Abstract
Aims
In this secondary analysis of the EMPEROR-Reduced trial, we sought to evaluate whether the benefits of empagliflozin varied by baseline health status and how empagliflozin impacted patient-reported outcomes in patients with heart failure with reduced ejection fraction.
Methods and results
Health status was assessed by the Kansas City Cardiomyopathy Questionnaires-clinical summary score (KCCQ-CSS). The influence of baseline KCCQ-CSS (analyzed by tertiles) on the effect of empagliflozin on major outcomes was examined using Cox proportional hazards models. Responder analyses were performed to assess the odds of improvement and deterioration in KCCQ scores related to treatment with empagliflozin. Empagliflozin reduced the primary outcome of cardiovascular death or heart failure hospitalization regardless of baseline KCCQ-CSS tertiles [hazard ratio (HR) 0.83 (0.68–1.02), HR 0.74 (0.58–0.94), and HR 0.61 (0.46–0.82) for <62.5, 62.6–85.4, and ≥85.4 score tertiles, respectively; P-trend = 0.10]. Empagliflozin improved KCCQ-CSS, total symptom score, and overall summary score at 3, 8, and 12 months. More patients on empagliflozin had ≥5-point [odds ratio (OR) 1.20 (1.05–1.37)], 10-point [OR 1.26 (1.10–1.44)], and 15-point [OR 1.29 (1.12–1.48)] improvement and fewer had ≥5-point [OR 0.75 (0.64–0.87)] deterioration in KCCQ-CSS at 3 months. These benefits were sustained at 8 and 12 months and were similar for other KCCQ domains.
Conclusion
Empagliflozin improved cardiovascular death or heart failure hospitalization risk across the range of baseline health status. Empagliflozin improved health status across various domains, and this benefit was sustained during long-term follow-up.
Clinical trial registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03057977.
Keywords: Empagliflozin, Heart failure, Health status, Quality of life, SGLT2 inhibitors
Graphical abstract
See page 1213 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab057)
Introduction
Besides the risk for mortality and recurrent hospitalizations, patients with heart failure with reduced ejection fraction (HFrEF) also suffer from impaired health status.1 , 2 Improvements in physical functioning and symptoms constitute major treatment goals in these patients as reflected by the guidance statements from regulatory agencies and the recognition of Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Minnesota Living with Heart Failure Questionnaire by the Food and Drug Administration as a clinical trial endpoint, or component of a combined endpoint to evaluate devices or drugs for heart failure.3 , 4
The sodium glucose co-transporter 2 (SGLT2) inhibitors empagliflozin and dapagliflozin have been shown to reduce the composite of heart failure hospitalizations or cardiovascular mortality.5–8 In the Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial, dapagliflozin improved cardiovascular outcomes across the range of baseline KCCQ scores and improved health status of patients compared with placebo.7 As compared with DAPA-HF, the EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction) trial was designed to be enriched for higher-risk patients with HFrEF, with lower left ventricular ejection fraction, higher natriuretic peptide levels, and worse renal function.8 It is important to assess the benefit of novel therapies on clinically relevant endpoints across the spectrum of disease severity, as has been done previously with angiotensin-converting enzyme inhibitors, beta-blockers, and mineralocorticoid receptor antagonists in HFrEF. Patients with more advanced disease may or may not respond similarly to those with milder symptoms and functional impairment.
In this secondary analysis of the EMPEROR-Reduced trial, we evaluated the effects of health status on the benefits of empagliflozin with respect to major clinical outcomes as well as the effects of empagliflozin on health status.
Methods
Study design and patient population
The design and primary results of EMPEROR-Reduced have been published previously.8 , 9 Briefly, EMPEROR-Reduced was a randomized, double-blind, parallel-group, placebo-controlled, event-driven study that enrolled adult patients who had chronic heart failure with New York Heart Association (NYHA) functional class II–IV symptoms with a left ventricular ejection fraction ≤40%. To enrol patients at increased risk of events, the number of patients with an ejection fraction of >30% was limited by requiring that they had been hospitalized for heart failure within 12 months or had exceptionally high levels of N-terminal pro-hormone B-type natriuretic peptide (NT-proBNP), i.e. >1000 or >2500 pg/mL in those with an ejection fraction of 31–35% or 36–40%, respectively. NT-proBNP level thresholds were doubled in patients with atrial fibrillation. Key exclusion criteria included symptomatic hypotension or a systolic blood pressure of <100 mmHg and an estimated glomerular filtration rate (eGFR) of <20 mL/min/1.73 m2 body surface area or requiring dialysis. The Ethical Committee of each of the 520 sites in 20 countries approved the protocol, and all patients gave written informed consent.
Quality of life outcome measures
The KCCQ-23 is a 23-item, disease-specific measure that assesses the impact of heart failure on the perspective of patients of their health status.10 The KCCQ-23 has been shown to be valid, reliable, and sensitive to clinical changes, and KCCQ scores are associated with death, hospitalization, and costs.11 , 12 The KCCQ-23 was completed via paper-and-pen version in person by patients at randomization, 3, 8, and 12 months (more specifically, at 12, 32, and 52 weeks) without assistance by site study staff. In the KCCQ-23, the clinical summary score (CSS) includes the physical function and symptoms domains; the total symptom score (TSS) quantifies symptom frequency and severity; and the overall summary score (OSS) is derived from TSS, physical function, quality of life, and social function.
Scores are transformed to a range of 0–100, where higher scores reflect better health status.
Statistical analysis
Patients were categorized into the pre-specified three groups according to tertiles of baseline KCCQ-CSS: (i) <62.5, (ii) 62.6–85.4, and (iii) ≥85.4 points. Baseline characteristics were summarized as means with standard deviation, medians with interquartile ranges, or frequencies and percentages. The rates of primary endpoint of cardiovascular death or heart failure hospitalization across KCCQ-CSS tertiles were compared using cumulative incidence curves. Hazard ratios (HRs) with 95% confidence intervals (CIs) and two-sided P-values were calculated using a Cox proportional hazards model. The pre-specified secondary outcomes were the occurrence of adjudicated total hospitalizations for heart failure (including first and recurrent events) and the slope of change in eGFR during double-blind therapy. These pre-specified secondary outcomes were also compared across KCCQ categories. For total hospitalizations for heart failure, a joint frailty model (with cardiovascular death as a competing risk) was used and for slope a random coefficient model was used based on on-treatment data.8 , 9 The differences in mean KCCQ-CSS, TSS, and OSS between empagliflozin and placebo were calculated based on all observed data (on- or off-treatment) using a mixed model for repeated measurements. No imputations were made for the occurrence of death. The least-squares mean differences between treatments were estimated following adjustment for baseline KCCQ values, age, eGFR, region, diabetes status, sex, and left ventricular ejection fraction.9 The adjustment for left ventricular ejection fraction was based on categories (≤30, >30 to ≤35, and >35%) that reflected the inclusion criteria for the trial.
