Abstract
This trial compared the rate and time of viral clearance in subjects receiving a combination of nitazoxanide, ribavirin, and ivermectin plus Zinc versus those receiving supportive treatment. This non‐randomized controlled trial included 62 patients on the triple combination treatment versus 51 age‐ and sex‐matched patients on routine supportive treatment. all of them confirmed cases by positive reverse‐transcription polymerase chain reaction of a nasopharyngeal swab. Trial results showed that the clearance rates were 0% and 58.1% on the 7th day and 13.7% and 73.1% on the 15th day in the supportive treatment and combined antiviral groups, respectively. The cumulative clearance rates on the 15th day are 13.7% and 88.7% in the supportive treatment and combined antiviral groups, respectively. This trial concluded by stating that the combined use of nitazoxanide, ribavirin, and ivermectin plus zinc supplement effectively cleared the SARS‐COV2 from the nasopharynx in a shorter time than symptomatic therapy.
Keywords: antiviral, COVID‐19, mild cases
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic caused by the novel Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) started in Wuhan, China, in December 2019, and spread worldwide 1 The mortality rate of COVID‐19 is one of the most important ways of measuring the disease's burden. Johns Hopkins resource data reveals the deaths per 100.000 population ranged from 10.1% to 3.5% in different localities, as updated on 20 October 2020. 2
The urgent need for a safe and effective treatment has encouraged researchers to initiate clinical trials evaluating the efficacy of many drugs targeting viral proteins, viral entry pathway or the immune regulatory pathways. 3 the following mentioned drugs were approved as safe and effective in other indications, now we redirect its use in combinations toward COVID‐19.
Nitazoxanide is an oral antiparasitic drug having activity against many protozoa and helminths. Recent studies suggested a potential antiviral activity for nitazoxanide and immune‐modulatory effect suppressing the proinflammatory cytokines including interleukin‐6 and tumor necrosis factor‐α. 4 , 5 , 6 , 7 In vitro studies suggested that nitazoxanide has activity against SARS‐COV‐2 replication but there is no clear evidence about its usefulness in the clinical setting. 2 , 8
Ribavirin is a guanosine analogue having a broad‐spectrum antiviral effect against RNA and DNA viruses. The mechanism of ribavirin action is not completely clear, but possible mechanisms include inhibition of mRNA capping and induction of mutations during viral replication. These mechanisms can limit viral replication and reduce the viral load. 9 , 10 Indirect antiviral activity of ribavirin mediated via immune regulatory pathways was also noted by many authors. 11 , 12 , 13 , 14
Previous clinical experience with ribavirin in the treatment of SARS‐COV and Middle‐East respiratory syndrome coronavirus has proved its efficacy against coronaviruses and encouraged researchers to evaluate ribavirin as a potentially effective antiviral in treatment of SARS‐COV‐2 infection. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 The Chinese governmental treatment plan recommended Ribavirin for SARS‐COV‐2 pneumonia and then many clinical trials started evaluating the drug in SARS‐COV‐2 infection. 23 , 24
Ivermectin is a broad‐spectrum antiparasitic drug belonging to the Ivermectin family having proved antiparasitic, antibacterial, and antiviral activity 25 , 26 Ivermectin has a broad range of antiviral activity against many RNA and DNA viruses in vitro. The in vivo antiviral potential of the drug was proved only against two RNA viruses, the West Nile virus, the Newcastle disease virus, and two DNA viruses the pseudorabies virus and parvoviruses. 27 , 28 , 29 , 30 A recent study reported an in vitro inhibition of SARS‐COV‐2 replication by Ivermectin, and so the drug is now a potential candidate for SARS‐COV‐2 treatment. 31
Zinc ions (Zn) play a pivotal role in the development and maturation of both the innate and acquired antiviral immune response and its deficiency is associated with immune dysregulation. 32 Zinc ions may also directly inhibit SARS‐CoV‐2 replication. Many authors hypothesized that zinc supplementation may have a potentially beneficial effect for treatment of SARS‐CoV‐2 infection. 33 , 34
2. STUDY RATIONALE
This is the first non‐randomized controlled trial on the triple combination of Ivermectin, nitazoxanide, and ribavirin compared to routine supportive treatment in the treatment of patients with COVID‐19. We hypothesized that treatment with a combination of multiple antiviral drugs with therapeutic minimum doses may be more effective than single‐drug treatments as this treatment regimen is safe with minor and self‐limiting gastrointestinal adverse events of diarrhea and vomiting.
