Abstract
Objective
Recommendations for widespread use of face mask, including suggested type, should reflect the current published evidence and concurrently be studied. This review evaluates the preclinical and clinical evidence on use of cloth and surgical face masks in SARS‐CoV‐2 transmission and proposes a trial to gather further evidence.
Methods
PubMed, EMbase, and the Cochrane Library were searched. Studies of SARS‐CoV‐2 and face masks and randomized controlled trials (RCTs) of n ≥ 50 for other respiratory illnesses were included.
Results
Fourteen studies were included in this study. One preclinical and 1 observational cohort clinical study found significant benefit of masks in limiting SARS‐CoV‐2 transmission. Eleven RCTs in a meta‐analysis studying other respiratory illnesses found no significant benefit of masks (±hand hygiene) for influenza‐like‐illness symptoms nor laboratory confirmed viruses. One RCT found a significant benefit of surgical masks compared with cloth masks.
Conclusion
There is limited available preclinical and clinical evidence for face mask benefit in SARS‐CoV‐2. RCT evidence for other respiratory viral illnesses shows no significant benefit of masks in limiting transmission but is of poor quality and not SARS‐CoV‐2 specific. There is an urgent need for evidence from randomized controlled trials to investigate the efficacy of surgical and cloth masks on transmission of SARS‐CoV‐2 and user reported outcomes such as comfort and compliance.
Keywords: COVID‐19, face masks, SARS‐CoV‐2, systematic review, trial proposal
1. INTRODUCTION
There has been significant debate amongst leading scientists about the benefit of face masks in SARS‐CoV‐2 and this question remains very important clinically and to the public. Globally, many countries have mandated wearing of face masks in certain public locations on the precautionary principle that face masks are beneficial and carry a low risk of harm. 1
Face masks include cloth masks, surgical fluid‐resistant masks and FFP3 respirators. The WHO states that although there is limited evidence, they recommend cloth masks for the public to control SARS‐CoV‐2 and to preserve surgical and FFP3 respirators for medical settings. 2 Any recommendation for widespread use of face masks, including type, should reflect the current published evidence whilst identifying gaps where evidence is lacking and plans research to fill them. This paper systematically reviews the published preclinical and clinical evidence for the use of face masks in SARS‐CoV‐2 and proposes a trial to holistically evaluate the evidence for masks in SARS‐CoV‐2.
The underlying logic behind use of face masks is that they are a physical barrier retaining the droplets, aerosols and particles, by which SARS‐CoV‐2 spreads. Droplets spread continuously in the flow of air a person creates when breathing and talking that can travel up to 8 m. 3 A recent study by the University of Edinburgh found all face mask materials, except those with valves, reduced the front flow of air from a modeled human by more than 90%. 4 A study published in Nature Medicine showed this barrier effect of surgical masks also significantly reduced detection of influenza, coronavirus and rhinovirus virus RNA in respiratory droplets and coronavirus RNA in aerosols of exhaled breaths of participants with laboratory confirmed illnesses. 5
SARS‐CoV‐2 has presymptomatic spread with carriers having maximal viral shedding prior to being ill, 6 a prolonged incubation period with a significant proportion of asymptomatic carriers capable of shedding the virus. 7 These transmission dynamics support precautionary universal masking of the public to prevent transmission.
Cloth masks are currently promoted by many governments to preserve surgical masks, but evidence of their equivalence to surgical masks is conflicting. One study comparing homemade cloth masks with additional kitchen roll versus N95 masks and surgical masks reported comparable efficacy of 95.15% versus 99.98% and 97.14%, respectively, in blocking avian influenza aerosols. 8 However, another study comparing the number of microorganisms isolated from a cough, found cotton cloth masks were 1/3rd as effective as surgical masks. Cloth masks still significantly reduced the number of microorganisms compared to the control of no mask. 9 Cloth masks are not fluid resistant so liable to get damp with prolonged use, which may reduce their barrier function. Their use in SARS‐CoV‐2 is important to study to inform public and manufacturing guidance given the burgeoning face mask market.
Indirect evidence that face masks may be an effective source control tool for SARS‐CoV‐2 comes from observations from surveys of household contacts of index cases and case number trends in different countries. Li et al 10 found in 105 cases and 392 household contacts, the secondary attack rate in households where the index patients quarantined upon symptom development (n = 14 used masks, dined separately, and distanced within the home). A study in Taiwan that found universal masking and hand‐hygiene during the COVID‐19 pandemic resulted in a 50% decline of infectious respiratory diseases compared to previous years. 11 Similarly, countries that practiced tight infectious control measures including universal masking and social distancing including China, Vietnam and South Korea and had significantly fewer cases and mortality, when compared to countries with more lax health precaution measures. 12 These findings give rationale to the policy of universal face masks for the general public but evidence from direct study is important evaluate efficacy of such policies to inform future strategy in the ongoing SARS‐CoV‐2 pandemic.
2. METHODS
2.1. Search
A systematic review of the literature was performed using PubMed, Cochrane CENTRAL, and EMbase with the last search being performed on 15 August 2020. The search terms for each search can be found in supporting information. Except for English language, no further restrictions were added to the search. References of the articles acquired were also searched by hand. Results were imported into the reference manager Mendeley and then screened initially by abstract and title and then full text screening.
2.2. Inclusion criteria
To enter the analysis, studies were required to fulfill one of the following criterion: any preclinical directly studying SARS‐CoV‐2 transmission and mask use, any published in practice studies (RCTs or observational studies) of mask use by humans in SARS‐CoV‐2, any RCT with more than 50 participants of face mask use compared with no mask or any RCT of cloth mask use compared with any control in any respiratory viral illness.