We performed responder analyses to investigate the proportion of patients on empagliflozin and placebo who had ≥5-, ≥10-, and ≥15-point improvement or ≥5-point deterioration in KCCQ scores at 3, 8, and 12 months; these thresholds are generally regarded as clinically meaningful changes.13 , 14 Odds ratios (ORs) along with 95% CI and two-sided P-values were calculated using logistic regression models, which included baseline scores, age, eGFR, region, diabetes status, sex, and left ventricular ejection fraction. Values for patients who were lost to follow-up or dropped out before 3, 8, or 12 months were imputed and estimates were combined using Rubin’s rules. Patients who died before 3, 8, or 12 months were counted as not improved in the analysis of improvement and deteriorated in the analysis of deterioration. To accommodate for the fact that patients cannot have KCCQ scores that exceed 100 (the so-called ‘ceiling effect’), patients with a baseline value of ≥95 or ≥90 or ≥85 points in KCCQ domains were considered to have 5- or 10- or 15-point improvement if their values remained ≥95 or 90 or 85. Similarly, patients with a KCCQ score at baseline that was ≤5 points were defined as deteriorated if their score remained ≤5 points.7 Details about the methods used for multiple imputation and for correction for the ceiling effect are shown in Supplementary material online, Appendix S1. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). A P-value of <0.05 was considered statistically significant.
Results
Patient population
Baseline characteristics of the patients according to KCCQ-CSS are shown in Table 1. Overall, the mean KCCQ-CSS was 70.7 (21.9). Patients with lower KCCQ-CSS results were more often women, obese, white, enrolled in Latin America, had higher NT-proBNP concentrations, and were more likely to have NYHA class III symptoms and history of diabetes or atrial fibrillation. Left ventricular ejection fraction, blood pressure, and proportion of patients with implantable cardioverter defibrillator were similar across the tertiles of KCCQ-CSS. Supplementary material online, Figure S1 provides an overview of the availability of KCCQ-CSS data at each time point in this analysis for the empagliflozin and placebo group. Twenty-five patients (10 on empagliflozin and 15 on placebo) had missing KCCQ-CSS data at baseline. In patients who were alive and where the time point of randomization allowed for a 3-, 8-, and 12-month follow-up assessment, KCCQ-CSS data were available for 3498 (95%), 3201 (93%), and 2472 (92%) patients, respectively. Baseline characteristics for patients with missing KCCQ-CSS data at baseline are shown in Supplementary material online, Table S1. Supplementary material online, Figure S2 shows the histogram for KCCQ-CSS at baseline.
Table 1.
Baseline characteristics according to Kansas City Cardiomyopathy Questionnaire-clinical summary score scores at baseline
| KCCQ-CSS at baseline |
P-value | |||
|---|---|---|---|---|
| Tertile <62.5 (N = 1220) | Tertile 62.6–85.4 (N = 1253) | Tertile ≥85.4 (N = 1232) | ||
| Age (years) | 66.6 (11.4) | 67.3 (10.5) | 66.7 (11.1) | 0.7545 |
| Women | 393 (32.2%) | 292 (23.3%) | 200 (16.2%) | <0.0001 |
| Race | <0.0001 | |||
| Asian | 104 (8.5%) | 209 (16.7%) | 348 (28.2%) | |
| Black | 112 (9.2%) | 79 (6.3%) | 66 (5.4%) | |
| White | 952 (78.0%) | 909 (72.5%) | 754 (61.2%) | |
| Other or missing | 52 (4.3%) | 56 (4.5%) | 64 (5.2%) | |
| Geographic region | <0.0001 | |||
| Asia | 63 (5.2%) | 143 (11.4%) | 286 (23.2%) | |
| Europe | 472 (38.7%) | 488 (38.9%) | 384 (31.2%) | |
| North America | 140 (11.5%) | 147 (11.7%) | 137 (11.1%) | |
| Latin America | 508 (41.6%) | 409 (32.6%) | 365 (29.6%) | |
| Others | 37 (3.0%) | 66 (5.3%) | 60 (4.9%) | |
| Systolic blood pressure (mmHg) | 121.7 (16.0) | 122.3 (15.4) | 122.0 (15.5) | 0.6425 |
| Diastolic blood pressure (mmHg) | 74.0 (11.1) | 74.1 (10.4) | 73.6 (10.8) | 0.3552 |
| Pulse (bpm) | 72.4 (11.9) | 71.3 (11.9) | 70.0 (11.3) | <0.0001 |
| Body mass index (kg/m²) | 28.8 (5.6) | 28.0 (5.4) | 26.9 (5.0) | <0.0001 |
| Body mass index ≥30 (kg/m²) | 461 (37.8%) | 411 (32.8%) | 288 (23.4%) | <0.0001 |
| Estimated glomerular filtration rate (mL/min/1.73 m²) | 60.8 (21.8) | 61.8 (21.4) | 63.3 (21.3) | 0.0040 |
| Estimated glomerular filtration rate <60 (mL/min/1.73 m²) | 605 (49.6%) | 623 (49.7%) | 559 (45.4%) | 0.0379 |