This trial aims to compare the rate and time of viral clearance in subjects receiving the combination of nitazoxanide, ribavirin, and ivermectin plus zinc versus those receiving supportive treatment.
3. PATIENTS AND METHODS
3.1. Study locality and duration
This study was carried out at the outpatients’ clinic of COVID‐19 at Mansoura University Hospital from May 15, 2020 to October 15, 2020. The COVID‐19 clinic was founded for triage and treatment of affiliated staff of Mansoura University and their families (both academic staff and administrative employees).
3.2. Study design
Non‐randomized phase I clinical trial.
3.3. Target population
Adult patients with suspected COVID‐19 as manifested by signs and symptoms who had become confirmed cases by positive reverse‐transcription polymerase chain reaction (RT‐PCR) of a nasopharyngeal swab.
Inclusion criteria: mild and early moderate cases with home treatment. With no associated co‐morbidities.
3.3.1. Sample size
A convenient sample of 113 patients who completed the study (51 and 62 in the supportive and combined antiviral groups, respectively) was included in the final analysis. Seventeen patients were excluded from the analysis as they dropped out from follow‐up.
3.3.2. Patients' allocation
Patients were self‐allocated to the treatment groups; the first 3 days of the week for the intervention arm while the other 3 days for symptomatic treatment. Patients were informed about the drugs included in each arm of the study and informed consent was signed from each case.
3.4. Data collection
A questionnaire was constructed to collect:
*Sociodemographic data, for example, age, sex, residence, occupation.
*Clinical data: exposure to a possible source of infection, signs and symptoms, severity of COVID‐19 classified according to the following; Asymptomatic illness: individuals who test positive for SARS‐CoV‐2 using a virologic test with no symptoms consistent with COVID‐19; Mild Illness: individuals who have any of following signs and symptoms of COVID‐19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell) but who do not have shortness of breath, dyspnea, or abnormal chest imaging; Moderate Illness: Individuals with lower respiratory affection and who have saturation of oxygen (SpO2) ≥ 94% in room air at sea level; Severe Illness: Individuals who have SpO2 < 94% in room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mmHg, respiratory frequency > 30 breaths per minute, or lung infiltrates > 50%; critical illness: individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction. 35
*Laboratory and radiological findings, if any, as well as associated comorbidities.
* The Coronavirus COVID‐19 Real‐Time PCR Assay is an in vitro diagnostic test based on real‐time PCR technology, developed for specific detection of SARS‐CoV‐2 viral RNA. The probe system is based on the standard hydrolysis probe system known as TaqMan® Technology. The COVID‐19‐specific probe is labelled with the FAM fluorophore and the internal control is labelled with the HEX fluorophore (genesig; Z‐Path‐COVID‐19‐CE‐IFU Issue 3.0 Published Date, Primerdesign Ltd.). The assay includes an internal control to identify possible PCR inhibition, measure extraction purity, and confirm the integrity of the PCR run.
*Follow‐up: both groups were followed up at weekly intervals to assesses the viral load using quantitative RT‐PCR in nasopharyngeal swab and resupply with drugs and record any side effects. Patients could consult the treating physicians by telephone or WhatsApp Web if there any complaints.
3.5. Treatment
Supportive symptomatic treatment (controlled or named white arm): in the form of paracetamol tablets (three times/day), zinc supplements (twice/day), good nutrition and hydration, and azithromycin capsules once may be added on a case by case basis.
Combined drugs as antivirals (intervention or named yellow arm): in the form of nitazoxanide 500 mg rapid release formula/6 h, ribavirin 1200 mg (400 mg divided doses); ivermectin in dose according to the following weight schedules: less than 60 kg or 60–90 kg 3 tables (200–300 μg/kg) (6 mg each table), 90–120 kg 4 tables (300–400 μg/kg), more than 120 kg 5 tables (30 mg fixed dose); all ivermectin doses taken singly after meals (due to long half live 12 to 54 h) were taken every 72 h till the end of 2 weeks according to each case; plus zinc supplement 30 mg twice daily.
Patients adherence: The best way to assess adherence is to discuss medication‐taking behaviors directly with the patient. The project clinical team revised the remaining pills before swabs plus followed up with the patients by telephone contact.
