Abstract
Data regarding antibody responses to severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) in patients infected with COVID‐19 are not yet available. In this study, we aimed to evaluate serum antibody responses in patients regardless of the outcome. We measured the circulating immunoglobulin G (IgG) antibody levels in 60 subjects with a certified history of SARS‐CoV‐2 infection by using immunoenzymatic, chemiluminescent, and Neutralization assays. Half patients had a severe infection, the other half were pauci‐symptomatic. We analyzed their antibody response to see the trend of the humoral response. Our results showed a significant difference in circulating IgG level among the two groups. The neutralizing antibody response against SARS‐CoV‐2 was significantly higher among those who had severe disease. Furthermore, ten subjects from each group were screened twice, and a declining antibody trend was observed in pauci‐symptomatic individuals. These findings provide evidence that humoral immunity against SARS‐CoV‐2 in pauci‐symptomatic people is weak and may not be long‐lasting. This may have implications for immunity strategy and prevention, since it is still not clear whether a time‐dependent decrease of both circulating and neutralizing antibodies to nonprotective levels could occur in a longer time span and whether potential vaccines are able to induce a herd immunity and a durable response.
Keywords: coronavirus, humoral immunity, neutralization
1. INTRODUCTION
In December 2019, a cluster of patients with pneumonia of unknown cause was identified in Wuhan, Hubei Province, China. 1 On January 7, 2020, China centers for disease control and prevention identified a novel beta‐coronavirus from lower respiratory tract samples of patients with pneumonia. 2 This novel coronavirus was later named “severe acute respiratory syndrome coronavirus‐2” (SARS‐CoV‐2). As an emerging acute respiratory infectious disease, SARS CoV‐2 primarily spreads through the respiratory tract, by droplets, respiratory secretions, and direct contact, 3 with a high human‐to‐human transmissibility.
Most adults or children with SARS‐CoV‐2 infection present mild flu‐like symptoms; only a minority of patients have a severe outcome and rapidly develop acute respiratory distress syndrome, respiratory and multiple organ failure, bleeding and coagulation dysfunction, even death. 4 So far, the golden clinical diagnostic method of COVID‐19 is nucleic acid detection in the nasopharyngeal swab or other lower respiratory tract samplings by real‐time PCR, which can be further confirmed by next‐generation sequencing.
Apart from RT‐PCR testing, serological testing is an additional emerging option in COVID‐19 diagnostics 5 primarily as a proof of past infection but also to support the diagnosis of suspected COVID‐19 patients. 6 , 7 Serological assays for the evaluation of the humoral responses against Spike (S) and Nucleoprotein (N) in COVID‐19 patients have been assessed, because of their high immunogenicity. Spike plays an important role in viral binding and entry into target cells, 8 while the Nucleoprotein in viral replication and assembly. 9 The kinetics of anti‐N response has been described as similar to that of the anti‐S, although N responses might appear earlier. 7 Anti‐SARS‐CoV‐2 antibody titers seem to correlate with disease severity, likely reflecting higher viral replication rates and/or immune activation in patients with severe outcome. 10
In hospitalized patients, seroconversion is typically detected between 5 and 14 days postsymptoms onset, with a median time of 5–12 days for anti‐S immunoglobulin M and 14 days for immunoglobulin G (IgG), and immunoglobulin A. 6 , 7 , 11 , 12
Neutralizing antibodies have been detected in symptomatic individuals 13 , 14 and their potency seems to be associated with high levels of circulating antibodies. On the other hand, despite representing the majority of SARS‐CoV‐2 infections, asymptomatic infections are currently poorly documented 15 and whether this immunity is mediated by neutralizing antibodies remains an outstanding question. 16
Moreover, it is still unknown how long SARS‐CoV‐2 infected subjects could maintain long‐term immunity and long‐lasting protective antibodies, regardless of the outcome.
In this study, we measured the circulating IgG antibody levels in 60 subjects with a certified history of SARS‐CoV‐2 infection, by using three different assays based on different methods. Subjects were equally divided into two groups: those hospitalized, who had a severe outcome, and those pauci‐symptomatic. We analyzed their antibody response to see the trend of the humoral response in individuals with different disease outcomes. Moreover, 10 patients of each group were screened a second time to evaluate the persistence of anti‐SARS‐CoV‐2 antibody 2 months after symptoms onset.