2.3. Exclusion criteria
Studies that failed to fulfill the inclusion criteria or studies where the outcomes of interest were not reported or if it was impossible to calculate these from the published reports were excluded. Registered trials with no results were not included in the analysis but mentioned in the discussion.
2.4. Data extraction
Each study was evaluated for inclusion or exclusion from the review and the following data were extracted: first author, year of publication, study design, number of participants, location, duration, disease/outcome studied, intervention and control, methods of study, compliance to interventions, and other significant details. One reviewer (AN) extracted data for all selected studies using RevMan software 5.0. 13 The accuracy of the extracted data was verified by the second reviewer (MD).
2.5. Risk of bias and quality of evidence
For assessing the risk of bias (ROB), the OHAT risk of bias tool 14 was used for preclinical studies, ROBINS‐1 tool for nonrandomized studies 15 and Cochrane's risk of bias tool for RCTs. 16 Clinical heterogeneity was assessed using the I 2 statistic and interpreted as per the Cochrane Handbook for Systematic Reviews of Interventions. The quality of the body of evidence was assessed as per the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) framework. Two reviewers (AN and MD) were responsible for the assessment.
2.6. Statistical analysis
RevMan 5.2 software was used for the quantitative analyses. Dichotomous outcomes were extracted as numerators and denominators and summarized using risk ratios (RRs) and 95% confidence intervals (CIs). RCTs were grouped by the outcome they assessed (laboratory confirmed respiratory virus and influenza like illness) and based on whether the intervention was a face mask alone or with hand hygiene. The random‐effects model was used to calculate the pooled outcome due to the studies sampling dissimilar populations and heterogeneity in the studies.
3. RESULTS
A total of 1499 studies were found in the search (Figure 1); after title, abstract and full text screening 14 studies were included in the review and 11 in the meta‐analysis. Of the studies found, there was 1 preclinical and 1 clinical study directly studying mask use in transmission of SARS‐CoV‐2, 11 randomized controlled trials studying transmission of other respiratory illnesses, and 1 randomized controlled trial comparing surgical and cloth masks in the prevention of respiratory illness.
FIGURE 1.
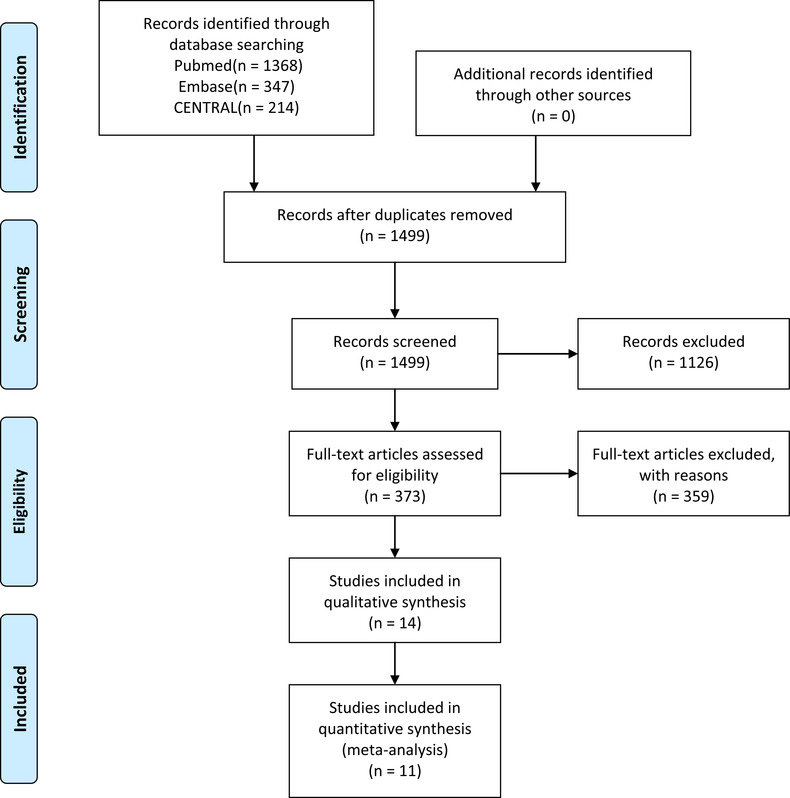
PRISMA flow diagram for study search and selection
3.1. Preclinical studies for masks in SARS‐CoV‐2
The preclinical study used hamsters infected with SARS‐CoV2 placed in cages adjacent to healthy hamsters to investigate noncontact transmission of SARS‐CoV‐2. 17 A fan was used to transmit the virus between the cages. In the control (no barrier between the cages), hamsters were infected at a 66.7% rate after 7 days (10/15) compared to 16.7 (2/12) when a barrier of surgical face masks was put on both cages. The rate rose to 25% (6/24) when masks were only placed on the cage of healthy hamsters. There was some concern over confounding bias that the authors could not be certain of the exact source of transmission and could not rule out transmission amongst hamsters in the same cage. They were unable to keep experimental conditions identical across study groups; for example, the speed of the unidirectional airflow could not be unified when the surgical mask partitions were installed—though this may simulate airflow when surgical masks are worn in practice. The risk of bias is shown in Figure 2.
FIGURE 2.

Risk of bias for preclinical studies included in the review
We found one other preclinical study in humans directly studying medical and cloth mask use in SARS‐CoV‐2; 18 however this study was retracted 19 due to errors in analysis and therefore not included in our analysis.
