Abstract
An outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is leading to an unprecedented worldwide health crisis. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2. Our objectives are to analysis the expression profile of ACE2 and TMPRSS2 in human spermatogenic cells, follicle cells, and preimplantation embryos, thereby providing mechanistic insights into viral entry and viral impact on reproduction. We found that ACE2 is mainly expressed during gametogenesis in spermatogonia and oocytes of antral follicles, granulosa cells of antral follicles and pre‐ovulatory follicles, while TMPRSS2 almost has no expression in spermatogenic cells, oocytes or granulosa cells. In preimplantation embryos, ACE2 is expressed in early embryos before eight‐cell stage, and trophectoderm of late blastocysts, while TMPRSS2 initiates its robust expression in late blastocyst stage. ACE2 and TMPRSS2 only show significant co‐expression in trophectoderm of late blastocysts in all above cell types. We speculate that trophectoderm of late blastocysts is susceptible to SARS‐CoV‐2, and that the chance of SARS‐CoV‐2 being passed on to offspring through gametes is very low. Therefore, we propose that fertility preservation for COVID‐19 patients is relatively safe and rational. We also recommend embryo cryopreservation and embryo transfer into healthy recipient mother at cleavage stage instead of blastocyst stage. Moreover, we unexpectedly found that co‐expression pattern of ACE2 and TMPRSS2 in oocytes and preimplantation embryos in human, rhesus monkey and mouse are totally different, so animal models have significant limitations for evaluating transmission risk of SARS‐CoV‐2 in reproduction.
Keywords: ACE2, assisted reproductive technology, reproduction, SARS‐CoV‐2, TMPRSS2
Abbreviations
- ART
assisted reproductive technology
- COVID‐19
coronavirus disease‐2019
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causing current coronavirus disease 2019 (COVID‐19) pandemic has received global attention. SARS‐CoV‐2 can target respiratory system and transmits via air droplets and contact. In addition to oropharyngeal swabs, the SARS‐CoV‐2 RNA has also been detected in blood, urine, and facial/anal swabs, suggesting other potential routes of transmission (Guan et al., 2020; Peng et al., 2020; Wang et al., 2020). Roujian Lu et al., 2020) found that SARS‐CoV‐2 is genetically close to SARS‐CoV (about 79% identity) and Middle East respiratory syndrome coronavirus (about 50% identity). Homology modeling has revealed that SARS‐CoV‐2 has a similar receptor‐binding domain structure to that of SARS‐CoV, which suggests that COVID‐19 infection might have a similar pathogenesis compared with SARS‐CoV infection (Hamming et al., 2004; Lu et al., 2020; To & Lo, 2004). These coronaviruses have very similar spike (S) protein 3‐D structures that are considered to have strong binding affinity to the human cell receptor, angiotensin‐converting enzyme 2 (ACE2). Through single‐cell RNA sequencing data analyses, Zou et al. (2020) identified the organs at risk, such as lung, heart, esophagus, kidney, bladder, and ileum, and located specific cell types, which are vulnerable to SARS‐CoV‐2 infection and might be related to the reported clinical symptoms of SARS‐CoV infection. Moreover, cell entry of coronaviruses depends not only on binding of the viral spike protein to cellular receptor, but also on S protein priming by host cell proteases (Hoffmann, Kleine‐Weber, Krüger, et al., 2020). Markus Hoffmann et al. (Hoffmann, Kleine‐Weber, Schroeder, et al., 2020) demonstrated that SARS‐CoV‐2 uses the SARS‐CoV receptor ACE2 for entry and the serine protease TMPRSS2 for S protein priming. TMPRSS2 is a prostate‐specific and androgen‐response gene that encodes a plasma membrane‐anchored serine protease that participates in proteolytic cascades of relevance for the normal physiologic function of the prostate (Lucas et al., 2014). High‐throughput single‐cell RNA sequencing data showed potential risk of SARS‐CoV‐2 infection in human testes (Fan et al., 2020; Wang & Xu, 2020). However, interpretation of high‐throughput single‐cell RNA sequencing is limited by its sequencing depth. To identify possibility of SARS‐CoV‐2 carrying and transmission through male reproductive system, several reports showed contradictory results for existence of SARS‐CoV‐2 in semen from men with COVID‐19 (Li et al., 2020; Pan et al., 2020; Song et al., 2020). Variation of these studies may be explained by timing of examination, sensitivity of testing, potential contamination, age of patients, etc. Further careful evaluation should be performed for potential infection in male reproductive system.