| N-terminal pro B-type natriuretic peptide (pg/mL) | 2227 (1280-4274) | 1846 (1115-3347) | 1679 (993-2912) | <0.0001 |
| Ischaemic etiology | 631 (51.7%) | 674 (53.8%) | 615 (49.9%) | 0.3677 |
| Left ventricular ejection fraction (%) | 27.3 (6.1) | 27.4 (5.9) | 27.7 (6.0) | 0.0600 |
| New York Heart Association class | <0.0001 | |||
| II | 670 (54.9%) | 990 (79.0%) | 1121 (91.0%) | |
| III | 532 (43.6%) | 263 (21.0%) | 109 (8.8%) | |
| IV | 18 (1.5%) | 0 | 2 (0.2%) | |
| Hypertension | 915 (75.0%) | 927 (74.0%) | 842 (68.3%) | 0.0002 |
| Diabetes | 656 (53.8%) | 595 (47.5%) | 593 (48.1%) | 0.0054 |
| Atrial fibrillation | 490 (40.2%) | 457 (36.5%) | 414 (33.6%) | 0.0005 |
| Heart failure hospitalization within 12 months | 384 (31.5%) | 382 (30.5%) | 378 (30.7%) | 0.6715 |
| Prior myocardial infarction | 555 (45.5%) | 547 (43.7%) | 513 (41.6%) | 0.0544 |
| Prior surgical or percutaneous coronary intervention | 485 (39.8%) | 529 (42.2%) | 504 (40.9%) | 0.5645 |
| Angiotensin converting enzyme inhibitor | 545 (44.7%) | 572 (45.7%) | 570 (46.3%) | 0.4283 |
| Angiotensin receptor blocker | 306 (25.1%) | 303 (24.2%) | 293 (23.8%) | 0.4536 |
| Angiotensin receptor neprilysin inhibitor | 226 (18.5%) | 241 (19.2%) | 258 (20.9%) | 0.1304 |
| Diuretica | 1107 (90.7%) | 1099 (87.7%) | 1020 (82.8%) | <0.0001 |
| Cardiac glycosides | 237 (19.4%) | 183 (14.6%) | 170 (13.8%) | 0.0001 |
| Beta-blocker | 1156 (94.8%) | 1186 (94.7%) | 1168 (94.8%) | 0.9544 |
| Mineralocorticoid receptor antagonist | 896 (73.4%) | 889 (70.9%) | 855 (69.4%) | 0.0272 |
| Anti-platelet | 658 (53.9%) | 666 (53.2%) | 651 (52.8%) | 0.5878 |
| Anti-coagulant | 490 (40.2%) | 493 (39.3%) | 465 (37.7%) | 0.2191 |
| Statin | 826 (67.7%) | 872 (69.6%) | 836 (67.9%) | 0.9390 |
| Implantable cardiac defibrillator | 379 (31.1%) | 431 (34.4%) | 357 (29.0%) | 0.2637 |
| Cardiac resynchronization therapy | 139 (11.4%) | 169 (13.5%) | 131 (10.6%) | 0.5547 |
Data are mean (SD), median (interquartile range), or number (%). Race was reported by the patients. Those who identified with more than one race or with no race were classified as ‘other’. Angiotensin receptor blocker is excluding valsartan when taken with sacubitril because sacubitril/valsartan is shown as angiotensin receptor neprilysin inhibitor.
KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire-clinical summary score.
Excluding mineralocorticoid receptor antagonists.
Effect of baseline health-related quality of life on pre-specified primary and secondary outcomes
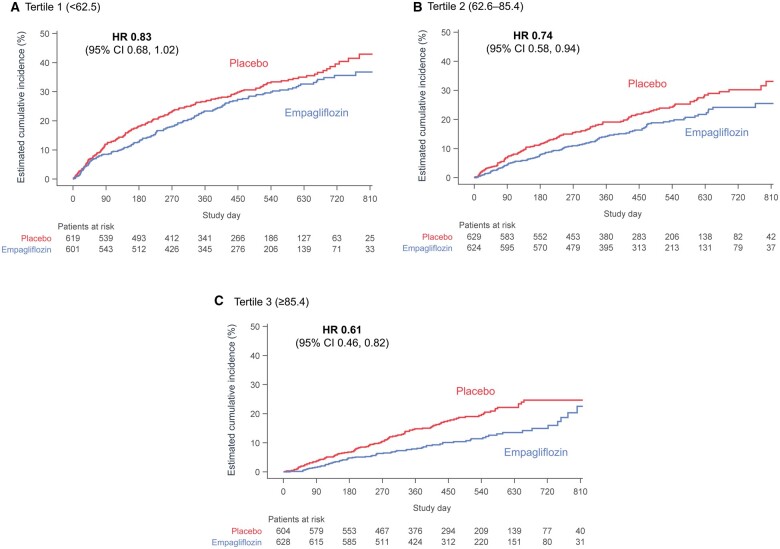
The incidence rate per 100 patient years at the risk of composite of cardiovascular death or heart failure hospitalization was higher in patients with lower baseline KCCQ-CSS (29.4, 19.8, and 15.0 per 100 patient years at risk on placebo for KCCQ-CSS score <62.5, 62.6–85.4, and ≥85.4, respectively). Empagliflozin reduced the primary outcome of cardiovascular death or heart failure hospitalization relative to placebo regardless of baseline KCCQ-CSS tertiles [HR 0.83 (0.68–1.02), HR 0.74 (0.58–0.94), and HR 0.61 (0.46–0.82) for <62.5, 62.6–85.4, and ≥85.4 score tertiles, respectively; P-trend = 0.10] (Figure 1). Results were similar for KCCQ-TSS and KCCQ-OSS (Supplementary material online, Figures S3 and S4).
Figure 1.
Effects of empagliflozin vs. placebo on time to first event of cardiovascular death or heart failure hospitalization according to baseline Kansas City Cardiomyopathy Questionnaires-clinical summary score tertile. Cumulative incidence curves for empagliflozin vs. placebo demonstrating time to composite of cardiovascular death or heart failure hospitalization in (A) lowest baseline Kansas City Cardiomyopathy Questionnaires-clinical summary score tertile, (B) middle baseline Kansas City Cardiomyopathy Questionnaires-clinical summary score tertile, and (C) highest baseline Kansas City Cardiomyopathy Questionnaires-clinical summary score tertile. CI, confidence interval; HR, hazard ratio.
Empagliflozin reduced the total number of heart failure hospitalizations across KCCQ-CSS tertiles [HR 0.80 (0.59–1.09); HR 0.65 (0.47, 0.91); and HR 0.59 (0.40, 0.85) for <62.5, 62.6–85.4, and ≥85.4 score tertiles, respectively; P-trend = 0.16]. Results were similar for KCCQ-OSS and KCCQ-TSS (Table 2).
Table 2.