3.2. In practice studies for masks in SARS‐CoV‐2
The clinical study was a nonrandomized retrospective observational cohort study. 20 The authors retrospectively analyzed 335 people from 124 families with proven SARS‐CoV‐2 to evaluate masking practices in the households to assess if they were predictors of secondary transmission. They determined that if one or more members of the household (either the index case or their contacts) wore a mask before development of symptoms, there was a 79% reduction in transmission (OR = 0.21, 95% CI: 0.06‐0.79). They counted all types of masks regardless of whether it was a N95 mask, disposable surgical mask, or cloth mask. Due to the retrospective, nonrandomize and observational nature of the study there were many areas for potential bias to arise summarized in Figure 3.
FIGURE 3.

Risk of bias for nonrandomized studies included in the review
3.3. Studies for mask use in preventing any other respiratory illness
For trials for surgical mask use in preventing any other respiratory illness; after title and abstract and full text screening. Eleven RCTs 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 were selected (see Table 1 for study characteristics and Figures 4 and 5 for summary of risk of bias).
TABLE 1.
Characteristics of randomized controlled trials included in the meta‐analysis
| Study | Design | Participants | Location | Duration | Disease/outcome studied | Interventions | Control | Compliance | Intervention details |
|---|---|---|---|---|---|---|---|---|---|
| Aiello (2010) | Cluster randomized open trial, randomizing all participants | 1437 | USA University Residence Halls | 6 weeks of term time (excluding spring break) | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) |
(1) Face mask (2) Face mask and hand hygiene |
Educational video and written material on infection control | Average hours of mask wearing in (1) 3.92 h vs (2) 2.99 h— asked to wear “as much as possible” in 24 h. FM use in control not assessed |
(1) 7 standard medical procedure masks with ear loops (TECNOL procedure masks; Kimberly Clark) (2) Alcohol hand sanitizer (62% ethyl alcohol in a gel base, portable squeeze bottle) Both came with education via video, website and written material |
| Aiello (2012) | Cluster randomized open trial, randomizing all participants | 1178 | USA University Residence Halls | 6 weeks of term time (excluding spring break) | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) |
(1) Face mask (2) Face mask and hand hygiene |
Educational video and written material on infection control | Staff observed very few participants wearing a mask properly for each hour of observation. FM use in control not assessed |
(1) 7 standard medical procedure masks with ear loops (TECNOL procedure masks; Kimberly Clark) with resealable plastic bags for mask storage when not in use (2) Alcohol hand sanitizer (62% ethyl alcohol in a gel base, portable squeeze bottle) Both came with education via video, website and written material |
| Barasheed (2014) | Cluster randomized open trial, randomizing contacts of index cases | 164 | Hajj mass gathering | 1‐week Hajj, 5 days follow up | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) | (1) Face mask | No intervention | 56/75 (76%) in the mask group and 11/89 (12%) in the control group, (P < .001). 10/75 in mask group wore masks whilst asleep also | Plain surgical face masks (3 M Standard Tie‐On Surgical Mask) |
| Canini (2010) | Cluster randomized open trial, randomizing contacts of index cases | 306 contacts in 105 households | Households in France | 5 days mask wearing, 21‐day follow up | Self‐reported Influenza like Illness (ILI) | (1) Face mask | No intervention | 34/51 (66%) wore masks > 80% of the time (self‐reported). Mask wearing in the control was not assessed | Surgical masks with ear loops, 3 ply, antifog (AEROKYN, LCH medical products, Paris, France). Children 5‐10 received face mask (Kimberly Clark). Plastic bags for disposal |
| Cowling (2009) | Cluster randomized open trial, randomizing contacts of index cases | 794 contacts in 407 households | Households in Hong Kong, China | 7 days follow up | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) |
(1) Face mask and hand hygiene (2) Hand hygiene |