SARS‐CoV was predominantly detected in lung, trachea and bronchus, but not in testis and ovary (Ding et al., 2004) and can cause orchitis indirectly (Xu et al., 2006). SARS‐CoV‐2 and SARS‐CoV have 76% of the amino acid sequence homology, perhaps relying on the same cellular receptor (ACE2 and TMPRSS2) to enter the cell (Hoffmann, Kleine‐Weber, Krüger, et al., 2020). SARS‐CoV‐2 is highly contagious and has multiple possible routes of transmission. A big concern is whether SARS‐CoV‐2 can be transmitted from the infected mother to the baby before birth. Dong et al. (2020) reported that a neonate born to a mother with COVID‐19 had elevated immunoglobulin M antibody levels 2 h after birth, considering the possibility of vertical transmission from mother to child. However, limitations of this report include the single case and that no polymerase chain reaction testing of amniotic fluid or placenta was performed. Moreover, Chen et al. (2020) reported no evidence of mother‐to‐child transmission. The latest research by Pique‐Regi et al. (2020) reported that co‐transcription of ACE2 and TMPRSS2 is negligible, thus not a likely path of vertical transmission for SARS‐CoV‐2 at any stage of pregnancy. Therefore, current clinical evidence indicates that SARS‐CoV‐2 is unlikely to infect the fetus. Until now, there are still limited published data on co‐expression of ACE2 and TMPRSS2 in the female reproductive system or in preimplantation embryo. It is important to evaluate the risk of SARS‐CoV‐2 infection in human reproductive cells and early embryos for potential viral infection and transmission, and this is especially valuable for safety assessment in assisted reproductive technology (ART).
2. RESULTS
Here, we used published RNA‐seq data (Jan et al., 2017; Yan et al., 2013; Zhang et al., 2018) with high sequencing depth to analyze the expression pattern of ACE2 and TMPRSS2 in all stages of spermatogenic cells, follicles and preimplantation embryos in human, to evaluate risk of SARS‐CoV‐2 infection in ART.
In human testis, we analyzed all stages of spermatogenic cells. We found that ACE2 is mainly expressed in spermatogonia, very low in leptotene/zygotene spermatocytes, and expressed in some pachytene spermatocytes and round spermatids, while TMPRSS2 was almost not expressed in any spermatogenic cell type (Figure 1). Our result shows that the chance of infection of human spermatogenic cells by SARS‐CoV‐2 is quite low. Since there are gap junction communications between granulosa cells and oocytes, with substances continuously exchanged between each other (Cecconi et al., 2004), SARS‐CoV‐2 may be transmitted to oocytes through granulosa cells and passed to further developmental stages. Therefore, both human granulosa cells and oocytes were analyzed for gene expression. Our analysis shows that during folliculogenesis ACE2 is mainly expressed in oocytes and granulosa cells of antral follicles and pre‐ovulation follicles, while TMPRSS2 has almost no expression in oocytes or granulosa cells (Figure 2). Therefore, oocytes seem to be unsusceptible to SARS‐CoV infection, which is consistent with a recent research that the viral RNA was undetectable in all the oocytes analyzed from the two SARS‐CoV‐2 positive women (Barragan et al., 2020). In preimplantation embryos, ACE2 is expressed in early embryos before eight‐cell embryo stage and in polar and mural trophectoderm of late blastocysts, and TMPRSS2 is initially expressed in all cell lineages at late blastocyst stage. ACE2 and TMPRSS2 are only robustly co‐expressed in the trophectoderm of late blastocysts. Both ACE2 and TMPRSS2 express at high levels in polar and mural trophectoderm (Figure 3). Therefore, we speculate that trophectoderm in late blastocysts is highly susceptible to SARS‐CoV‐2 infection, while other cell types in late blastocysts which contribute to fetus do not seem to be susceptible to SARS‐CoV infection.
Figure 1.