3.6. Ethical consideration
IRB approval at 10/5/2020 with code number RP.20.05.69, all included patients provided written informed consent before inclusion in the study. Also, this study is registered on ClinicalTrials.gov ID: NCT04392427.
3.7. Data analysis
Data were analyzed using statistical package for social sciences version 23. Quantitative variables were presented as mean and standard deviation and an unpaired t test was used for group comparison. Categorical variables were presented as number and percent. χ2 test or Fisher's exact test was used for the comparison between the two groups, as appropriate. p ≤ .05 was considered statistically significant.
4. RESULTS
Sociodemographic data described in Table 1 shows that both groups are matched by their age, sex, residence, and CT lung findings. However, patients on supportive treatment are more likely to be health care workers, unknown exposure, early moderate severity, and have low mean oxygen saturation than the other group. Patients flow charts in Figure 1 summarize the patients' selection criteria in both arms of the study.
Table 1.
Sociodemographic characteristics of both groups
| Supportive treatment (51) | Combined antiviral (62) | ||
|---|---|---|---|
| N (%) | N (%) | p | |
| Age | |||
| 35 and less | 24 (47.1) | 33 (53.2) | .5 |
| >35 | 27 (52.9) | 29 (46.8) | .9 |
| Mean (SD) | 37.5 (10.9) | 37.9 (11.9) | |
| Sex | |||
| Female | 29 (56.9) | 32 (51.6) | .6 |
| Male | 22 (43.1) | 30 (48.4) | |
| Residence | |||
| Urban | 38 (74.5) | 46 (74.2) | .97 |
| Rural | 13 (25.5) | 16 (25.8) | |
| Occupation | |||
| HCW | 48 (94.4) | 48 (77.4) | .01 |
| Others | 3 (5.9) | 14 (22.6) | |
| Exposure | |||
| Contact with case | 8 (15.7) | 23 (37.1) | .01 |
| Unknown | 43 (84.3) | 39 (62.9) | |
| Severity | |||
| Mild | 36 (70.6) | 54 (87.1) | .03 |
| Early moderate | 15 (29.4) | 8 (12.9) | |
| CT Lunga | |||
| Free | 26 (47.3) | 34 (81.0) | .5 |
| ≤50% | 9 (25.7) | 8 (38.1) | |
| O2 saturation | ≤.001 | ||
| Mean (SD) | 96.9 (1.1) | 97.7 (0.9) |
Abbreviation: HCW, Health care workers.
Done for 35/42 in supportive/combined antiviral.
Figure 1.
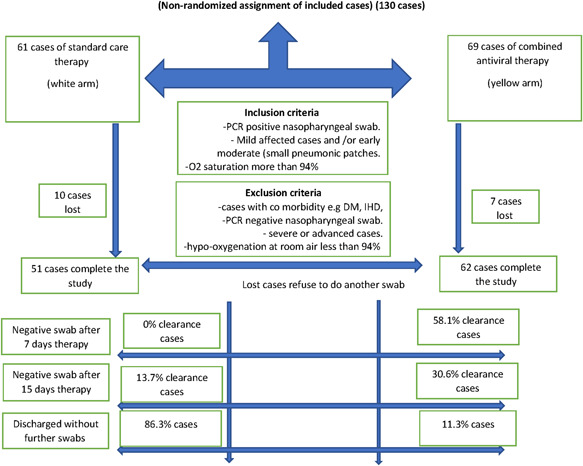
Flow chart of all COVID‐19 infected cases
Clinical symptomatology data shown in Table 2 reveals that no symptoms, abdominal pain, and nausea were significantly higher among the group of the combined antiviral than the supportive treatment. However, dyspnea is significantly higher among the supportive treatment group than the combined antiviral.
Table 2.
Clinical symptoms and signs of both groups (if any)
| Supportive treatment (51) | Combined antiviral (62) | p | |
|---|---|---|---|
| N (%) | N (%) | ||
| No symptoms | 2 (3.9) | 17 (27.4) | .001 |
| Fever | 35 (68.6) | 38 (61.3) | .4 |
| Cough | 35 (68.6) | 32 (51.6) | .07 |
| Sore throat | 14 (27.5) | 20 (32.3) | .6 |
| Malaise | 14 (27.5) | 10 (16.1) | .1 |
| Muscle pain | 13 (25.5) | 21 (33.9) | .3 |
| Headache | 3 (5.9) | 11 (17.7) | .06 |
| Dry mouth | 0 | 2 (3.2) | .5 |
| Dyspnea | 7 (13.7) | 1 (1.6) | .02 |
| Chest pain | 1 (2.0) | 1 (1.6) | 1.0 |
| Vomiting | 1 (2.0) | 1 (1.6) | 1.0 |
| Loss of smell/taste | 9 (17.6) | 3 (4.8) | .03 |
| Breathlessness | 7 (13.7) | 5 (8.1) | .3 |
| Myalgia | 3 (5.9) | 3 (4.8) | 1.0 |
| Diarrhea | 15 (29.4) | 17 (27.4) | .8 |
| Abdominal pain | 3 (5.9) | 12 (19.4) | .04 |
| Nausea | 3 (5.9) | 11 (17.7) | .06 |
Note: Categories are not mutually exclusive.