Effect of empagliflozin on pre-specified outcomes by baseline tertiles of Kansas City Cardiomyopathy
| Outcome | Empagliflozin | Placebo | HR (95% CI) | P-trend* |
|---|---|---|---|---|
| Cardiovascular death or heart failure hospitalization | ||||
| KCCQ-CSS Tertile 1 (<62.5) | 173/601 (29%) | 200/619 (32%) | 0.83 (0.68–1.02) | 0.100 |
| KCCQ-CSS Tertile 2 (62.6–85.4) | 115/624 (18%) | 148/629 (24%) | 0.74 (0.58–0.94) | |
| KCCQ-CSS Tertile 3 (≥85.4) | 72/628 (12%) | 112/604 (19%) | 0.61 (0.46–0.82) | |
| KCCQ-TSS Tertile 1 (<66.7) | 174/595 (29%) | 199/621 (32%) | 0.84 (0.69–1.04) | 0.065 |
| KCCQ-TSS Tertile 2 (66.8–89.6) | 112/623 (18%) | 146/622 (24%) | 0.73 (0.57–0.94) | |
| KCCQ-TSS Tertile 3 (≥89.6) | 74/635 (12%) | 115/609 (19%) | 0.61 (0.45–0.81) | |
| KCCQ-OSS Tertile 1 (<58.9) | 175/597 (29%) | 198/623 (32%) | 0.85 (0.70–1.05) | 0.102 |
| KCCQ-OSS Tertile 2 (59.0–80.7) | 114/621 (18%) | 157/630 (25%) | 0.68 (0.53–0.87) | |
| KCCQ-OSS Tertile 3 (≥80.7) | 71/635 (11%) | 105/599 (18%) | 0.65 (0.48–0.88) | |
| Total number of hospitalizations for heart failure | ||||
| KCCQ-CSS Tertile 1 (<62.5) | 195/601 | 235/619 | 0.80 (0.59–1.09) | 0.161 |
| KCCQ-CSS Tertile 2 (62.6–85.4) | 118/624 | 188/629 | 0.65 (0.47–0.91) | |
| KCCQ-CSS Tertile 3 (≥85.4) | 75/628 | 129/604 | 0.59(0.40–0.85) | |
| KCCQ-TSS Tertile 1 (<66.7) | 203/595 | 230/621 | 0.89 (0.66–1.21) | 0.033 |
| KCCQ-TSS Tertile 2 (66.8–89.6) | 114/623 | 186/621 | 0.58 (0.42–0.80) | |
| KCCQ-TSS Tertile 3 (≥89.6) | 71/635 | 136/609 | 0.56 (0.39–0.81) | |
| KCCQ-OSS Tertile 1 (<58.9) | 190/597 | 230/623 | 0.83 (0.61–1.12) | 0.277 |
| KCCQ-OSS Tertile 2 (59.0–80.7) | 121/621 | 199/630 | 0.60 (0.43–0.83) | |
| KCCQ-OSS Tertile 3 (≥80.7) | 77/635 | 123/599 | 0.65 (0.45–0.95) | |
| Slope of change in eGFR (mL/min/1.73 m2/year) for empagliflozin and placebo, with difference in slope (SE) | ||||
| KCCQ-CSS Tertile 1 (<62.5) | −1.0 (0.41) | −2.2 (0.42) | 1.25 (0.59) | 0.74 |
| KCCQ-CSS Tertile 2 (62.6–85.4) | −0.33 (0.40) | −2.6 (0.39) | 2.27 (0.56) | |
| KCCQ-CSS Tertile 3 (≥85.4) | −0.37 (0.38) | −1.9 (0.39) | 1.56 (0.54) | |
| KCCQ-TSS Tertile 1 (<66.7) | −0.98 (0.41) | −2.2 (0.42) | 1.25(0.59) | 0.54 |
| KCCQ-TSS Tertile 2 (66.8–89.6) | −0.42 (0.39) | −2.4 (0.40) | 2.08 (0.56) | |
| KCCQ-TSS Tertile 3 (≥89.6) | −0.29 (0.39) | −2.05 (0.39) | 1.76 (0.55) | |
| KCCQ-OSS Tertile 1 (<58.9) | −0.96 (0.42) | −2.2 (0.41) | 1.34 (0.59) | 0.44 |
| KCCQ-OSS Tertile 2 (59.0–80.7) | −0.48 (0.39) | −2.3 (0.40) | 1.77 (0.56) | |
| KCCQ-OSS Tertile 3 (≥80.7) | −0.26 (0.38) | −2.2 (0.39) | 1.97 (0.55) | |
CI, confidence interval; CSS, clinical summary score; eGFR, estimated glomerular filtration rate; HR, hazard ratio; KCCQ, Kansas City Cardiomyopathy; OSS, overall summary score; TSS, total symptom score.
P-value from trend test assuming ordering of the KCCQ tertiles and testing for a linear trend across subgroups.
The beneficial effect of empagliflozin relative to placebo on the rate of decline of eGFR was present across all tertiles of KCCQ-CSS [mean change for empagliflozin vs. placebo: 1.25 (0.59) mL/min/1.73 m2/year for tertile <62.5; 2.27 (0.56) mL/min/1.73 m2/year for tertile 62.6–85.4; and 1.56 (0.54) mL/min/1.73 m2/year for tertile ≥85.4; P-trend = 0.74]. Results were similar for KCCQ-OSS (P-trend = 0.44) and KCCQ-TSS (P-trend = 0.54) (Table 2).
Effect of empagliflozin on health-related quality of life outcomes
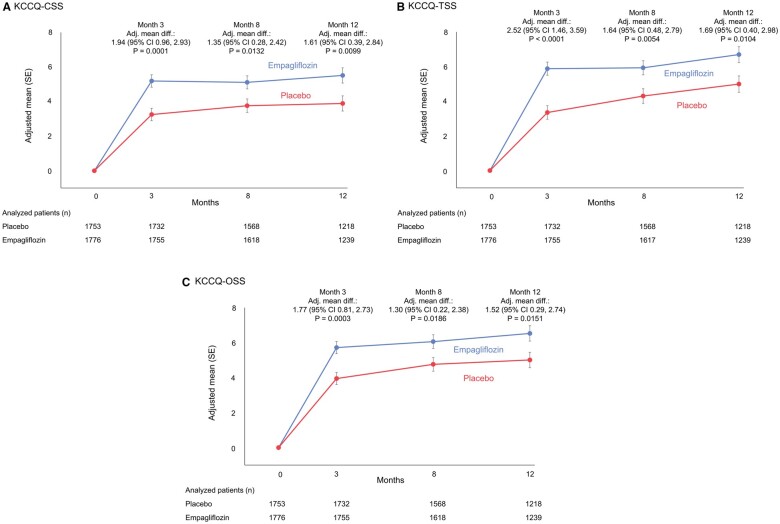
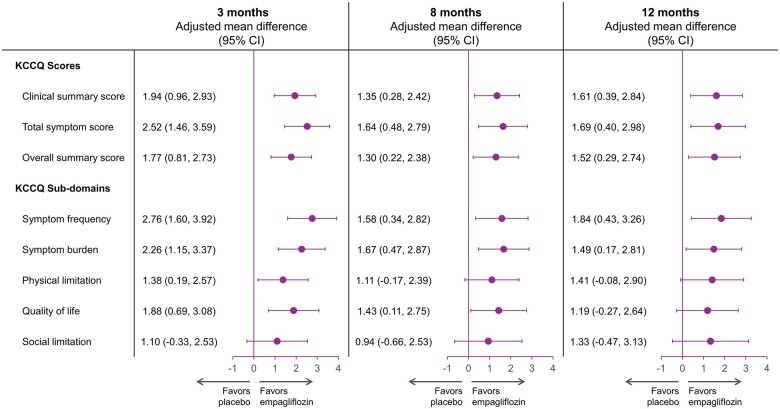
The mean changes in KCCQ-CSS, KCCQ-TSS and KCCQ-OSS by treatment arms over time are presented in Figure 2A–C, respectively. Empagliflozin significantly improved KCCQ-CSS (by 1.94, 1.35, and 1.61 points), TSS (2.52, 1.64, and 1.69 points), and OSS (1.77, 1.30, and 1.52 points) vs. placebo at 3, 8, and 12 months, respectively (P < 0.05 for all, Figure 3). The effect of empagliflozin on KCCQ-CSS, KCCQ-TSS, and KCCQ-OSS by tertiles of baseline score at 3, 8, and 12 months is shown in Table 3.