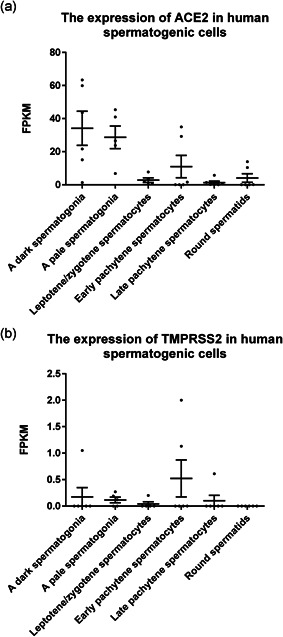
The expression of ACE2 and TMPRSS2 in human spermatogenic cells. Dot plots indicate (a) ACE2 and (b) TMPRSS2 expression in several types of human spermatogenic cells, including spermatogonia, spermatocytes, and spermatids. Data are presented as the mean ± SEM. FPKM, fragments per kilobase of transcript per million mapped reads
Figure 2.
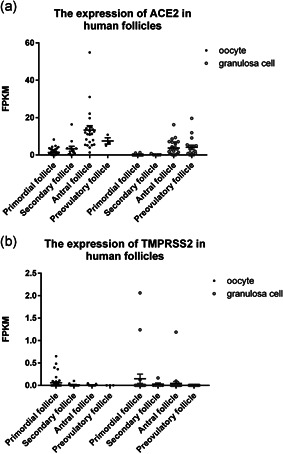
The expression of ACE2 and TMPRSS2 in human follicles. Dot plots indicate (a) ACE2 and (b) TMPRSS2 expression in oocyte and granulosa cells in different stages of human follicles. Data are presented as the mean ± SEM. FPKM, fragments per kilobase of transcript per million mapped reads
Figure 3.
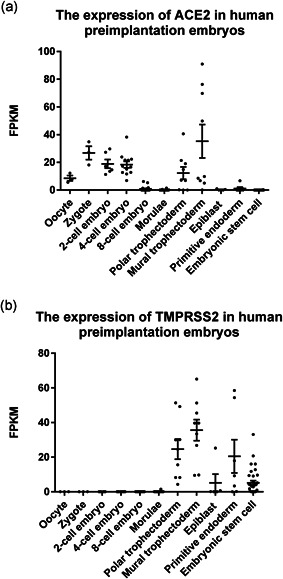
The expression of ACE2 and TMPRSS2 in human preimplantation embryos and embryonic stem cells. Dot plots indicate (a) ACE2 and (b) TMPRSS2 expression in all stages of human preimplantation embryos and in human embryonic stem cells. Data are presented as the mean ± SEM. FPKM, fragments per kilobase of transcript per million mapped reads
In addition, we analyzed the expression of ACE2 and TMPRSS2 in rhesus monkey and mouse oocytes and preimplantation embryos using published RNA‐seq data (Tang et al., 2011; Wang et al., 2017). We found that ACE2 and TMPRSS2 are highly co‐expressed in rhesus monkey oocytes and cleavage embryos (Figure 4a). Differently, Ace2 and Tmprss2 are not co‐expressed in any stage of mouse oocytes or preimplantation embryos (Figure 4b). Above results show that the co‐expression pattern of ACE2 and TMPRSS2 of human, rhesus monkey, and mouse in oocytes and preimplantation embryos are distinct. Therefore, to evaluate potential transmission risk of SARS‐CoV‐2 in human reproduction, animal models have limitations, and only human samples seem to be reliable for usage.
Figure 4.