Education about the importance of health and lifestyle with infection control for household contacts advice | Self‐reported adherence 49% index patients and 26% contact patients reported adherence to instructions in FM+HH group. 31% and 15% of index patients in HH and control wore masks, 5% and 7% of contacts, respectively | 50 surgical masks (Tecnol—The Lite One Kimberly Clark) ± 75 pediatric masks for 3‐7 and 221 mL Ivory liquid Soacp (Protector & Gamble) and 100 mL WHO recommended Vickmans alcohol gel 80% ethanol |
| Cowling (2008) | Cluster randomized open trial, randomizing contacts of index cases | 198 contacts in 121 households | Households in Hong Kong, China | 9 days follow up | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) |
(1) Face masks (2) Hand hygiene |
Education on health and lifestyle with infection control for household contacts advice | Self‐reported adherence 45% of index subject (21% of contacts) as often or always wearing the mask. In the control 30% (1%) and 28% (4%) in HH | 50 surgical masks (Tecnol—The Lite One Kimberly Clark) and 75 pediatric masks for 3‐7) 221 mL Ivory liquid Soacp (Protector & Gamble) and 100 mL WHO recommended Vickmans alcohol gel 80% ethanol |
| Larson (2010) | Cluster randomized open trial, randomizing contacts of index cases | 1842 contacts in 509 households | Households in Upper Manhattan | 19 months | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) |
(1) Hand hygiene (2) Face mask and hand hygiene |
Educational materials regarding the prevention and treatment of URIs and influenza | Compliance with the mask was poor—mean of only two masks/day/ILI episode. No data of FM use in control group |
(1) Purell, Johnson & Johnson hand sanitizer (2) As above + Procedure Face Masks for adults and children, KimberlyClark, Roswell, Georgia |
| MacIntyre (2009) | Cluster randomized open trial, randomizing contacts of index cases | 286 contacts in 145 households | Households in Sydney, Australia | 2 winter seasons of 2006 and 2007. Follow up 2 weeks after participant developed symptoms. Intervention for 5 days | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) |
(1) Face masks (2) P2 masks |
Health guidelines, pamphlets about infection control | Day 1 compliance for surgical FM 35/94 (38%) to day 5 29/94 (31%). Control group FM use not assessed |
(1) 3 M surgical mask, cat. No. 1820 USA for adults (2) P2 masks (3 M flat‐fold P2 mask cat no. 9320 UK) + pamphlets for both |
| MacIntyre (2016) | Cluster randomized open trial, randomizing contacts of index cases | 597 contacts in 245 households | Households in Beijing, China | 7 days follow up | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) | (1) Face mask | No intervention | Average mask uses 4.4 hours in mask group, 1.4 in control out of all contact time (10.4 and 11.1) hours respectively | (1) 21 medical masks (3 M 1817 surgical mask) |
| Simmerman (2011) | Cluster randomized open trial, randomizing contacts of index cases | 1147 members in 442 households | Household in Bangkok, Thailand | 21 days follow up | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) |
(1) Hand hygiene (2) Face mask and hand hygiene |
Nutritional and general health advice | Patients wore mask for median of 153 minutes/day. FM use in the control group not assessed. |
(1) 50 standard paper surgical face masks and 20 pediatric masks (Medcon company Thailand) (2) Liquid hand soap Teepol brand |
| Suess (2012) | Cluster randomized open trial, randomizing contacts of index cases | 218 contacts in 84 households | Households in Berlin, Germany | 8 days follow up over 2 flu seasons in total | Self‐reported Influenza like Illness (ILI) and laboratory polymerase chain reaction (PCR) |
(1) Face mask and hand hygiene (2) Face mask |
Written information on infection control | Daily adherence 50% in nearly all groups from the 3rd day onwards. Participants who wore masks in the control group were excluded from analysis | Surgical face masks (Child's Face Mask Kimberly‐Clark) and Adults (Aerokyn Masques) |
FIGURE 4.
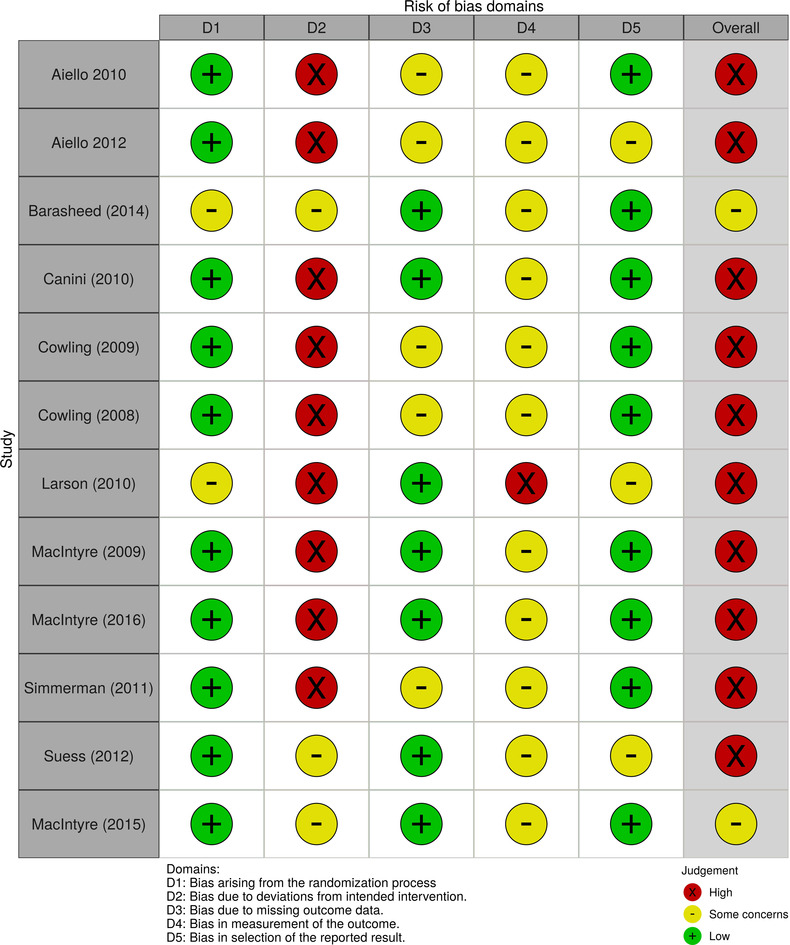
Risk of bias for randomized control studies included in review
FIGURE 5.
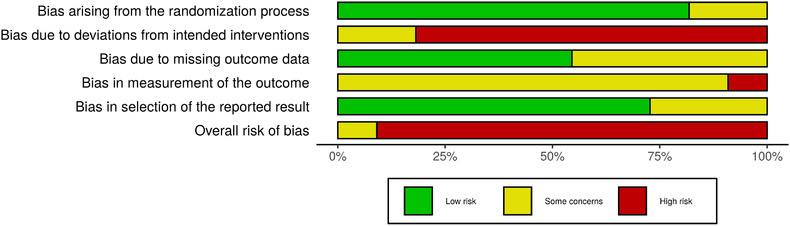
Overall risk of bias for studies included in the meta‐analysis
3.4. Laboratory confirmed respiratory viral illness
When combined, the 10 RCTs that looked at face mask use with or without hand hygiene (FM ± HH) had moderate heterogeneity that was significant (I 2 = 54%, P = .02) (Figure 6). In the random‐effects model, no significant difference was demonstrated between mask and no mask groups for the outcome of laboratory confirmed respiratory viral illness (RR = 0.99, 95% CI: 0.98‐1.01).