2. MATERIALS AND METHODS
2.1. Study design and participants
The participants in this study were subjects with an assessed history of SARS‐CoV‐2 infection, between March and May 2020. Half of them were hospitalized in “Santa Maria alle Scotte” University Hospital, in Siena with a severe outcome. Instead, the other half consisted of pauci‐symptomatic subjects reporting mild signs compatible with COVID‐19 (fever, cough), who were placed in isolation at home. All infections were confirmed by RT‐qPCR Test (nasopharyngeal swab) (in case of current infection) and/or by serological testing (for past infections). This research was carried out according to the principles of Helsinki declaration, with reference to BIOBANK‐MIU‐2010 document approved by the Ethics Committee with amendment No 1, on February 17, 2020 in terms of General Data Protection and Regulation. A total of 60 subjects, 30 who had a severe outcome and 30 who had mild infection were screened for the presence of anti‐SARS‐CoV‐2 IgG antibodies. Moreover, 10 subjects selected from each group were screened twice, respectively, 30 and 60 days after symptoms, to evaluate the trend of their immune response.
2.2. Cells and viruses
Vero E6 cells (ATCC CRL‐1586M) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Euroclone) supplemented with 100 U/ml penicillin/streptomycin and 5% heat‐inactivated fetal calf serum (Euroclone) at 37°C in a humidified 5% CO2 atmosphere. SARS‐CoV‐2 virus (SARS‐CoV‐2/human/ITA/Siena‐1/2020; GenBank: MT531537.2), isolated from a COVID infected patient in the Virology lab at “S. Maria alle Scotte” Hospital, was propagated on Vero E6 cells until a cytopathic effect (CPE) appeared. Viral stocks were prepared, titrated on Vero E6 cells, and stored at −80°C.
2.3. SARS‐CoV‐2 IgG antibody detection
Subjects' sera were analyzed using two separate immunoassays. The Abbott SARS‐CoV‐2 IgG chemiluminescent microparticle immunoassay (CMIA) (Abbott Laboratories) was performed on an Abbott Architect i2000 (Abbott Diagnostics) according to the manufacturer's instructions. This method is a qualitative assay that detects IgG binding to an undisclosed epitope of the SARS‐CoV‐2 nucleocapsid protein, with the results expressed as relative light units. The other assay was the Enzywell SARS‐CoV‐2 IgG (DIESSE Diagnostica Senese; Monteriggioni), an enzyme‐linked immunosorbent assay (ELISA)‐based 96‐well plate format assay which detects anti‐SARS‐CoV‐2 IgG directed against the inactivated native virus, with the result given in optical density at 450 nm. The final interpretation of positivity was determined by the ratio above a threshold value, with positive ratio ≥1.4 or negative ratio less than 1.4 for CMIA assay, and a positive ratio ≥1.1, borderline ratio more than 0.9 and less than 1.1, or negative ratio less than 0.9 for the ELISA. Each value represented the mean of triplicate determinations.
2.4. SARS‐CoV‐2 microneutralization test
SARS‐CoV‐2 virus neutralization assay was carried out on Vero E6 cells in a 96‐well microplate. Twenty‐five microliters of twofold serial dilutions (1:8–1:1024) of sera samples were added to an equal volume of the SARS‐CoV‐2 strain containing 150 TCID50 and incubated for 90 min at 37°C. Finally, 50 μl of Vero E6 cells suspension (2 × 105 cells/ml) prepared in complete DMEM were added to each well. After incubation at 37°C, the cultures were daily examined at the microscope (Olympus IX51) for the presence of the CPE. The 50% end point titer was calculated using the Reed‐Muench method. 17 A positive and negative control serum were included in each assay. Geometric mean titers (GMTs) of the neutralization assay were calculated.
2.5. Statistical analysis
The differences between age, time of blood sample collection, circulating IgG levels, and neutralizing titers were evaluated and the statistical significances assessed with two‐tailed χ 2 test. Results were considered statistically significant at p < .05. Spearman's rank correlation coefficient was used to assess correlations of log‐transformed continuous variables between the groups.