Viral clearance comparison in Table 3 shows that the clearance rates were 0% and 58.1% on the 7th day and 13.7% and 73.1% on the 15th day in the supportive treatment and combined antiviral groups, respectively. The cumulative clearance rates on the 15th day are 13.7% and 88.7% in supportive treatment and combined antiviral groups, respectively.
Table 3.
Viral clearance (negative PCR swab) at follow up of both groups
| Supportive treatment | Combined antiviral | p | ||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||||
| Number tested | Clearance | Cumulative clearance | Number tested | Clearance | Cumulative clearance | Clearance | Cumulative clearance | |
| Days after therapy | N (%) | N (%) | N (%) | N (%) | ||||
| 7 days | 51 | 0 | 62 | 36 (58.1) | ≤.001 | |||
| 15 days | 51 | 7 (13.7%) | 7 (13.7%) | 26 | 19 (73.1%) | 55 (88.7%) | ≤.001 | ≤.001 |
| No further swabs | 44 (86.3%) | 7 (11.3%) | ||||||
Basic biochemical data in Table 4 shows more leukocytopenias and lymphopenias in the combined antiviral group while the liver biochemical profile had no difference and renal functions in all included patients were normal.
Table 4.
Basic biochemical data of both groups
| Biochemical result | Supportive treatment | Combined antiviral | Significance test |
|---|---|---|---|
| Hemoglobin (mg/dl) | 12.7 ± 1.6 | 12.6 ± 1.6 | t = 0.2, p = .9 |
| Platelets (103/dl) | 261.2 ± 84.1 | 236.6 ± 71.8 | t = 1.5, p = .1 |
| Total leucocyte count (103/dl) | 6.5 ± 2.4 | 5.1 ± 2.2 | t = 3.1, p = .002 |
| Neutrophils (103/dl) | 3.8 ± 1.8 | 3.0 ± 1.4 | t = 2.3, p = .02 |
| Lymphocytes (103/dl) | 2.1 ± 0.9 | 1.6 ± 0.8 | t = 2.4, p = .02 |
| Alanine transaminases (IU/L) | 32.0 ± 22.5 | 26.1 ± 8.1 | t = 1.9, p = .1 |
| Aspartate transaminases (IU/L) | 30.7 ± 20.0 | 25.4 ± 8.1 | t = 1.8, p = .1 |
| Albumin (mg/dl) | 3.8 ± 0.3 | 3.9 ± 0.2 | t = 1.8, p = .1 |
| Total bilirubin (mg/dl) | 0.7 ± 0.2 | 0.7 ± 0.1 | t = 12, p = .2 |
Note: t = t test; p = p value; data presented as the mean ± SD.
Drugs' side effect data in Table 5 reveals that 58.1% and 41.9% of patients on the combined antivirals received treatment for 7 and 15 days; respectively. The commonest side effects are GIT upsets, colored urine, and palpitation (22.6%, 22.6%, and 19.4%) No mortality was recorded during the follow‐up duration (15 days).
Table 5.
Duration of treatment and side effect in the combined antiviral arm
| N (%) | |
|---|---|
| Treatment duration: 7 days | 36 (58.1) |
| 15 days | 26 (41.9) |
| No side effect | 32 (51.6) |
| High liver enzymes | 7 (11.3) |
| GIT upsets | 14 (22.6) |
| Itching | 1 (1.6) |
| Headache | 3 (4.8) |
| Colored urine | 14 (22.6) |
| Palpitation | 12 (19.4) |
5. DISCUSSION
It is well known that it takes years before the approval of a new antiviral for clinical use, so urgency is needed for providing highly active antiviral drugs for any novel emerging infectious disease. In such a pandemic as COVID‐19, researchers were obliged to test the existing broad‐spectrum antiviral drugs that have been used to treat other viral infections for drug repurposing. So, we used a combination of antiviral drugs with well‐known efficacy against other viral infections.