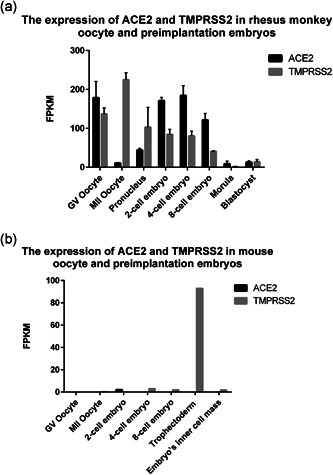
The expression of ACE2 and TMPRSS2 in rhesus monkey and mouse oocytes and preimplantation embryos. Bar charts indicate ACE2 and TMPRSS2 expression in oocytes and preimplantation embryos in (a) rhesus monkey and (b) mouse. Data are presented as the mean ± SEM. FPKM, fragments per kilobase of transcript per million mapped reads
Taken together, we give suggestions on ART procedures for safety concern based on expression profile of ACE2 and TMPRSS2 in current COVID‐19 pandemic. (a) We propose that fertility preservation for ART patients even COVID‐19 carriers is relatively safe and rational. (b) For operations in the laboratory environment which may be contaminated by SARS‐CoV‐2 (such as medium or liquid nitrogen), cleavage‐stage embryos are not supposed to be susceptible to the coronavirus based on our analysis and are safer to be operated in vitro than blastocysts. Therefore, we recommend that cleavage‐stage embryo rather than blastocyst should be selected for cryopreservation during the COVID‐19 epidemic, to avoid any possible infection from outside, such as liquid nitrogen which could be contaminated by SARS‐CoV‐2 from the operator or the container. It is known that people positive for virus are contagious by spreading virus in small aerosols. Viruses in a 1 μm thin layer of water will be vitrified upon cooling into liquid nitrogen (Adrian et al., 1984). Therefore, if aerosol drops containing viruses touch liquid nitrogen, they will be vitrified and contaminate the liquid nitrogen (Arav. 2020). (c) Meanwhile, we strongly recommend using fresh or frozen cleavage‐stage embryo for embryo transfer into women tested for negative of SARS‐CoV‐2 to avoid any possible infection from as early as blastocyst stage, although pregnant COVID‐19 female patients are still recommended to born children. Besides, to avoid possible cross‐contamination among gametes and embryos, more modifications of operations may be useful in regular embryology practice (Anifandis et al., 2020; Arav. 2020).
3. DISCUSSION
Because of the risk of viral transmission between ART patients, staff, physicians, and viral carriers, and to comply with local restrictions for nonemergency surgeries, many programs of assisted reproduction have suspended procedures throughout the globe during the height of the pandemic. Recently, ART programs gradually resume operations globally, especially in China because of declined spreading of COVID‐19. However, there is evidence that more than 60% of COVID‐19 patients were identified to be asymptomatic and may spread virus (Qiu, 2020). Therefore, as Andrabi SW et al. (Andrabi et al., 2020) reported, some stricter processes need to be implemented and our suggestions can be used as a supplement. Although the levels of messenger RNA might not be absolutely equal to protein, our finding supports the hypothesis that the chance of SARS‐CoV‐2 passing through germ cells to offspring is very low. Moreover, trophectoderm in blastocyst is susceptible to SARS‐Cov‐2 infection, but trophectoderm does not contribute to fetus tissues, so the chance of fetus infection through this route is also low. Notably, infection of trophectoderm in blastocyst may impact fetus development and therefore clinical examination and animal models for further evaluation are needed.
4. MATERIALS AND METHODS
4.1. RNA sequencing data analysis
The raw RNA sequencing data were downloaded from GEO dataset or NCBI Sequence Read Archive. Generally, raw reads were processed with cutadapt to remove adapters and perform quality trimming with default parameters. Trimmed reads were mapped to human genome (hg19), using STAR with default settings. RSEM program was used to calculate fragments per kilobase of transcript per million mapped reads values. Reported RNA sequencing data was from SRP069329 (human spermatogenic cells; Jan et al., 2017), GSE107746 (human follicle cells; Zhang et al., 2018) and GSE36552 (human preimplantation embryos and embryonic stem cells; Yan et al., 2013), GSE86938 (analyzed RNA‐seq data of rhesus monkey oocyte and preimplantation embryos; Wang et al., 2017), GSE22182 and GSE119906 (analyzed RNA‐seq data of mouse oocyte and preimplantation embryos; Tang et al., 2011).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
This study was supported by National Key R&D Program of China (2018YFC1004502, 2018YFC1004001) and the National Natural Science Foundation of China (NSFC31771661).
Cheng G‐p, Guo S‐m, Zhou L‐q. Suggestions on cleavage embryo and blastocyst vitrification/transfer based on expression profile of ACE2 and TMPRSS2 in current COVID‐19 pandemic. Mol Reprod Dev. 2021;88:211–216. 10.1002/mrd.23456
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in GEO at https://www.ncbi.nlm.nih.gov/geo/, reference number SRP069329, GSE107746, GSE36552, GSE86938, GSE22182, and GSE119906. These data were derived from the following resources available in the public domain: NCBI, https://www.ncbi.nlm.nih.gov.