Figure 2.
Effects of empagliflozin vs. placebo on mean Kansas City Cardiomyopathy Questionnaire scores. Changes in (A) Kansas City Cardiomyopathy Questionnaire-clinical summary score, (B) Kansas City Cardiomyopathy Questionnaire-total symptom score, and (C) Kansas City Cardiomyopathy Questionnaire-overall summary score from baseline to 3, 8, and 12 months for empagliflozin vs. placebo. All observed data were used regardless whether on- or off-treatment. Adj. mean diff., adjusted mean difference; CI, confidence interval; CSS, clinical summary score; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, overall summary score; TSS, total symptom score.
Figure 3.
Adjusted mean difference in Kansas City Cardiomyopathy Questionnaire-clinical summary score, total symptom score, overall summary score, and sub-domains for empagliflozin vs. placebo at 3, 8, and 12 months. All observed data were used regardless whether on- or off-treatment. CI, confidence interval; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Table 3.
Effect of empagliflozin on Kansas City Cardiomyopathy scores at 3, 8, and 12 months
| Placebo-adjusted mean change (95% CI) | P-trend* | |
|---|---|---|
| 3 months | ||
| KCCQ-CSS Tertile 1 (<62.5) | 2.95 (1.15 to 4.75) | 0.215 |
| KCCQ-CSS Tertile 2 (62.6–85.4) | 2.05 (0.32 to 3.78) | |
| KCCQ-CSS Tertile 3 (≥85.4) | 1.33 (−0.41 to 3.08) | |
| KCCQ-TSS Tertile 1 (<66.7) | 4.41 (2.47 to 6.36) | 0.036 |
| KCCQ-TSS Tertile 2 (66.8–89.6) | 2.62 (0.73 to 4.51) | |
| KCCQ-TSS Tertile 3 (≥89.6) | 1.45 (−0.43 to 3.34) | |
| KCCQ-OSS Tertile 1 (<58.9) | 2.42 (0.68 to 4.16) | 0.205 |
| KCCQ-OSS Tertile 2 (59.0–80.7) | 2.64 (0.96 to 4.32) | |
| KCCQ-OSS Tertile 3 (≥80.7) | 0.82 (−0.87 to 2.51) | |
| 8 months | ||
| KCCQ-CSS Tertile 1 (<62.5) | 1.32 (−0.64 to 3.28) | 0.927 |
| KCCQ-CSS Tertile 2 (62.6–85.4) | 1.92 (0.05 to 3.79) | |
| KCCQ-CSS Tertile 3 (≥85.4) | 1.18 (−0.70 to 3.06) | |
| KCCQ-TSS Tertile 1 (<66.7) | 1.71 (−0.42 to 3.83) | 0.613 |
| KCCQ-TSS Tertile 2 (66.8–89.6) | 3.06 (1.02 to 5.11) | |
| KCCQ-TSS Tertile 3 (≥89.6) | 0.94 (−1.11 to 2.98) | |
| KCCQ-OSS Tertile 1 (<58.9) | 1.28 (−0.70 to 3.26) | 0.943 |
| KCCQ-OSS Tertile 2 (59.0–80.7) | 1.88 (−0.01 to 3.77) | |
| KCCQ-OSS Tertile 3 (≥80.7) | 1.33 (−0.57 to 3.23) | |
| 12 months | ||
| KCCQ-CSS Tertile 1 (<62.5) | 1.77 (−0.48 to 4.03) | 0.751 |
| KCCQ-CSS Tertile 2 (62.6–85.4) | 2.24 (0.09 to 4.39) | |
| KCCQ-CSS Tertile 3 (≥85.4) | 1.26 (−0.86 to 3.39) | |
| KCCQ-TSS Tertile 1 (<66.7) | 3.03 (0.63 to 5.43) | 0.260 |
| KCCQ-TSS Tertile 2 (66.8–89.6) | 1.94 (−0.35 to 4.24) | |
| KCCQ-TSS Tertile 3 (≥89.6) | 1.03 (−1.25 to 3.31) | |
| KCCQ-OSS Tertile 1 (<58.9) | 0.98 (−1.26 to 3.23) | 0.733 |
| KCCQ-OSS Tertile 2 (59.0–80.7) | 2.65 (0.51 to 4.79) | |
| KCCQ-OSS Tertile 3 (≥80.7) | 1.50 (−0.62 to 3.62) | |
CI, confidence interval; CSS, clinical summary score; KCCQ, Kansas City Cardiomyopathy; OSS, overall summary score; TSS, total symptom score.
P-value from trend test assuming ordering of the KCCQ tertiles and testing for a linear trend across subgroups.