FIGURE 6.
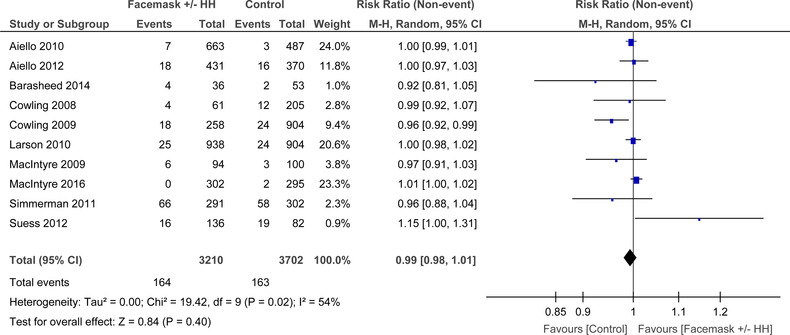
Forest plot for RCTs comparing face masks ± hand hygiene to no masks for laboratory confirmed virus
For face masks alone (Figure 7) there was moderate heterogeneity that was not significant (I 2 = 53%, P = .05), among the seven RCTs. In the random‐effects model, there was no difference demonstrated between mask and no mask groups for the outcome of laboratory confirmed respiratory viral illness (RR = 1.00, 95% CI: 0.98‐1.02).
FIGURE 7.
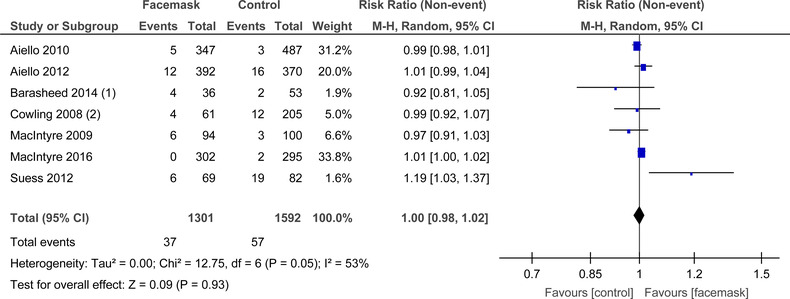
Forest plot for RCTs comparing face masks alone to no masks for laboratory confirmed virus
For face masks and hand hygiene (FM+HH) as the intervention (Figure 8) there was moderate heterogeneity that was not significant (I 2 = 40%, P = .14) among the six RCTs. In the fixed‐effects model demonstrating no significant benefit of FM+HH in lowering laboratory confirmed respiratory virus symptoms (RR = 1.01, 95% CI: 0.99‐1.02).
FIGURE 8.

Forest plot for RCTs comparing face masks + hand hygiene to no masks for laboratory confirmed virus
3.5. Influenza like illness symptoms
When combined, the 11 RCTs that looked at FM ± HH there was significant heterogeneity (I 2 = 84%, P < .001) (Figure 9). In the random‐effects model there was no difference in mask use and no mask groups for the outcome of influenza like illness (ILI) symptoms (RR = 0.89, 95% CI: 0.98‐1.07).
FIGURE 9.
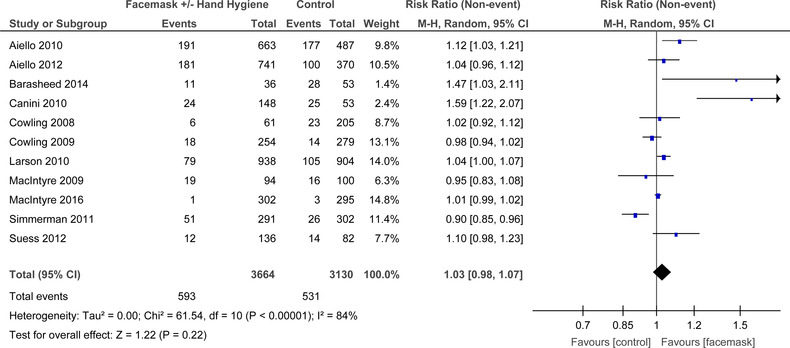
Forest plot for RCTs comparing face masks ± hand hygiene to no masks for influenza‐like‐illness symptoms
For FM alone (Figure 10) there was substantial heterogeneity that was significant (I 2 = 72%, P < .0008), amongst the eight RCTs. In the random‐effects model, there was no significant difference demonstrated between mask and no mask groups for the outcome of laboratory confirmed respiratory viral illness (RR = 1.03, 95% CI: 0.97‐1.09).
FIGURE 10.
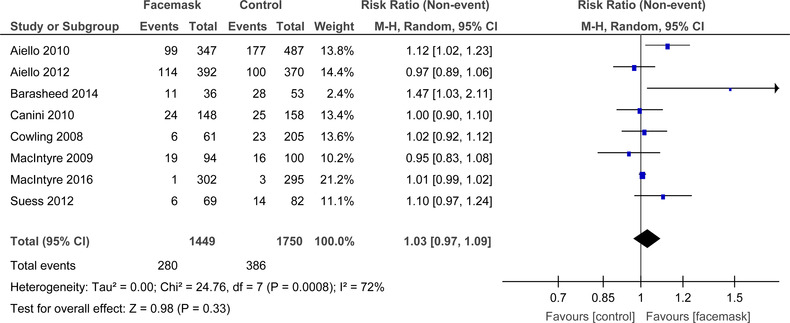
Forest plot for RCTs comparing face masks alone to no masks for influenza‐like‐illness symptoms
For FM+HH (Figure 11), there was substantial heterogeneity that was significant (I 2 = 81%, P < .0001) amongst the six RCTs. The random effects model demonstrated no significant benefit of masks plus hand hygiene in lowering influenza like symptoms; however, the studies have significant clinical heterogeneity (RR = 1.02, 95% CI: 0.96‐1.08).