To our knowledge, this is the first controlled trial on this triple unique combination of nitazoxanide, ribavirin, and ivermectin plus zinc in the treatment of patients with COVID‐19. This study demonstrated that the combination of nitazoxanide, ribavirin, and ivermectin plus zinc was effective in suppressing the shedding of SARS‐CoV‐2 in nasopharyngeal swabs among mild and early moderate cases receiving home treatment compared to those receiving routine supportive symptomatic treatment alone. This could be helpful towards a safe and effective treatment combination for COVID‐19. Most patients (88%) treated with this combination were RT‐PCR negative on the 15th day.
This combination seems to be safe with minor side effects and no reported mortality at all during the follow‐up duration (15 days plus post‐therapy for 1 month). The commonest side effects were GIT upsets, colored urine, and palpitation.
Many studies proved that Ribavirin was effective against COVID‐19 when used in combination with interferon‐α or lopinavir–ritonavir. 3 , 36 , 37 In contrast; when used as a single agent in vitro studies it showed decreased potency compared to its comparative therapeutic agents. 38 , 39 This suggests that ribavirin when used alone has limited therapeutic efficacy against COVID‐19. Moreover; dose‐dependent adverse drug reactions, including hematologic and liver toxicity, were reported. 3
In in vitro studies (Vero E6 cells), nitazoxanide inhibited SARS‐CoV‐2 at a low micromolar concentration. 2 Nitazoxanide was suggested as a protocol for early cases of COVID‐19 in combination with azithromycin. 40 Many countries have started clinical trials for nitazoxanide such as Egypt, the United States, Brazil, and Mexico. 41
Recently, Caly et al. 31 reported in vitro potent inhibition of COVID‐19 replication by ivermectin. But, still the problem remains as to how to calculate the most effective accepted dose against SARS‐CoV‐2. The ivermectin concentrations used in this in vitro study are 50‐ to 100‐fold the peak concentration (C max) achieved in plasma after the single dose of 200 μg/kg commonly used for the control of onchocerciasis. 42 Although, single doses up to 120 mg of ivermectin (which is 10‐fold greater than those approved by the US Food and Drug Administration) can be safe and well‐tolerated; the C max values reported were ∼250 ng/ml, one order of magnitude lower than effective in vitro concentrations against SARS‐CoV‐2. 43 A recent phase III clinical trial in dengue patients (DNV) in Thailand revealed that lower doses of ivermectin can be effective. A once‐daily dose of 400 µg/kg for 3 days was found to be safe but did not produce any clinical benefit, and showed a modest and indirect in vivo effect against DNV. 44
The antioxidant, anti‐inflammatory, immunomodulatory and antiviral activities of Zn are well known. Its antiviral effect is mediated by suppressing RNA‐dependent RNA polymerase (RdRp). 45 Zinc ions (Zn2+) are closely involved in the normal development, differentiation, and function of immune cells, thus considered critical for generating both innate and acquired (humoral) antiviral responses. 32 The synergistic effect of zinc, if combined with antiviral treatment, has been proved previously with hepatitis C virus, human papillomavirus, viral diarrhea in children, and human immunodeficiency virus. 46 , 47 , 48 , 49 Short‐term treatment with zinc in therapeutic doses is completely safe. Zn toxicity rarely occurs in very sporadic cases unlike many other metal ions with similar chemical properties. 50
Study limitation: the groups were not randomized and the drug combination does not have an established in vitro mechanism of action and remains exploratory.