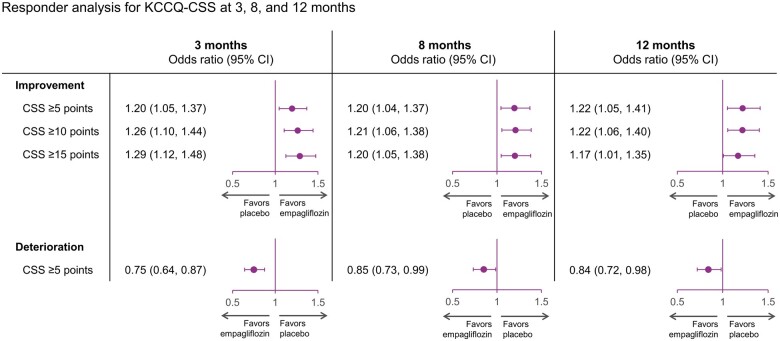
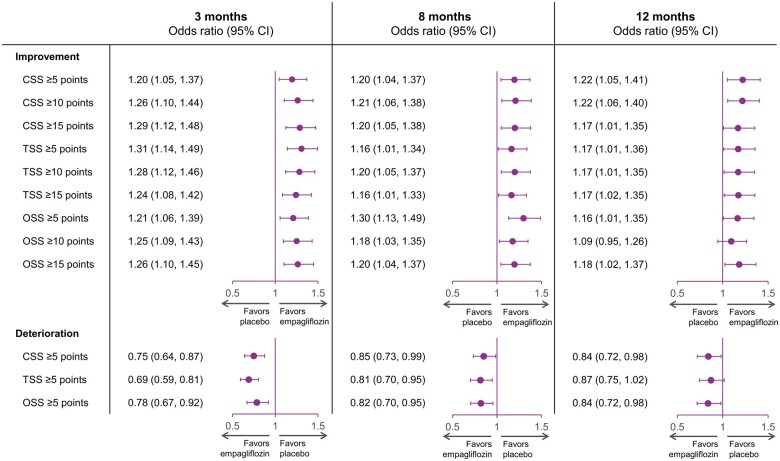
Responder analysis
The results of the responder analyses are shown in Figure 4. At all time points, patients in the empagliflozin group were more likely to show improvement and less likely to experience deterioration in KCCQ-CSS. At 3 months, the ORs for the effect of empagliflozin vs. placebo were 1.20 (95% CI 1.05–1.37) for a ≥5-point improvement, 1.26 (95% CI 1.10–1.44) for a ≥10-point improvement, 1.29 (95% CI 1.12–1.48) for a ≥15-point improvement, and 0.75 (95% CI 0.64–0.87) for a ≥5-point deterioration (all P < 0.05). At 8 months, the ORs for the effect of empagliflozin vs. placebo were 1.20 (95% CI 1.04–1.37) for a ≥5-point improvement, 1.21 (95% CI 1.06–1.38) for a ≥10-point improvement, 1.20 (95% CI 1.05–1.38) for a ≥15-point improvement, and 0.85 (95% CI 0.73–0.99) for a ≥5-point deterioration (all P < 0.05). A similar pattern was observed at these time points for KCCQ-TSS and KCCQ-OSS.
Figure 4.
Responder analyses of clinically meaningful improvement and deterioration in Kansas City Cardiomyopathy Questionnaire-clinical summary score, Kansas City Cardiomyopathy Questionnaire-total symptom score, and Kansas City Cardiomyopathy Questionnaire-overall summary score with empagliflozin vs. placebo over time. Odds ratios for ≥5-, ≥10-, and ≥15-point improvement and ≥5-point deterioration with empagliflozin vs. placebo at 3, 8, and 12 months. CI, confidence interval; CSS, clinical summary score; OSS, overall summary score; TSS, total symptom score.
Discussion
We report several key findings in this secondary analysis of the EMPEROR-Reduced trial. First, empagliflozin reduced the primary outcome of cardiovascular death or heart failure hospitalization across the range of KCCQ-23 scores. Second, empagliflozin significantly improved clinically relevant domains of health status including the KCCQ-CSS, KCCQ-TSS, and KCCQ-OSS scores; these benefits were observed at the first post-randomization assessment and were sustained over the first year of double-blind therapy. Third, using clinically relevant thresholds of a 5-, 10-, or 15-point increase and a 5-point decline, patients treated with empagliflozin were significantly more likely to show improvement and less likely to experience deterioration, when compared with placebo. These findings on patient centred outcomes, when taken together with the benefits of SGLT2 inhibitors to reduce the risk of major heart failure and serious adverse renal events, support a role for SGLT2 inhibitors as a key component of foundational therapy for patients with HFrEF.
Previous analyses have raised the possibility that patients with milder severity of symptoms of heart failure may show a particularly pronounced response to SGLT2 inhibitors.5 , 8 In the large-scale DAPA-HF trial, SGLT2 inhibition reduced the risk of cardiovascular death or worsening heart failure requiring hospitalization or urgent care by 37% in patients with class II symptoms, but by only 10% in patients with class III–IV symptoms.5 The difference in the magnitude of the benefit on the risk of cardiovascular death and hospitalization for heart failure between patients with milder and more severe symptoms was less striking with empagliflozin in the EMPEROR-Reduced trial, with risk reductions of 29% and 17% for class II and class III, respectively.8 In the current analysis, as compared with patients with relatively poor health status, those with better health status at baseline showed a numerically greater benefit on the risk of cardiovascular death and hospitalization for heart failure with empagliflozin. However, in the current trial, neither NYHA class nor KCCQ health status at baseline exerted a statistically significant influence on the magnitude of the response to empagliflozin.8 , 15 Importantly, even in patients with the worst KCCQ health status at baseline, the effect of empagliflozin to reduce the risk of heart failure hospitalizations and slow the decline in glomerular filtration rate remained clinically important.
When compared with placebo, empagliflozin improved health status as assessed by the KCCQ by 1.5–2.0 points, an effect that was statistically significant regardless of the KCCQ domain; the effect was seen at the first double-blind assessment and was sustained for 52 weeks. These results are strikingly similar, both in magnitude and time course, to the effects of dapagliflozin reported in the DAPA-HF trial.7 Although changes in KCCQ scores of at least 5 points are often considered to be clinically meaningful when assessed in individual patients, this threshold is not applicable to the assessment of between-group differences in populations of patients, especially when many patients have reasonably high KCCQ scores at the time of enrolment in the trial.14 It is therefore, noteworthy that, in trials with sacubitril/valsartan and ivabradine, meaningful decreases in the risk of cardiovascular death or hospitalization for heart failure have generally been accompanied by between-group differences of 1.5–2.5 points in favor of active treatment.16 , 17 Nevertheless, if 5- and 10-point thresholds are applied to the participants in the EMPEROR-Reduced trial, patients in the empagliflozin groups were 15–20% more likely to show meaningful improvement and 15–20% less likely to show meaningful deterioration in health status. These ORs in favor of empagliflozin with respect to health status are also similar to those reported with dapagliflozin in the DAPA-HF trial. However, such cross-trial comparisons should be carried out with caution since different trials may focus on different KCCQ domains and may differ with respect to their handling of missing data due to patient dropout or death. Furthermore, it is understood that patients with a reasonably high KCCQ scores at baseline cannot show an improvement in KCCQ score even if they were to experience symptomatic benefits, and different trialists often take different approaches to the analysis of these ceiling effects.
