To the Editors:
The efficacy of proning for intubated and sedated patients with ARDS (Acute Respiratory Distress Syndrome) is well established. 1 Proning of non‐intubated coronavirus disease 2019 (COVID‐19) patients has, therefore, emerged as a potential early treatment of respiratory deterioration. 2 , 3 , 4 However, there are limited data on proning for non‐intubated patients with COVID‐19. Thus, in non‐intubated COVID‐19 patients, we aimed to examine whether, compared to the first proning treatment, subsequent proning treatments lead to a similar magnitude of change for key respiratory observations.
This audit was performed during the second wave of COVID‐19 in Victoria, Australia (1 July 1–30 September 2 020). Following ethics approval, we used clinical records to retrospectively identify patients where proning was deemed clinically indicated. This applied to 27 spontaneously breathing adults with COVID‐19 admitted to the Austin Hospital. Proning was considered clinically indicated if the patient required supplemental oxygen or was tachypnoeic (respiratory rate ≥ 25 breaths per minute). We applied linear mixed effect modelling with the patient as a random effect for data analysis. We compared the changes in oxyhaemoglobin saturation (SpO2), measured with pulse oximetry, and in respiratory rate (breaths per minute) induced by the first proning treatment with those induced by subsequent proning treatments. We performed a sensitivity analysis with events where oxygen flow rate (litres per minute) remained constant during proning to determine whether changes in supplemental oxygen during proning per se modified the findings. Statistical significance was set at P < 0.05 and analyses were performed using Stata 16.1. 5
The median age was 57 years (interquartile range (IQR): 51, 73), most were male (n = 21, 78%) and chronic disease burden was low (Charlson comorbidity index score median 1, IQR: 0–1). Twenty (74%) patients received proning at least once, six (22%) never received proning despite clinical indication (e.g. refused) and for one patient proning was documented but no data were available. There were 94 documented proning events in 20 patients, the majority occurred in the intensive care unit (n = 67, 71%) and the remaining in the COVID‐19 ward (n = 27, 29%). For the patients who received proning (n = 20), the median (IQR) number of treatments per patient and their duration (min) were 3 (1, 6) and 105 (57, 170), respectively. Overall, the median SpO2 change per proning treatment was −1% (−2, 2) and 0 (−3, 2) breaths per minute for respiratory rate. There was no statistically significant effect of subsequent proning treatment when compared to the first proning treatment for either change in SpO2 or respiratory rate (SpO2: β = 0.10 (95% CI: −1.77 to 1.97); respiratory rate: β = −1.66 (95% CI: −4.21 to 0.89)). Plotting differences in SpO2 and respiratory rate for the proning event sequence in each patient did not reveal any clear response trajectories, that is, ‘responders’ (Fig. 1).
Figure 1.
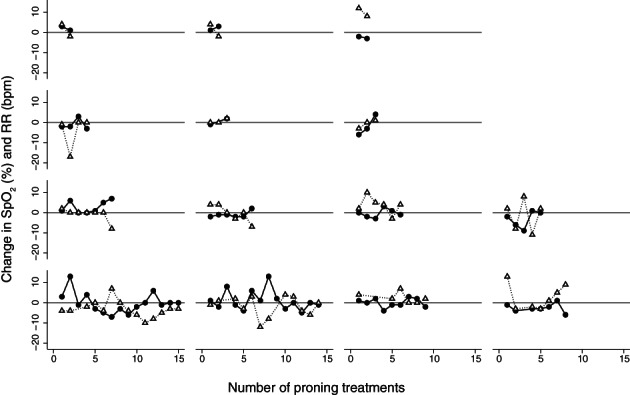
Spontaneously breathing patients with COVID‐19 who received at least two proning treatments (n = 14); changes in oxyhaemoglobin saturation measured with pulse oximetry and RR per patient for each proning treatment are displayed. Reference line (zero) indicates no change in oxyhaemoglobin saturation or RR.  , Change in SpO2 (%) per treatment;
, Change in SpO2 (%) per treatment;  , change in RR (bpm) per treatment. bpm, breaths per minute; COVID‐19, coronavirus disease 2019; RR, respiratory rate; SpO2, peripheral oxyhaemoglobin saturation.
, change in RR (bpm) per treatment. bpm, breaths per minute; COVID‐19, coronavirus disease 2019; RR, respiratory rate; SpO2, peripheral oxyhaemoglobin saturation.
The sensitivity analysis revealed that during the majority of proning treatments, patients were receiving supplemental oxygen (n = 83, 88%), predominantly via nasal prongs (n = 55 events, 59%). When oxygen flow rate remained constant (n = 61), SpO2 decreased in 52% of treatments (pre‐ and post‐proning difference < 0%, range: −9% to −1%) and increased in 39% of treatments (pre‐ and post‐proning difference > 0%, range: 1% to 13%). In all these patients, there was no effect of subsequent proning treatment compared to the first for both SpO2 and respiratory rate.