6. CONCLUSION
The results of this study confirm that combined use of nitazoxanide, ribavirin, and ivermectin plus zinc supplement effectively cleared the SARS‐COV2 from the nasopharynx in a shorter time than the symptomatic therapy with a few side effects, mostly gastrointestinal upset, with no reported mortality over the follow‐up period.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Hatem Elalfy and Tarek Besheer shared the idea and research design of the clinical trial. The manuscript was written, and clinical assessment, COVID‐19 classification, and data collection done by teamwork of the following doctors: Hatem Elalfy, Tarek Besheer, Ahmed El‐Mesery, Ahmed Alhawarey, and Mohamed Alegezy; while Tamer Elhadidy and Asem A. Hewidy were responsible for the pulmonary evaluation. Hossam Zaghloul and his colleague, Douaa Raafat, Wafaa M. El‐Emshaty, and Nermin Y. Abo El Kheir were responsible for the swab and PCR test for SARS‐Co‐V2. Abdel‐Hady El‐Gilany was responsible for statistical analysis and study design. Data analysis and interpretation were done by all research team members. Mahmoud El‐Bendary, Mustafa Ahmed Mohamed Neamatallah, and Mahmoud Abdel‐Aziz Soliman supervised all these processes.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26880
ACKNOWLEDGMENTS
Great thanks to resident doctors at the front line of this pandemic, Manar Metwalle, Mahmoud Samir, Ahmed Ezzate, who participated in data collection. This study is part of an official grant from the Mansoura University Funding Research Unit.
Elalfy H, Besheer T, El‐Mesery A, et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID‐19 J Med Virol. 2021;93:3176–3183. 10.1002/jmv.26880
DATA AVAILABILITY STATEMENT
data can be requested from the hospital administration after approval from Institutional Review Board (IRB) due to ethical & confidentiality reasons.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;323(18):1824‐1836. [DOI] [PubMed] [Google Scholar]
- 4. Fox LM, Saravolatz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clin Infect Dis. 2005;40(8):1173‐1180. [DOI] [PubMed] [Google Scholar]
- 5. Rossignol JF. Nitazoxanide: a first‐in‐class broad‐spectrum antiviral agent. Antiviral Res. 2014;110:94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossignol JF. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9(3):227‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hong SK, Kim HJ, Song CS, Choi IS, Lee JB, Park SY. Nitazoxanide suppresses IL‐6 production in LPS‐stimulated mouse macrophages and TG‐injected mice. Int Immunopharmacol. 2012;13(1):23‐27. [DOI] [PubMed] [Google Scholar]
- 8. Cao J, Forrest JC, Zhang X. A screen of the NIH clinical collection small molecule library identifies potential anti‐coronavirus drugs. Antiviral Res. 2015;114:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci U S A. 2001;98(12):6895‐6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16(1):37‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hultgren C, Milich DR, Weiland O, Sallberg M. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus‐specific immune responses. J Gen Virol. 1998;79(Pt 10):2381‐2391. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi T, Nakatsuka K, Shimizu M, et al. Ribavirin modulates the conversion of human CD4(+) CD25(‐) T cell to CD4(+) CD25(+) FOXP3(+) T cell via suppressing interleukin‐10‐producing regulatory T cell. Immunology. 2012;137(3):259‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Z, Ping Y, Yu Z, et al. Dynamic changes in CD45RA(‐)Foxp3(high) regulatory T‐cells in chronic hepatitis C patients during antiviral therapy. Int J Infect Dis. 2016;45:5‐12. [DOI] [PubMed] [Google Scholar]
- 14. Tam RC, Pai B, Bard J, et al. Ribavirin polarizes human T cell responses towards a Type 1 cytokine profile. J Hepatol. 1999;30(3):376‐382. [DOI] [PubMed] [Google Scholar]
- 15. Al‐Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Booth CM. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801‐2809. [DOI] [PubMed] [Google Scholar]
- 17. Chong YP, Song JY, Seo YB, et al. Antiviral treatment guidelines for Middle East respiratory syndrome. Infect Chemother. 2015;47(3):212‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Habib AMG, Ali MAE, Zouaoui BR, Taha MAH, Mohammed BS, Saquib N. Clinical outcomes among hospital patients with Middle East respiratory syndrome coronavirus (MERS‐CoV) infection. BMC Infect Dis. 2019;19(1):870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt TS, Al Mutairy E. Ribavirin and interferon‐α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir Ther. 2015;20(1):87‐91. [DOI] [PubMed] [Google Scholar]
- 20. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986‐1994. [DOI] [PubMed] [Google Scholar]
- 21. Peiris J, Chu C, Cheng V, et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767‐1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348(20):1995‐2005. [DOI] [PubMed] [Google Scholar]