FIGURE 11.
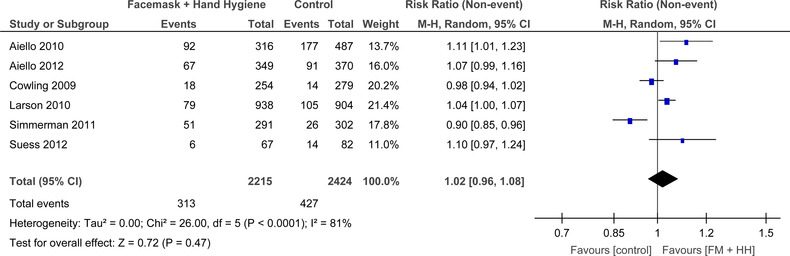
Forest plot for RCTs comparing face masks + hand hygiene to no masks for influenza‐like‐illness symptoms
3.6. Quality of evidence
The quality of evidence was moderate or low quality primarily due to risk of bias, small effect magnitudes, substantive inconsistency of the results and differences in the population groups and study designs included in the various studies. Therefore, confidence in the effect estimate is limited and the true effect may be substantially different from the estimate of the effect. A funnel plot was done for all studies using the influenza like illness outcome for FM ± HH versus control, which shows that publication bias cannot be ruled out.
3.7. Cloth mask
One cluster RCT 32 was found comparing the efficacy of cloth face mask and medical face masks in protecting the wearer. The study, conducted over 4 weeks in 14 hospitals in Hanoi, Vietnam with 1607 healthcare workers, compared locally manufactured medical with two layer cotton cloth masks made of cotton and a control arm of usual practice (245 wore cloth or surgical masks, 3 used N95s, 2 used no mask). They found significant benefit of surgical masks (1/580 and 19/580) compared to cloth masks (13/569 and 31/569) in reducing ILI (RR = 6.64, 95% CI: 1.45‐28.65) and laboratory confirmed virus (RR = 1.72, 95% CI: 1.01‐2.94), respectively.
4. DISCUSSION
4.1. Studies for mask use in SARS‐CoV‐2
The published preclinical body of evidence that directly investigates SARS‐CoV‐2 and masks is limited. This is likely due to the difficult of directly studying SARS‐CoV‐2 and masks in an experimental set up and push for clinical data. Overall, the preclinical study was of high quality in a verified animal model for SARS‐CoV‐2 and suggests benefit of surgical masks in limiting the transmission of SARS‐CoV‐2. No such study with cloth masks has been performed to date but would be useful to perform.
The only clinical study showed there was significantly less transmission of SARS‐CoV‐2 between index cases and household members when at least one participant wore a mask but only before development of symptoms not upon. The study had complete follow up of participants therefore secondary attack rate is well calculated, but it is limited due to its study design resulting in high risk of bias and therefore limits the conclusions we can draw from it. It is difficult to extract the exact effect of masks on transmission due to the observational and noninterventional nature of the study. This study supports the precautionary use and concomitant study of mask use in humans to prevent transmission of SARS‐CoV‐2
There is currently no published evidence from randomized trials studying face masks to prevent SARS‐CoV‐2 transmission. This finding is important as it shows we have no in practice evidence and identifies a gap in the research.
There are only two trials on the centralized WHO COVID‐19 trials register investigating the use of face masks in the community to prevent SARS‐CoV‐2 transmission. A Danish study (NCT04337541) 33 is investigating reduction in COVID‐19 infection using medical grade face masks outside the healthcare system. It will compare medical grade face mask use to the control of “government advice,” where it is currently not mandatory. As this study is not a cluster randomized control trial, it will not see the effects of being surrounded by other mask wearers in the protection from COVID‐19 but can investigate protection for the wearer only. The Bandim Health Project is setting up a cluster‐RCT (NCT04471766) 34 in Guinea Bissau studying locally made the effect of cloth face masks versus no masks on incidence of COVID‐19 in an urban population. It is not clear how they will cluster patients yet and this study is not currently recruiting.
4.2. Studies for surgical mask use in preventing any other respiratory illness
A total of 11 cluster randomized control trials (c‐RCTs) studying mask use in preventing transmission of respiratory illnesses 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 were identified and synthesized in a meta‐analysis. The results of the meta‐analysis show no statistically significant benefit of surgical‐mask use when used with or without hand hygiene for influenza like illness symptom reporting nor laboratory confirmed viral illnesses.
The study quality is low with confounding factors such as adherence, affecting the overall conclusion. Although adherence to mask use makes the results difficult to interpret, it may be that this is the reality of how effective this intervention would be in real world application. However, results from observational studies in the time of SARS, suggest adherence was better than the influenza trials as the perceived threat is greater. 35 , 36 Behavioral studies support the idea that individuals were more likely to wear face masks when the perceived susceptibility and severity of being afflicted with life‐threatening diseases was high. 37 None of the studies look at the unintended harms of the intervention, for example, discomfort, reactive dermatitis, distress, breathing difficulties, etc, which are important as they may affect adherence to the intervention.