In spontaneously breathing patients with COVID‐19, the novel and clinically important findings of this research were that there was no evidence of a consistent response to proning treatment and that the magnitude of any response to proning was not indicative of any subsequent response to another proning treatment. Studies of proning in non‐ventilated patients have reported improvement in oxygenation following proning and a lower incidence of intubation. 3 , 4 , 6 , 7 However, none of these were controlled, let alone randomized. Moreover, all these studies had small sample sizes (n = 10–56) with limited or no data on subsequent proning outcomes. In contrast, a recent observational cohort study (n = 199) reported no difference in clinical outcomes for patients receiving proning in addition to high‐flow nasal oxygen therapy. In fact, proning delayed but did not avoid intubation. 8 We observed a heterogeneous response to proning and were unable to identify responders and non‐responders.
In summary, in spontaneously breathing patients with COVID‐19, on an analysis of close to 100 treatments, we found no evidence of reproducible response to proning and no relationship between the effect of proning on first treatment with subsequent treatments. Our findings imply uncertainty about the benefit of this intervention.
Author contributions
Conceptualization: Z.A., T.R., D.J.B. Formal analysis: J.R.A.J., M.G., D.J.B. Investigation: Z.A., N.B., A.D., T.R. Methodology: J.R.A.J., R.B., M.G., T.R., D.J.B. Supervision: R.B., D.J.B. Validation: N.B., A.D. Writing—original draft: J.R.A.J. Writing—review and editing: J.R.A.J., R.B., M.G., T.R., D.J.B.
Acknowledgement
We are appreciative of the statistical support received from the Melbourne Statistical Consulting Centre.
Jones JRA, Attard Z, Bellomo R, et al. Repeated proning in non‐intubated patients with COVID‐19. Respirology. 2021;26:279–280. 10.1111/resp.14008
Received 9 December 2020; accepted 22 December 2020
Handling Editor: Philip Bardin
Contributor Information
Jennifer R.A. Jones, Email: aujrjones@student.unimelb.edu.
Zachary Attard, Email: zachary.ATTARD@austin.org.au.
Rinaldo Bellomo, Email: rinaldo.BELLOMO@austin.org.au.
Nicola Burgess, Email: nicola.BURGESS@austin.org.au.
Ashleigh Donovan, Email: ashleigh.DONOVAN@austin.org.au.
Thomas Rollinson, Email: thomas.ROLLINSON@austin.org.au.
David J. Berlowitz, Email: david.berlowitz@austin.org.au.
REFERENCES
- 1. Sud S, Friedrich JO, Adhikari NK, Taccone P, Mancebo J, Polli F, Latini R, Pesenti A, Curley MA, Fernandez R et al. Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: a systematic review and meta‐analysis. CMAJ. 2014; 186: E381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Damarla M, Zaeh S, Niedermeyer S, Merck S, Niranjan‐Azadi A, Broderick B, Punjabi N. Prone positioning of nonintubated patients with COVID‐19. Am. J. Respir. Crit. Care Med. 2020; 202: 604–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng Z, Tay WC, Ho CHB. Awake prone positioning for non‐intubated oxygen dependent COVID‐19 pneumonia patients. Eur. Respir. J. 2020; 56: 2001198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson AE, Ranard BL, Wei Y, Jelic S. Prone positioning in awake, nonintubated patients with COVID‐19 hypoxemic respiratory failure. JAMA Intern. Med. 2020; 180: 1537–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. StataCorp . Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC, 2019.
- 6. Sartini C, Tresoldi M, Scarpellini P, Tettamanti A, Carcò F, Landoni G, Zangrillo A. Respiratory parameters in patients with COVID‐19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA 2020; 323: 2338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coppo A, Bellani G, Winterton D, Di Pierro M, Soria A, Faverio P, Cairo M, Mori S, Messinesi G, Contro E et al. Feasibility and physiological effects of prone positioning in non‐intubated patients with acute respiratory failure due to COVID‐19 (PRON‐COVID): a prospective cohort study. Lancet Respir. Med. 2020; 8: 765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrando C, Mellado‐Artigas R, Gea A, Arruti E, Aldecoa C, Adalia R, Ramasco F, Monedero P, Maseda E, Tamayo G et al. Awake prone positioning does not reduce the risk of intubation in COVID‐19 treated with high‐flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit. Care 2020; 24: 597. [DOI] [PMC free article] [PubMed] [Google Scholar]