3. RESULTS
3.1. Study group
We analyzed sera from 60 subjects with a certified history of SARS‐CoV‐2 infection. Half of them had a severe infection and needed hospital recovery (H) and the other half was pauci‐symptomatic with mild signs (fever and/or cough). Mean age was 66.1 years for the hospitalized (95% confidence interval [CI]: 61.0–71.0) and 45.0 for the pauci‐symptomatic (95% CI: 38.6–51.4) subjects (p < .0001). All these subjects were screened for the presence of specific anti‐SARS‐CoV‐2 IgG antibodies either by indirect ELISA or CMIA. Blood samples were collected about 30 days since symptoms onset (T0). Finally, only 10 subjects of each group were screened twice (T0 = average 30 days; T1 = average 60 days) for the presence of both circulating IgG levels and specific anti‐SARS‐CoV‐2 neutralizing antibodies.
3.2. Anti‐SARS‐CoV‐2 specific IgG
Results obtained by ELISA and CMIA, respectively, showed a significant difference in circulating IgG level among patients with a severe outcome and those with mild symptoms at T0 (n = 30; 7.31 vs. 4.06; p = .0018 and 6.21 vs. 4.95; p = .048) (Table 1A).
Table 1.
Serological analysis of the study population according to the clinical outcome in subjects screened once (Table 1A) and twice (Table 1B)
| Severe cases (H) | Pauci‐symptomatic (P) | |
|---|---|---|
| Table 1A | T0 (30) | T0 (30) |
| CMIA IgG (S/SCO) | 6.21 (5.47–6.95) | 4.95 (3.97–5.93) |
| ELISA IgG (AU) | 7.31 (5.82–8.80) | 4.06 (2.81–5.31) |
| NT Ab (GMT) | 87.7 (54.9–121.0) | 23.3 (10.4–36.2) |
| Severe cases (H) | Pauci‐symptomatic (P) | |||
|---|---|---|---|---|
| Table 1B | T0 (10) | T1 (10) | T0 (10) | T1 (10) |
| CMIA IgG (S/SCO) | 5.63 (4.06–7.22) | 5.54 (4.19–6.89) | 6.46 (5.23–7.69) | 4.94 (3.61–6.27) |
| ELISA IgG (AU) | 6.16 (3.31–9.01) | 6.28 (3.68–8.88) | 5.01 (2.49–7.53) | 3.23 (2.01–4.45) |
| NT Ab (GMT) | 65.38 (0–254.0) | 100.51 (14.6–186.0) | 29.21 (8.61–49.8) | 21.52 (0–45.4) |
Note: 95% CI values were indicated below each result.
Abbreviations: AU, arbitrary units; CI, confidence interval; GMT, geometric mean titer; S/SCO, signal/signal cut off.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Concerning those subjects who were screened twice (n = 10+10), no significant differences in IgG levels were found between the two samplings (T0, T1) of both the groups, using both ELISA and CMIA (p > .05), although a lower IgG level was noticed among those with mild symptoms (Table 1B).
On the contrary, regarding the neutralizing activity, an evident GMT difference was found between the two groups at T0 (Figure 1); indeed, a higher titer was present in severe cases in comparison with those having mild disease (87.7 vs. 23.3; p = .0002). This difference was also confirmed for the patients tested twice (p = .046), although no significant difference in neutralizing antibody titer was found between the first and the second samples drawn 1 month apart from the same subjects, probably due to the limited number of samples (Table 1B). It is worthy mentioning that a different trend of antibody response was observed in the H group, where the tendency of neutralizing antibodies was increasing over time, while it was decreasing in pauci‐symptomatic individuals.
Figure 1.
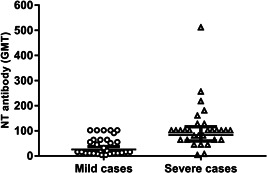
Differences in neutralizing antibody titers between SARS‐CoV‐2 infected patients with mild or severe outcome. The whiskers represent the values from the 5th to the 95th percentiles; the GMTs are depicted by the horizontal lines in the boxes. Individual data points are shown. The p value of the GMT between the two groups is 0.0002. GMT, Geometric mean titer; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
3.3. Neutralizing antibody titer and correlation with circulating IgG levels
We correlated the IgG titers obtained in the two serological assays, ELISA and CMIA, to the neutralizing antibody titers, to evaluate whether circulating IgG antibody levels could partly be associated to a neutralizing activity. As expected, we observed a moderate positive correlation between the neutralizing response and circulating IgG by using the whole virus proteins‐based ELISA (r = .60) and a weak correlation using SARS CoV‐2 N antigen‐based CMIA (r = .44) (data not shown).