The results of this analysis should be interpreted in light of the fact that they represent secondary findings and that the KCCQ-23 data were missing for some patients at baseline and at follow-up. Moreover, the analysis of KCCQ-23 following randomization did not take into account the occurrence of deaths since there were more deaths in patients on placebo, any analysis that imputed for death would have led to larger estimated treatment effects. Furthermore, as with other trials, our results may not be generalizable to patients who did not fulfil the eligibility criteria for participation in the EMPEROR-Reduced trial.
In conclusion, empagliflozin significantly improved cardiovascular outcomes across the range of baseline KCCQ-23 domains and improved health status in patients with HFrEF. Treatment with empagliflozin was accompanied by a higher likelihood of improvement and a lower likelihood of deterioration in health status. The highly concordant findings on patient-reported health status in the EMPEROR-Reduced and DAPA-HF trials support a role for SGLT2 inhibitors as a part of foundational treatment of HFrEF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors were fully responsible for all content and editorial decisions, were involved at all stages of development, and have approved the final version. Graphical assistance, supported financially by Boehringer Ingelheim, was provided by Paul Lidbury of Elevate Scientific Solutions.
Funding
The EMPEROR-Reduced trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
Declaration
The trial was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of interest: J.B. reports consulting fees from BI, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave Ltd, and Vifor. S.D.A. reports grants and personal fees from Vifor Int. and Abbott Vascular and personal fees from AstraZeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Servier and Vifor Int. G.F. reports lectures and/or Committee Member contributions in trials sponsored by Medtronic, Vifor, Servier, Novartis, Bayer, Amgen, and Boehringer Ingelheim. M.S.K. reports no relevant disclosures. J.P.F. is a consultant for Boehringer Ingelheim. S.J.P. is a consultant for Boehringer Ingelheim. N.G. reports personal fees from AstraZeneca, BMS/Pfizer, Medtronic, Novartis, Pfizer, Servier, Abbott, Boehringer Ingelheim, Amgen, V-wave, Merck, and Sanofi and research grants from AstraZeneca, Medtronic, Novartis, and Servier. J.L.J. reports grant support, consulting income, and participation in clinical endpoint committees/data safety monitoring boards from Janssen, participation in clinical endpoint committees/data safety monitoring boards from Boehringer Ingelheim, grant support from Novartis, Innolife, Applied Therapeutics, and Siemens Diagnostics, and consultancy fees from Novartis, Roche Diagnostics, and Abbott Diagnostics. I.L.P. reports personal fees from Boehringer Ingelheim, during the conduct of the study; personal fees from Boehringer Ingelheim, outside the submitted work. C.S.P.L. reports research grants from Bayer, Boston Scientific, Roche Diagnostic, Medtronic, Vifor Pharma, and AstraZeneca, consulting fees from Merck, Bayer, Boston Scientific, Roche Diagnostic, Vifor Pharma, AstraZeneca, Novartis, Amgen, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, Novo Nordisk, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, Cytokinetics, WebMD Global LLC, Radcliffe Group Ltd, and Corpus and serves as co-founder & non-executive director of eKo.ai. P.P. reports personal fees from Boehringer Ingelheim, AstraZeneca, Servier, BMS, Amgen, Novartis, Merck, Pfizer, and Berlin Chemie and grants and personal fees from Vifor Pharma. N.S. reports personal fees from Amgen, Astrazeneca, Boehringer Ingelheim, Eli-Lilly, MSD, Novo Nordisk, Pfizer, and Sanofi and grant income from Boehringer Ingelheim. S.V. holds a Tier 1 Canada Research Chair in Cardiovascular Surgery and reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli-Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Sun Pharmaceuticals, PhaseBio, and the Toronto Knowledge Translation Working Group. He is a member of the scientific excellence committee of the EMPEROR-Reduced trial and served as a national lead investigator of the DAPA-HF and EMPEROR-Reduced trials. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. M.B., W.J., B.P., C.Z., and O.V. are employees of Boehringer Ingelheim. F.Z. has recently received steering committee or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cardior, CVRx, Janssen, Livanova, Merck, Mundipharma, Novartis, Novo Nordisk, and Vifor Fresenius. M.P. reports personal fees from Boehringer Ingelheim, during the conduct of the study, and personal fees from Abbvie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk, outside the submitted work.
Contributor Information
Javed Butler, Department of Medicine, University of Mississippi School of Medicine, Jackson, MS, USA.
Stefan D Anker, Department of Cardiology (CVK); and Berlin Institute of Health Center for Regenerative Therapies (BCRT); German Centre for Cardiovascular Research (DZHK) partner site Berlin; Charité Universitätsmedizin Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany.
Gerasimos Filippatos, Heart Failure Unit, National and Kapodistrian University of Athens School of Medicine, Athens University Hospital Attikon, 2 Thivon Street, Athens 157 72, Greece.
Muhammad Shahzeb Khan, Department of Medicine, University of Mississippi School of Medicine, Jackson, MS, USA.
João Pedro Ferreira, Department of Cardiothoracic Physiology and Surgery, Cardiovascular R&D Unit, Institut Lorrain du Coeur et des Vaisseaux, 5 Rue du Morvan, 54500 Vandeuvre-lès-Nancy, France.
Stuart J Pocock, Department of Medical Statistics, London School of Hygiene & Tropical Medicine, Keppel Street, London WCIE 7HT, UK.
Nadia Giannetti, Division of Cardiology, McGill University Health Center, 1001 Decarie Blvd.Royal Victoria Hospital, D05.5115 Montreal, Quebec H4A 3J1, Canada.
James L Januzzi, Cardiology Division, Harvard Medical School, Massachusetts General Hospital, 25 Shattuck St, Boston, MA 02115, USA.
Ileana L Piña, Department of Medicine, Wayne State and Central Michigan Universities, 540 E. Canfield Ave, Detroit, MI 48201, USA.
Carolyn S P Lam, National Heart Centre Singapore & Duke-National University of Singapore, 8 College Rd, Singapore 169857, Singapore.
Piotr Ponikowski, Centre for Heart Diseases, Wroclaw Medical University, Borowska 213, 50-556 Wroclaw, Poland.
