predictors of overall mortality. The presence of diabetes mellitus further potentiates this relationship.1 In chronic kidney disease (CKD) stages 4–5, ∼50% of patients suffer from cardiovascular disease, and cardiovascular mortality accounts for ∼40–50% of all deaths in patients with CKD stage 4 as well as patients with end-stage kidney disease (ESKD), compared with 26% in controls with normal kidney function.2 In terms of cardiovascular disease and mortality, the relative importance of heart failure, valvular and sudden cardiac death, but not ischaemic heart disease, increases as CKD progresses.2 In a multitude of studies in subjects with advanced CKD, correction for classical cardiovascular risk factors, such as hypertension, diabetes mellitus, and dyslipidaemia, did not neutralize the impact of CKD on cardiovascular risk,3 supporting the conclusions of a large meta-analysis that diabetes and kidney disease serve as independent predictors of clinical outcomes.1 These observations underline the importance of non-traditional, CKD-specific cardiovascular risk factors and may explain at least in part why traditional strategies to improve cardiovascular outcome have largely failed in the context of CKD.4 Also, this emphasizes the need not only to identify pathological mechanisms adversely affecting the cardiovascular system in CKD but also to develop novel therapeutic strategies. However, in most outcome trials conducted in the different fields of cardiology, patients with advanced CKD have been excluded, resulting in an unfortunate and serious lack of evidence-based recommendations for this particular high-risk group.
In this issue of the European Heart Journal, Heerspink et al.5 present a pre-specified analysis from the randomized, placebo-controlled DAPA-CKD (Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease) trial,6 in which they studied the effects of the sodium–glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin on morbidity and mortality in patients with CKD. The trial had been performed in a population of diabetic but also non-diabetic CKD patients with a mean eGFR of 43 mL/min/1.73 m2. In DAPA-CKD, dapagliflozin as compared with placebo led to a significant reduction in the primary composite endpoint of a sustained ≥50% eGFR decline, ESKD, renal or cardiovascular death, as well as hospitalization for heart failure. In the present analysis, the authors now show that among the 247 deaths in DAPA-CKD (i.e. 5.7% of the overall population), 37% were due to cardiovascular causes, 41% due to non-cardiovascular causes, and 22% were of undetermined cause. Over a median follow-up of 2.4 years, dapagliflozin led to a highly significant 31% risk reduction of all-cause mortality with a significant reduction in patients both with and without diabetes; moreover, the results were consistent across all other pre-specified subgroups. Interestingly, the effect on all-cause mortality was largely driven by a significant 46% relative risk reduction of non-cardiovascular death, while the effect on cardiovascular death was not significant. Albeit only seen in a small number of patients and only described as a post-hoc analysis, the reduction in non-cardiovascular deaths by dapagliflozin was mainly driven by a reduction in deaths due to infections and malignancies.
The authors should be congratulated for conducting this landmark trial in the underinvestigated population of high-risk patients with advanced CKD and for providing these pre-specified analyses to the scientific community. The results of this mortality analysis are important for several reasons. First, our current knowledge of the causes of death in patients with CKD is mainly based on observational studies with the inherent limitations of medical databases and registers, such as lack of adjudication and potential misclassification. In the DAPA-CKD trial, mortality data were adjudicated by an independent events committee and they are derived from a very well characterized study population. The DAPA-CKD data thus extend our knowledge on the causes of death in patients with advanced CKD in that non-cardiovascular deaths occurred at a similar frequency to cardiovascular deaths (see above). Among the non-cardiovascular deaths, patients mainly died because of infection (19%) and malignancies (11%), while cardiovascular deaths mostly resulted from sudden cardiac death (21%) followed by 5.5% due to heart failure and only in 4.5% of the cases from acute myocardial infarction. This is remarkably similar to observations in diabetic patients on dialysis, for example in the 4D study7 where sudden cardiac death was the most prominent cause of cardiovascular mortality. The important insight from DAPA-CKD is, thus, that in CKD the shift towards more and more sudden cardiac deaths occurs long before the ESKD state has been reached.
Interestingly, in the subgroup of patients without diabetes, non-cardiovascular death occurred more often than cardiovascular death (54% vs. 30%), a result not observed in subjects with diabetes (38% vs. 39%), underscoring epidemiological data showing that diabetes is an additional cardiovascular risk promoter in CKD patients.1 DAPA-CKD enrolled two-thirds of all patients with diabetes and one-third without a diagnosis of type 2 diabetes, but dapagliflozin led to a significant reduction in all-cause mortality in both subgroups. The second important message is therefore that in DAPA-CKD, SGLT2 inhibitor treatment, a therapy originally developed for the treatment of patients with type 2 diabetes, was life-saving in all CKD patients—irrespective of their diabetes status.
In a very large cohort study on non-cardiovascular mortality in advancing CKD, the relative importance of malignancy diminished, whereas infectious and diabetic complications increasingly contributed to deaths.2 While these Canadian data mostly have been reproduced in subsequent studies, it is important to note that such studies are all based on ICD-9 or -10 codes, and causes of death have hardly ever been adjudicated in an independent fashion. The more important are the adjudicated DAPA-CKD non-cardiovascular deaths (41% of the overall mortality). The beneficial effect of dapagliflozin on deaths due to infections raises the hypothesis that SGLT2 inhibitors may exhibit modulatory effects on inflammatory processes and immune function beyond the cardiovascular system and the kidney.