All but two of these studies 21 , 22 identified index cases and studied secondary attack rates, which does not account for spread of the respiratory virus before randomization. The other two studies looked at all respiratory viral rates in a student cohort over several months. The studies that masked index cases 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 can inform how masking both the wearer and the contact can limit transmission. It is difficult to elucidate whether the effect is due to infection prevention in someone protecting themselves from others, or others from themselves. None of the studies focused on SARS‐CoV2 or focus on beta‐coronaviruses so the generalizability in the current pandemic is limited.
4.3. Studies for cloth mask use in preventing any other respiratory illness
One c‐RCT 32 found rates of all infection outcomes (ILI and laboratory confirmed) were higher in the cloth mask arm compared with the surgical mask arm. The authors could not determine whether this is because of reduced benefit of the cloth masks in comparison to surgical masks or a detrimental effect of cloth masks because they did not have enough non mask wearers in the control group. The authors hypothesize that the poor performance of cloth masks could be due to their inferior filtration potential and the act of doffing, washing, and reusing the mask. The question arises whether we should be wary of the message that cloth masks offer equal protection in transmission as surgical masks without evidence to support its use. The current recommendation is also based resource allocation and prioritizing high risk such as healthcare settings. With more evidence to support the benefits of surgical mask, efforts to increase the supply of surgical masks and education on its proper use may be more impactful.
Compliance was similar in both arms of the study (56.8% with cloth masks and 56.6% with surgical masks). Although in a healthcare setting in nonpandemic times, this suggests adherence to either mask will be similar. However, there is a trend of media outlets purporting the “comfiest” masks suggesting comfort is important for the general public. 38 , 39 An overview of 84 articles 40 found surgical masks negatively impacted thermoregulation in humans thus making them hard to wear constantly. This highlights the need to study the side effects and user reported outcomes of each mask type and whether they affect compliance. A compromise in efficacy for gain in user‐comfort and compliance may be beneficial in terms of public health.
5. TRIAL PROPOSAL
The existing evidence is poor and highlights the need for further study. We propose a randomized controlled trial where patients are consented, randomized by cluster (eg, by workplace). To account for varying government guidance across countries on face masks mandates; the arms of randomization should be a control of normal behavior according to the authority's recommendations mask and experimental arms of normal behavior plus a face mask (each arm with a different type of face mask). An alternative method of randomizing would be to cluster household contacts of a confirmed index case of SARS‐CoV‐2. Once a patient had confirmed SARS‐CoV‐2, their household members will receive either surgical masks, cloth face masks, or education on other infection control methods. All households will be asked to follow current recommended advice of isolating the index case and minimizing contact.
All participants will be asked to self‐report their symptoms with interval testing of SARS‐CoV‐2 RT‐PCR by nasopharyngeal swab to measure the secondary attack rates. Participants should be tested for antibodies before the start date and after study completion. The participants should also be asked to report on user reported outcomes such as comfort, effects on quality of life and adherence to the masks. If both masks are found to be equivalent in safety and efficacy, then the findings on which mask type is more acceptable to wear to the participant and if this affects compliance will be important outcomes to assess.
In this way, a definitive answer with a high powered RCT can answer whether surgical mask or use of cloth face covering can limit SARS‐CoV‐2 transmission in community applications. As the world comes out of lockdown, now is the time for a randomized trial to establish the evidence of cloth and surgical masks in the prevention of transmission in SARS‐CoV‐2.
6. CONCLUSION
The available preclinical findings limited clinical and indirect evidence suggests biological plausibility that face masks may reduce the spread of SARS‐CoV‐2. The available clinical trial evidence shows no significant difference in limiting transmission respiratory viral illnesses, but the evidence is of poor quality. All current evidence focuses on protection for the wearer not on controlling spread. There is an urgent need for randomized controlled trials to investigate the impact of surgical and cloth masks on transmission of SARS‐CoV‐2 and user reported outcomes such as comfort and compliance.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary Material
Nanda A, Hung I, Kwong A, et al. Efficacy of surgical masks or cloth masks in the prevention of viral transmission: systematic review, meta‐analysis and proposal for future trial. J Evid Based Med. 2021;14:97–111. 10.1111/jebm.12424
REFERENCES
- 1. Masks4All . What countries require masks in public or recommend masks? 2020. Available from: https://masks4all.co/what-countries-require-masks-in-public/. Accessed August 16, 2020.
- 2. WHO . Coronavirus disease (COVID‐19) advice for the public: when and how to use masks. 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks. Accessed August 16, 2020.
- 3. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID‐19. J Am Med Assoc. 2020;323(18):1837‐1838. [DOI] [PubMed] [Google Scholar]
- 4. Viola IM, Peterson B, Pisetta G, et al. Face coverings, aerosol dispersion and mitigation of virus transmission. physics.med‐ph arXiv; 2020. Available from: https://arxiv.org/abs/2005.10720. Accessed August 17, 2020. [DOI] [PMC free article] [PubMed]
- 5. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676‐680. http://www.nature.com/nm/index.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS‐CoV‐2‐Singapore. Morb Mortal Wkly Rep. 2020;69(14):411‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Javid B, Weekes MP, Matheson NJ. Covid‐19: should the public wear face masks? BMJ. 2020;369:m1442. http://www.bmj.com/. [DOI] [PubMed] [Google Scholar]
- 8. Ma Q‐X, Shan H, Zhang H‐L, Li G‐M, Yang R‐M, Chen J‐M. Potential utilities of mask‐wearing and instant hand hygiene for fighting SARS‐CoV‐2. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies A, Thompson KA, Giri K, Kafatos G, Walker J, Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic?. Disaster Med Public Health Prep. 2013;7(4):413‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li W, Zhang B, Lu J, et al. The characteristics of hous6ehold transmission of COVID‐19. Clin Infect Dis. 2020;71(8):6‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsieh C‐C, Lin C‐H, Wang WYC, Pauleen DJ, Chen JV. The outcome and implications of public precautionary measures in Taiwan‐declining respiratory disease cases in the COVID‐19 pandemic. Int J Environ Res Public Health. 2020;17(13):1‐10. http://www.ncbi.nlm.nih.gov/pubmed/32640752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng V C‐C, Wong S‐C, VW‐M C. The role of community‐wide wearing of face mask for control of coronavirus disease 2019 (COVID‐19) epidemic due to SARS‐CoV‐2. J Infect. 2020;81(1):107‐114. http://www.elsevier.com/inca/publications/store/6/2/3/0/5/4/index.htt [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Nordic Cochrane Centre TCC. Review Manager (RevMan) [Computer program]. Version 5.3, The Cochrane Collaboration, 2020.