Naveed Sattar, Institute of Cardiovascular and Medical Sciences, University of Glasgow, BHF Glasgow Cardiovascular Research Centre (GCRC), 126 University Place, Glasgow G12 8TA, UK.
Subodh Verma, Division of Cardiac Surgery, St Michael’s Hospital, University of Toronto, 30 Bond Street, Toronto, ON, M5B 1W8, Canada.
Martina Brueckmann, Boehringer Ingelheim International GmbH, Binger Strasse 173 Ingelheim am Rhein, 55216, Germany; Faculty of Medicine Mannheim, University of Heidelberg, Ludolf-Krehl-Straße 13-17, 68167 Mannheim, Germany.
Waheed Jamal, Boehringer Ingelheim International GmbH, Binger Strasse 173 Ingelheim am Rhein, 55216, Germany.
Ola Vedin, Boehringer Ingelheim AB, Hammarby allé 29, 120 32 Stockholm, Sweden.
Barbara Peil, Boehringer Ingelheim Pharma GmbH & Co. KG, Binger Strasse 173 Ingelheim am Rhein, 55216, Germany.
Cordula Zeller, Boehringer Ingelheim Pharma GmbH & Co. KG, Birkendorfer Str. 65, 88397 Biberach an der Riß, Germany.
Faiez Zannad, Department of Cardiothoracic Physiology and Surgery, Cardiovascular R&D Unit, Institut Lorrain du Coeur et des Vaisseaux, 5 Rue du Morvan, 54500 Vandeuvre-lès-Nancy, France.
Milton Packer, Cardiovascular Science, Baylor Heart and Vascular Institute, Baylor University Medical Center, 621 N. Hall Street, Dallas, TX 75226, USA; Faculty of Medicine, National Heart and Lung Institute, Imperial College, Guy Scadding Building, Cale Street, SW3 6LY London, UK.
Data availability
The sponsor of the EMPEROR-Reduced trial (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient level clinical study data. Researchers are invited to submit inquiries via the following website: https://trials.boehringer-ingelheim.com.
References
- 1. Lewis EF. Assessing the impact of heart failure therapeutics on quality of life and functional capacity. Curr Treat Options Cardiovasc Med 2013;15:425–436. [DOI] [PubMed] [Google Scholar]
- 2. Vaishnava P, Lewis EF. Assessment of quality of life in severe heart failure. Curr Heart Fail Rep 2007;4:170–177. [DOI] [PubMed] [Google Scholar]
- 3.Clinical Outcome Assessments (COA) Qualification Submissions Office of Cardiology Hematology, Endocrinology, and Nephrology (OCEHM) Division of Cardiovascular and Nephrology (DCN). DDT COA #000084: Kansas City Cardiomyopathy Questionnaire (KCCQ). 2020. https://www.fda.gov/drugs/clinical-outcome-assessment-coa-qualification-program/ddt-coa-000084-kansas-city-cardiomyopathy-questionnaire-kccq (14 December 2020).
- 4.US-FDA. Treatment for Heart Failure: Endpoints for Drug Development Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/treatment/heart-failure-endpoints-drug-development-guidance-industry (14 December 2020).
- 5. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 6. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, Drazner MH, Fong MW, Givertz MM, Gordon RA, Jermyn R, Katz SD, Lamba S, Lanfear DE, LaRue SJ, Lindenfeld JAnn, Malone M, Margulies K, Mentz RJ, Mutharasan RK, Pursley M, Umpierrez G, Kosiborod M, Malik AO, Wenger N, Ogunniyi M, Vellanki P, Murphy B, Newman J, Hartupee J, Gupta C, Goldsmith M, Baweja P, Montero M, Gottlieb SS, Costanzo MR, Hoang T, Warnock A, Allen L, Tang W, Chen HH, Cox JM; On behalf of the DEFINE-HF Investigators. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation 2019;140:1463–1476. [DOI] [PubMed] [Google Scholar]
- 7. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJV. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation 2020;141:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F, Packer M, Anker SD, Butler J, Filippatos G, Zannad F, George J, Brueckmann M, Perrone S, Nicholls S, Janssens S, Bocchi E, Giannetti N, Verma S, Jian Z, Spinar J, Seronde M‐F, Böhm M, Merkely B, Chopra V, Senni M, Taddei S, Tsutsui H, Choi D‐J, Chuquiure E, La Rocca HPB, Ponikowski P, Juanatey JRG, Squire I, Butler J, Januzzi J, Pina I, Pocock SJ, Carson P, Doehner W, Miller A, Haas M, Pehrson S, Komajda M, Anand I, Teerlink J, Rabinstein A, Steiner T, Kamel H, Tsivgoulis G, Lewis J, Freston J, Kaplowitz N, Mann J, Petrie M, Bernstein R, Cheung A, Green J, Januzzi J, Kaul S, Ping CLS, Lip G, Marx N, McCullough P, Mehta C, Ponikowski P, Rosenstock J, Sattar N, Scirica B, Tsutsui H, Verma S, Wanner C, Welty FK, Parhofer KG, Clayton T, Pedersen TR, Lees KR, Konstam MA, Greenberg B, Palmer M; EMPEROR-Reduced Trial Committees and Investigators. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail 2019;21:1270–1278. [DOI] [PubMed] [Google Scholar]
- 10. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 11. Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones SG, Spertus JA. Association of serial Kansas City cardiomyopathy questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol 2017;2:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015;8:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 14. Butler J, Khan MS, Mori C, Filippatos GS, Ponikowski P, Comin-Colet J, Roubert B, Spertus JA, Anker SD. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2020;22:999–1005. [DOI] [PubMed] [Google Scholar]
- 15. Packer M, Anker SD, Butler J, Filippatos GS, Ferreira JP, Pocock S, Carson PE, Anand IS, Doehner W, Haass M, Komajda M, Miller AB, Pehrson S, Teerlink JR, Brueckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F; EMPEROR-Reduced Trial Committees and Investigators. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation 2020;doi:10.1161/CIRCULATIONAHA.120.051783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ekman I, Chassany O, Komajda M, Bohm M, Borer JS, Ford I, Tavazzi L, Swedberg K. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J 2011;32:2395–2404. [DOI] [PubMed] [Google Scholar]
- 17. Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD, Swedberg K. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail 2017;10:e003430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sponsor of the EMPEROR-Reduced trial (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient level clinical study data. Researchers are invited to submit inquiries via the following website: https://trials.boehringer-ingelheim.com.