To the Editor,
In the wake of the COVID‐19 pandemic, the antibody responses to the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus have received a huge interest for diagnostic or epidemiological purposes. 1 Therefore, a range of serological tests detecting specific antibodies to SARS‐CoV‐2 have emerged, but their performances depend on several factors, such as the methodology of the immunoassay, the viral antigen used for antibody binding, and the isotype detection. 2 In this study, the detection of total antibodies (including immunoglobulin G [IgG] and IgM) to SARS‐CoV‐2 has been performed on the Advia Centaur XP platform by using a new commercial immunoassay from Siemens Healthineers®. This chemiluminescent technique allows the detection of antibodies that recognized a recombinant receptor binding domain (RBP) protein from the coronavirus Spike protein S1. Antibodies targeting the viral RBP tend to have neutralizing capacities and to confer protective immunity. 3 The Siemens SARS‐CoV‐2 assay was carried out according to the manufacturer's instructions and its recommended cutoff of 1 was applied for results interpretation (index ≥ 1 means positive while index <1 is negative).
The local ethical committee of the CHU Tivoli approved this study.
The sensitivity was determined by investigating 246 residual serums collected longitudinally over the course of time from 81 SARS‐CoV‐2‐infected patients with a positive reverse‐transcription polymerase chain reaction (RT‐PCR; or COVID‐antigen in three cases) on nasopharyngeal swab at the time of diagnosis. The performances were analyzed by receiver operating characteristic curves at different times between the PCR and blood sampling.
Since the test detects both IgG and IgM, the first‐week post‐PCR was divided into two parts to specify the sensitivity during the early phase of infection. The samples were classified into five categories: 0–2 days (n = 58), 3–6 days (n = 48), 7–13 days (n = 63), 14–20 days (n = 44), and 21–28 days (n = 33) after the positive RT‐PCR. The diagnostic sensitivity was 18.97% (95% confidence interval [CI]: 9.9%–31.4%) at 0–2 days, 52.08% (95% CI: 37.2%–66.7%) at 3–6 days, 79.37% (95% CI: 67.3%–88.5%) at 7–13 days, 90.91% (95% CI: 78.3%–97.5%) at 14–20 days, and 93.94% (95% CI: 79.8%–99.3%) at 21–28 days post‐RT‐PCR. Figure 1 shows that 4/44 patients remained negative 2 weeks after RT‐PCR. In two cases, the inability to detect anti‐SARS‐CoV‐2 persisted
Figure 1.
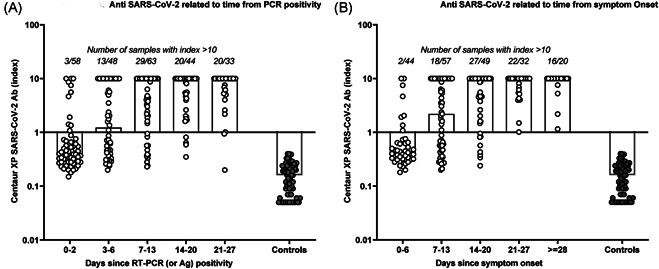
Clinical performance of the Centaur XP anti‐SARS‐CoV‐2 immunoassay: kinetics of the antibody response in COVID patients relative to time since positive RT‐PCR/or COVID‐antigen (A) and to time since symptom onset (B) as compared with prepandemic controls. RT‐PCR, reverse transcription polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
On late samples taken until Days 32 and 37. For the other two, delayed samples were not available. All but one were also negative for anti‐SARS‐CoV‐2 IgG when serum samples were analyzed using another serological assay (Liaison® SARS‐CoV‐2 IgG, Diasorin® measuring anti‐S1/S2 IgG). The antibody response remains unclear for asymptomatic subjects. 4 At 14–20 days post‐RT‐PCR, we observed a higher proportion of false‐negative among asymptomatic carriers (2/11), as compared with symptomatic patients (2/33) so that the sensitivity reached 94.29% (95% CI: 80.8%–99.3%) in this latter group.
The timeframe between the first clinical manifestations and the completion of the nasopharyngeal swab was quite variable (median: 5 days, range: 0–14 days). Therefore, for 65/70 symptomatic patients for whom the beginning of the infection was mentioned in the medical records, the sensitivity was also calculated considering the time since symptom onset. The sensitivity was 18.18% (95% CI: 8.2%–32.7%) at 0–6 days, 59.65% (95% CI: 45.8%–72.4%) at 7–13 days, 83.67% (95% CI: 70.3%–92.7%) at 14–20 days, and 100% for samples collected ≥21 days after the first symptoms. It means that all but one (for whom a follow‐up sample was not available) false‐negative patients at J14–J20 developed antibodies beyond 21 days after the first clinical manifestations.
To assess the specificity, 82 residual serum fractions collected before November 2019 were studied. It included 26 prepandemic clinical samples and 56 samples with possible confounding factors, such as Mycoplasma pneumoniae IgM (n = 15), HBsAg (n = 8), hepatitis C virus antibodies (n = 4), cytomegalovirus IgM (n = 7), EBV IgM (n = 10), toxoplasma IgM (n = 3), rheumatoid factor (n = 2), antinuclear antibodies >1/1280 (n = 4), and monoclonal immunoglobulins (n = 3). No false positive was detected and the results were clearly below the positivity threshold with a median index of 0.16 (range: <0.05–0.4). Based on Youden's index (sensitivity + specificity − 1), the specificity remained excellent up to a cutoff of 0.4. Considering this threshold, the sensitivity was 97.73% (95% CI: 88.0%–99.9%) at 14–20 days post‐RT‐PCR. However, further studies on larger cohorts are mandatory to confirm this hypothesis.
The Siemens assay automated on a Centaur XP platform appears to be a promising serological test to detect total anti‐SARS‐CoV‐2 antibodies that provides within the second week after the RT‐PCR, a sensitivity of 90.91% and even 94.29%, when only symptomatic patients are included. All controls were tested negative, leading to a specificity of 100% in our pre‐COVID cohort. Hence, this test allows reliable and rapid detection of antibodies generated secondarily to COVID‐19 infection.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the clinical laboratory staff from the CHU Tivoli for their technical assistance. Kits were kindly provided by Siemens Healthineers®.
REFERENCES
- 1. Farnsworth CW, Anderson NW. SARS‐CoV‐2 serology: much hype, little data. Clin Chem. 2020;66(7):875‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang ZL, Hou YL, Li DT, Li FZ. Diagnostic efficacy of anti‐SARS‐CoV‐2 IgG/IgM test for Covid‐19: a meta‐analysis [published online ahead of print]. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wanbo T, Xiujuan Z, Yuxian H, Shibo J, Lanying D. Identification of SARS‐CoV RBD‐targeting monoclonal antibodies with cross‐reactive or neutralizing activity against SARS‐CoV‐2. Antiviral Res. 2020;179:104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yongchen Z, Shen H, Wang X, et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):833‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