A limitation of the present post-hoc analysis of the DAPA-CKD trial is that the findings are based on a relatively low number of events, in particular in non-diabetic patients, as 80% of deaths were clustered in the subgroup with diabetes. Thus, in non-diabetic patients in particular, the effects of an SGLT2 inhibitor on infection- or cancer-related mortality can at best be hypothesis generating but deserve further evaluation in clinical studies and experimental approaches. While the authors propose potential mechanisms by which dapagliflozin or, more probably, SGLT2 inhibitors in general might contribute to such benefits, there is a significant likelihood for chance findings given the low event rates. Finally, as with all large clinical trials, a common limitation is that ‘healthier’ patients tend to be included, which renders an extrapolation to all patients with advanced CKD difficult. Nevertheless, we eagerly await subgroup analyses of particular, more homogeneous, DAPA-CKD patients, such as the 270 patients with IgA nephropathy.
Taken together, the data from DAPA-CKD, in conjunction with the results from other recently published SGLT2 inhibitor trials in patients with heart failure and reduced ejection fraction, position the class of SGLT2 inhibitors far beyond their original use in patients with type 2 diabetes (Graphical Abstract).5 , 6 , 8 For the cardiovascular high-risk population of patients with CKD, SGLT2 inhibition with dapagliflozin provides for the first time a treatment option to significantly improve the prognosis and to reduce mortality—independent of the presence of diabetes. Within the next few years, additional studies with SGLT2 inhibitors in CKD patients will report (e.g. EMPA-KIDNEY with empagliflozin, NCT03594110), but now it is on us—nephrologists, cardiologists, and all healthcare providers treating patients with CKD—to ensure that these life-saving therapies are implemented. For the treatment of patients with diabetes, a subset of members of the writing groups of the 2019 American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) consensus document and the 2019 European Society of Cardiology (ESC) guidelines have recently published a call for action to ensure that clinical inertia no longer leads to the low prescription of evidence-based life-saving therapies in high risk patients.9 The data published by Heerspink et al. extend this call to patients with CKD: ‘Apply the evidence originating from large studies and provide the best therapy possible in daily practice’.
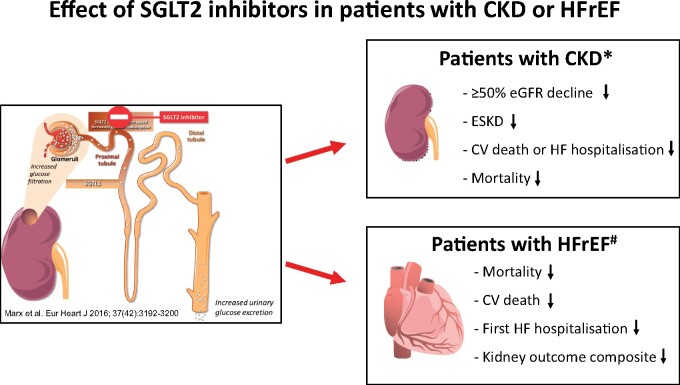
Graphical Abstract.
Effect of sodium–glucose co-transporter 2 (SGLT2) inhibitors in patients with chronic kidney disease (CKD) or heart failure with reduced ejection fraction (HFrEF). CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HF, heart failure. *Data from DAPA-CKD5 , 6. #Data from a meta-analysis from DAPA-HF and EMPEROR-Reduced.8
Funding
N.M. and J.F. are supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TRR 219; Project-ID 322900939 [C01, M01, M03, M05].
Conflict of interest: N.M. has received support for clinical trial leadership from Boehringer Ingelheim and Novo Nordisk; served as a consultant to Boehringer Ingelheim, Merck, Novo Nordisk, AstraZeneca, and BMS; received grant support from Boehringer Ingelheim, Merck, and Novo Nordisk; and served as a speaker for Boehringer Ingelheim, Merck, Novo Nordisk, Lilly, BMS, and AstraZeneca. N.M. declines all personal compensation from pharma or device companies. J.F. served as a consultant to Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk.
Footnotes
† doi:10.1093/eurheartj/ehab094.
Contributor Information
Nikolaus Marx, Department of Internal Medicine I (Cardiology), University Hospital, RWTH Aachen University, Aachen, Germany.
Jürgen Floege, Division of Nephrology and Clinical Immunology, University Hospital, RWTH Aachen University, Aachen, Germany.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015;26:2504–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vanholder R, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Massy ZA, Rodriguez M, Stegmayr B, Stenvinkel P, Wratten ML. Uremic toxicity: present state of the art. Int J Artif Organs 2001;24:695–725. [PubMed] [Google Scholar]
- 4. Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, Kanbay M, Mallamaci F, Massy ZA, Rossignol P, Vanholder R, Wiecek A, Zoccali C, London GM; Board of the EURECA-m Working Group of ERA-EDTA. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014;383:1831–1843. [DOI] [PubMed] [Google Scholar]
- 5.Heerspink HJL, Sjöström CD, Jongs N, Chertow GM, Kosiborod M, Hou FF, McMurray JJV, Rossing P, Correa-Rotter R, Kurlyandskaya R, Stefansson BV, Toto RD, Langkilde AM, Wheeler DC; for the DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis fromthe DAPA-CKD randomized controlled trial. Eur Heart J 2021;doi:10.1093/eurheartj/ehab094.
- 6. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 7. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 8. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 9. Marx N, Davies MJ, Grant PJ, Mathieu C, Petrie JR, Cosentino F, Buse JB. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol 2021;9:46–52. [DOI] [PubMed] [Google Scholar]