- 14. National Toxicology Program , US Department of Health and Human Services. OHAT Risk of Bias Tool; 2015. Available from: https://ntp.niehs.nih.gov/go/riskbias. Accessed August 20, 2020.
- 15. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016:355:i 4919 https://www.bmj.com/content/355/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 17. Chan JF‐W, Yuan S, Zhang AJ, et al. Surgical mask partition reduces the risk of non‐contact transmission in a golden Syrian hamster model for Coronavirus Disease 2019 (COVID‐19). Clin Infect Dis Off Publ Infect Dis Soc Am. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bae S, Kim M‐C, Kim JY, et al. Effectiveness of surgical and cotton masks in blocking SARS–CoV‐2: a controlled comparison in 4 patients. Ann Intern Med. 2020;April:4‐5.Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emexb&NEWS=N&AN=631714504 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Bae S, Kim M‐C, Kim JY, et al. Notice of retraction: effectiveness of surgical and cotton masks in blocking SARS‐CoV‐2. Ann Intern Med. 2020;172(1):.79 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS‐CoV‐2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Heal. 2020;5(5):2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aiello AE, Murray GF, Perez V, et al. Mask use, hand hygiene, and seasonal influenza‐like illness among young adults: a randomized intervention trial. J Infect Dis. 2010;201(4):491‐498. [DOI] [PubMed] [Google Scholar]
- 22. Aiello AE, Perez V, Coulborn RM, Davis BM, Uddin M, Monto AS. Facemasks, hand hygiene, and influenza among young adults: a randomized intervention trial. PLoS One. 2012;7(1):e29744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suess T, Remschmidt C, Schink SB, et al. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009–2011. BMC Infect Dis. 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barasheed O, Almasri N, Badahdah A‐M, et al. Pilot randomised controlled trial to test effectiveness of facemasks in preventing influenza‐like illness transmission among Australian hajj pilgrims in 2011. Infect Disord—Drug Targets. 2014;14(2):110‐116.Available from: http://www.benthamdirect.org/pages/all_b_bypublication.php [DOI] [PubMed] [Google Scholar]
- 25. Canini L, Andréoletti L, Ferrari P, et al. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PLoS One. 2010;5(11):e13998. Available from: http://www.plosone.org/article/fetchObjectAttachment.action?uri=info%3Adoi%2F10.1371%2Fjournal.pone.0013998&representation=PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cowling BJ, Ip DKM, Fang VJ, et al. Modes of transmission of influenza B virus in households. PLoS One. 2014;9(9):e108850. Available from: http://www.plosone.org/article/fetchObject.action?uri=info%3Adoi%2F10.1371%2Fjournal.pone.0108850&representation=PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cowling BJ, Chan K‐H, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151(7),437‐446. [DOI] [PubMed] [Google Scholar]
- 28. Larson EL, Ferng Y, Wong‐McLoughlin J, Wang S, Haber M, Morse SS. Impact of non‐pharmaceutical interventions on URIs and influenza in crowded, urban households. Public Health Rep. 2010;125(2):178‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacIntyre CR, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. 2009;15(2):233‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. MacIntyre CR, Zhang Y, Chughtai AA, et al. Cluster randomised controlled trial to examine medical mask use as source control for people with respiratory illness. BMJ Open. 2016;6(12):e012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simmerman JM, Suntarattiwong P, Levy J, et al. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok, Thailand. Influenza Other Respi Viruses. 2011.;5(4):256‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MacIntyre CR, Seale H, Dung TC, et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5(4):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bundgaard H. Reduction in COVID‐19 infection using surgical facial masks outside the healthcare system. clinicaltrials.gov. 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04337541. Accessed August 22, 2020.
- 34. Project BH. Locally produced cloth face mask and COVID‐19 like illness prevention; 2020. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04471766. Accessed August 22, 2020.
- 35. Jefferson T, Del Mar C, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Datab Syst Rev. 2010;7:CD006207 [DOI] [PubMed] [Google Scholar]
- 36. Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta‐analysis. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2017;65(11):1934‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sim SW, Moey KSP, Tan NC. The use of facemasks to prevent respiratory infection: a literature review in the context of the Health Belief Model. Singapore Med J. 2014;55(3):160‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Besser R. 41 breathable face masks, just in time for summer. Vogue; 2020. Available from: https://www.vogue.com/slideshow/breathable-masks. Accessed August 22, 2020.
- 39. Varghese D. The best breathable face masks will help you stay safe in the heat. GQ; 2020. Available from https://www.gq.com/story/the-best-breathable-face-masks. Accessed August 22, 2020.
- 40. Roberge RJ, Kim, J‐H , Coca A. Protective facemask impact on human thermoregulation: an overview. Ann Occup Hyg. 2012;56(1):102‐112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