REFERENCES
- Adrian, M. , Dubochet, J. , & Lepault, J. (1984). Cryo‐electron microscopy of viruses. Nature, 308, 32–36. [DOI] [PubMed] [Google Scholar]
- Andrabi, S. W. , Jaffar, M. , & Arora, P. R. (2020). COVID‐19: New adaptation for IVF laboratory protocols. JBRA Assisted Reproduction, 24(3), 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anifandis, G. , Messini, C. I. , Daponte, A. , & Messinis, I. E. (2020). COVID‐19 and fertility: A virtual reality. Reproductive BioMedicine Online, 41(2), 157–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arav, A. (2020). A recommendation for IVF lab practice in light of the current COVID‐19 pandemic. Journal Of Assisted Reproduction And Genetics, 37(7), 1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan, M. , Guillen, J. J. , Martin‐Palomino, N. , Rodriguez, A. , & Vassena, R. (2020). Undetectable viral RNA in oocytes from SARS‐CoV‐2 positive women. Human Reproduction, 36, 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi, S. , Ciccarelli, C. , Barberi, M. , Macchiarelli, G. , & Canipari, R. (2004). Granulosa cell‐oocyte interactions. EUR J OBSTET GYN R B, 115, S19–S22. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Guo, J. , Wang, C. , Luo, F. , Yu, X. , Zhang, W. , Li, J. , Zhao, D. , Xu, D. , Gong, Q. , Liao, J. , Yang, H. , Hou, W. , & Zhang, Y. (2020). Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: A retrospective review of medical records. The Lancet, 395(10226), 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Y. Q. , He, L. , Zhang, Q. L. , Huang, Z. X. , Che, X. Y. , Hou, J. L. , Wang, H. J. , Shen, H. , Qiu, L. W. , Li, Z. G. , Geng, J. , Cai, J. J. , Han, H. X. , Li, X. , Kang, W. , Weng, D. S. , Liang, P. , & Jiang, S. B. (2004). Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. Journal of Pathology, 203(2), 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, L. , Tian, J. , He, S. , Zhu, C. , Wang, J. , Liu, C. , & Yang, J. (2020). Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA: Journal of American Medical Association, 323(18), 1846–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, C. , Li, K. , Ding, Y. , Lu, W. L. , & Wang, J. (2020). ACE2 Expression in kidney and testis may cause kidney and testis damage after 2019‐nCoV infection, Cold Spring Harbor Laboratory. [Google Scholar]
- Guan, W. J. , Ni, Z. Y. , Hu, Y. , Liang, W. H. , Ou, C. Q. , He, J. X. , Liu, L. , Shan, H. , Lei, C. L. , Hui, D. , Du, B. , Li, L. J. , Zeng, G. , Yuen, K. Y. , Chen, R. C. , Tang, C. L. , Wang, T. , Chen, P. Y. , Xiang, J. , … Zhong, N. S. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. , Lely, A. T. , Navis, G. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology, 203(2), 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Krüger, N. , Müller, M. , Drosten, C. , & Pöhlmann, S. (2020). The novel coronavirus 2019 (2019‐nCoV) uses the SARS‐coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells, Cold Spring Harbor Laboratory. [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Kruger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N. H. , Nitsche, A. , Muller, M. A. , Drosten, C. , & Pohlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan, S. Z. , Vormer, T. L. , Jongejan, A. , Roling, M. D. , Silber, S. J. , de Rooij, D. G. , Hamer, G. , Repping, S. , & van Pelt, A. M. M. (2017). Unraveling transcriptome dynamics in human spermatogenesis. Development, 144(20), 3659–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. , Jin, M. , Bao, P. , Zhao, W. , & Zhang, S. (2020). Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Network Open, 3(5), e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , Wang, W. , Song, H. , Huang, B. , Zhu, N. , Bi, Y. , Ma, X. , Zhan, F. , Wang, L. , Hu, T. , Zhou, H. , Hu, Z. , Zhou, W. , Zhao, L. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 395(10224), 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, J. M. , Heinlein, C. , Kim, T. , Hernandez, S. A. , Malik, M. S. , True, L. D. , Morrissey, C. , Corey, E. , Montgomery, B. , Mostaghel, E. , Clegg, N. , Coleman, I. , Brown, C. M. , Schneider, E. L. , Craik, C. , Simon, J. A. , Bedalov, A. , & Nelson, P. S. (2014). The androgen‐regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Dicovery, 4(11), 1310–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, F. , Xiao, X. , Guo, J. , Song, Y. , Li, H. , Patel, D. P. , Spivak, A. M. , Alukal, J. P. , Zhang, X. , Xiong, C. , Li, P. S. , & Hotaling, J. M. (2020). No evidence of severe acute respiratory syndrome‐coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertility and Sterility, 113(6), 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L. , Liu, J. , Xu, W. , Luo, Q. , Chen, D. , Lei, Z. , Huang, Z. , Li, X. , Deng, K. , Lin, B. , & Gao, Z. (2020). SARS‐CoV‐2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. Journal of Medical Virology, 92(9), 1676–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique‐Regi, R. , Romero, R. , Tarca, A. L. , Luca, F. , Xu, Y. , Alazizi, A. , Leng, Y. , Hsu, C. , & Gomez‐Lopez, N. (2020). Does the human placenta express the canonical cell entry mediators for SARS‐CoV‐2? eLife, 9:e58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, J. (2020). Covert coronavirus infections could be seeding new outbreaks. Nature. [DOI] [PubMed] [Google Scholar]
- Song, C. , Wang, Y. , Li, W. , Hu, B. , Chen, G. , Xia, P. , Wang, W. , Li, C. , Diao, F. , Hu, Z. , Yang, X. , Yao, B. , & Liu, Y. (2020). Absence of 2019 novel coronavirus in semen and testes of COVID‐19 patients. Biology of Reproduction, 103(1), 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, F. , Barbacioru, C. , Nordman, E. , Bao, S. , Lee, C. , Wang, X. , Tuch, B. B. , Heard, E. , Lao, K. , & Surani, M. A. (2011). Deterministic and stochastic allele specific gene expression in single mouse blastomeres. PLOS One, 6(6), e21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. F. , & Lo, A. W. (2004). Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS‐CoV) and its putative receptor, angiotensin‐converting enzyme 2 (ACE2). Journal of Pathology, 203(3), 740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Xu, Y. , Gao, R. , Lu, R. , Han, K. , Wu, G. , & Tan, W. (2020). Detection of SARS‐CoV‐2 in different types of Clinical specimens. JAMA: Journal of American Medical Association, 323(18), 1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Liu, D. , He, D. , Suo, S. , Xia, X. , He, X. , Han, J. J. , & Zheng, P. (2017). Transcriptome analyses of rhesus monkey preimplantation embryos reveal a reduced capacity for DNA double‐strand break repair in primate oocytes and early embryos. Genome Research, 27(4), 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , & Xu, X. (2020). scRNA‐seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS‐CoV‐2 infection in spermatogonia, leydig and sertoli cells. Cells‐Basel, 9(9204), 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Qi, L. , Chi, X. , Yang, J. , Wei, X. , Gong, E. , Peh, S. , & Gu, J. (2006). Orchitis: A complication of severe acute respiratory syndrome 1 (SARS 1). Biology of Reproduction, 74(2), 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. , Yang, M. , Guo, H. , Yang, L. , Wu, J. , Li, R. , Liu, P. , Lian, Y. , Zheng, X. , Yan, J. , Huang, J. , Li, M. , Wu, X. , Wen, L. , Lao, K. , Li, R. , Qiao, J. , & Tang, F. (2013). Single‐cell RNA‐Seq profiling of human preimplantation embryos and embryonic stem cells. Nature Structural & Molecular Biology, 20(9), 1131–1139. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Yan, Z. , Qin, Q. , Nisenblat, V. , Chang, H. , Yu, Y. , Wang, T. , Lu, C. , Yang, M. , Yang, S. , Yao, Y. , Zhu, X. , Xia, X. , Dang, Y. , Ren, Y. , Yuan, P. , Li, R. , Liu, P. , Guo, H. , … Yan, L. (2018). Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Molecular Cell, 72(6), 1021–1034. [DOI] [PubMed] [Google Scholar]
- Zou, X. , Chen, K. , Zou, J. , Han, P. , Hao, J. , & Han, Z. (2020). Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Frontiers in Medicine, 14(2), 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in GEO at https://www.ncbi.nlm.nih.gov/geo/, reference number SRP069329, GSE107746, GSE36552, GSE86938, GSE22182, and GSE119906. These data were derived from the following resources available in the public domain: NCBI, https://www.ncbi.nlm.nih.gov.