- 23. Hung IFN, Lung KC, Tso EYK, et al. Triple combination of interferon beta‐1b, lopinavir‐ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: an open‐label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng YM, Xu XL, He XQ, et al. Comparative effectiveness and safety of ribavirin plus interferon‐alpha, lopinavir/ritonavir plus interferon‐alpha, and ribavirin plus lopinavir/ritonavir plus interferon‐alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol. Chin Med J. 2020;133(9):1132‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell WC, Benz GW. Ivermectin: a review of efficacy and safety. J Vet Pharmacol Ther. 1984;7(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 26. Crump A, Ōmura S. Ivermectin, 'wonder drug' from Japan: the human use perspective. Proc Jpn Acad Ser B. 2011;87(2):13‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lv C, Liu W, Wang B, et al. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res. 2018;159:55‐62. [DOI] [PubMed] [Google Scholar]
- 28. Wang X, Lv C, Ji X, Wang B, Qiu L, Yang Z. Ivermectin treatment inhibits the replication of porcine circovirus 2 (PCV2) in vitro and mitigates the impact of viral infection in piglets. Virus Res. 2019;263:80‐86. [DOI] [PubMed] [Google Scholar]
- 29. Raza S, Shahin F, Zhai W, et al. Ivermectin inhibits bovine herpesvirus 1 DNA polymerase nuclear import and interferes with viral replication. Microorganisms. 2020;8(3):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lundberg L, Pinkham C, Baer A, et al. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antiviral Res. 2013;100(3):662‐672. [DOI] [PubMed] [Google Scholar]
- 31. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Res. 2020;178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Overbeck S, Rink L, Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp. 2008;56(1):15‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar A, Kubota Y, Chernov M, Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID‐19. Med Hypotheses. 2020;144:109848‐109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman MT, Idid SZ. Can Zn be a critical element in COVID‐19 treatment? Biol Trace Elem Res. 2020;199:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clinical management of COVID‐19 interim guidance. May 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 36. Yousefi B, Valizadeh S, Ghaffari H, Vahedi A, Karbalaei M, Eslami M. A global treatments for coronaviruses including COVID‐19. J Cell Physiol. 2020;235(12):9133‐9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhong H, Wang Y, Zhang Z‐L, et al. Efficacy and safety of current therapeutic options for COVID‐19—lessons to be learnt from SARS and MERS epidemic: a systematic review and meta‐analysis. Pharmacol Res. 2020;157:104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelleni MT. Nitazoxanide/azithromycin combination for COVID‐19: a suggested new protocol for early management. Pharmacol Res. 2020;157:104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahmoud DB, Shitu Z, Mostafa A. Drug repurposing of nitazoxanide: can it be an effective therapy for COVID‐19? J Genet Eng Biotechnol. 2020;18(1):35‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chaccour C, Hammann F, Rabinovich NR. Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar J. 2017;16(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guzzo CA, Furtek CI, Porras AG, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42(10):1122‐1133. [DOI] [PubMed] [Google Scholar]
- 44. Yamasmith E. Efficacy and Safety of Ivermectin against dengue Infection: a phase III, randomized, double‐blind, placebo‐controlled trial. The 34th Annual Meeting of the Royal College of Physicians of Thailand‐ ‘Internal Medicine and One Health’: Chonburi, Thailand. Registry. 2018. https://clinicaltrials.gov/ct2/show/NCT02045069. Accessed November 4, 2020. [Google Scholar]
- 45. te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLOS Pathog. 2010;6(11):e1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bae SN, Lee KH, Kim JH, Lee SJ, Park LO. Zinc induces apoptosis on cervical carcinoma cells by p53‐dependent and ‐independent pathway. Biochem Biophys Res Commun. 2017;484(1):218‐223. [DOI] [PubMed] [Google Scholar]
- 47. Simonart T, de Maertelaer V. Systemic treatments for cutaneous warts: a systematic review. J Dermatolog Treat. 2012;23(1):72‐77. [DOI] [PubMed] [Google Scholar]
- 48. Bhutta ZA, Bird SM, Black RE, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr. 2000;72(6):1516‐1522. [DOI] [PubMed] [Google Scholar]
- 49. Asdamongkol N, Phanachet P, Sungkanuparph S. Low plasma zinc levels and immunological responses to zinc supplementation in HIV‐infected patients with immunological discordance after antiretroviral therapy. Jpn J Infect Dis. 2013;66(6):469‐474. [DOI] [PubMed] [Google Scholar]
- 50. Pal A, Squitti R, Picozza M, et al. Zinc and COVID‐19: basis of current clinical trials. Biol Trace Elem Res. 2020:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
data can be requested from the hospital administration after approval from Institutional Review Board (IRB) due to ethical & confidentiality reasons.