4. DISCUSSION
In this study, we analyzed the titer of anti‐SARS‐CoV‐2 IgG antibodies with three different serological assays in a cohort of subjects with a certified history of COVID‐19, equally distributed with a severe outcome or mild symptoms.
Despite the limited number of subjects, the most remarkable finding of this study was the significantly lower antibody titer in patients who experienced mild infection with respect to those affected by a severe respiratory syndrome. Both ELISA and CMIA, although based on different antigens, such as all virus proteins in the first assay and the nucleoprotein in the second one, showed an antibody response, which was significantly higher in patients with severe disease than in pauci‐symptomatic subjects in the same time frame since symptoms onset.
Previous studies on humoral response in SARS and MERS demonstrated that the humoral response could wane over time. 18 , 19 We do not know how long this immunity could last in individuals affected by COVID‐19. 20 However, although the decay of total specific IgG was similar in both the groups, we noticed that the neutralizing antibodies, representing the protective response, only raised in severe cases 2 months after symptoms onset. On the contrary, the neutralizing response was very low in pauci‐symptomatic individuals (GMT: 29.2) with an evident decrease after 2 months (GMT: 21.52). These data raise concern that humoral immunity against SARS‐CoV‐2 may not be long‐lasting in people with mild illness, threatening their protective status. Moreover, neutralizing antibody titers from all study subjects did not show a good correlation with the level of circulating IgG antibodies evaluated in ELISA or CMIA. In particular, only a modest correlation (r = .60) was found with ELISA values, while weak correlation (r = .44) was shown with CMIA. This can be easily explained on the basis of the antigen used in each test. Indeed, ELISA was based on all the viral proteins, including the Spike protein, responsible for cell binding to the receptor and containing the sequence recognized by neutralizing antibodies. CMIA was only using the immunogenic nucleoprotein, to which the humoral response is promptly mounted in the host, but it is not involved in the neutralizing activity. The observational time considered in this study was quite short, but the preliminary results indicated that a part of the population, particularly young people who presented a very mild disease, developed a weak humoral response, mainly characterized by a low neutralizing activity that could wane over time. For this reason, patients with high levels of circulating antibodies, especially those who had a severe outcome, could be more likely protected, while subjects with a favorable outcome, who showed low levels of neutralizing antibodies, may not maintain a long‐lasting response and be susceptible to reinfection. Therefore, it could be important to keep monitoring both kind of subjects and see whether a time‐dependent decrease of both circulating and neutralizing IgG antibodies to nonprotective levels could occur in a longer time course.
This finding may have implications for immunity strategy and prevention, since it is still not clear whether the immunity is long‐lasting and the potential vaccines, based on the spike antigen, are able to induce a durable response and herd immunity. Further studies are necessary to understand the role of cellular immune response and identify the correlates of protection for COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Maria Grazia Cusi. Data curation: Maria Grazia Cusi, Gabriele Anichini. Investigation: Gianni Gori Savellini. Methodology: Gabriele Anichini, Claudia Gandolfo, Chiara Terrosi and Shibily Prathyumnan. Resources: Simonetta Fabrizi, Giovanni Battista Miceli and Federico Franchi. Supervision: Maria Grazia Cusi. Writing—original draft: Gabriele Anichini. Writing—review and editing: Maria Grazia Cusi. All authors revised and approved the final version of the manuscript.
Anichini G, Gandolfo C, Terrosi C, et al. Antibody response to SARS‐CoV‐2 in infected patients with different clinical outcome. J Med Virol. 2021;93:2548–2552. 10.1002/jmv.26789
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X, Wang W, Zhao X, et al. Transmission dynamics and evolutionary history of 2019‐nCoV. J Med Virol. 2020;92:501‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 92, 2020:401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel R, Babady E, Theel ES, et al. Report from the American Society for Microbiology COVID‐19 International Summit, 23 March 2020: value of diagnostic testing for SARS‐CoV‐2/COVID‐19. mBio. 2020;11:e00722‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020;71:778‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease. Clin Infect Dis. 2020:28ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nat Commun. 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeng W, Liu G, Ma H, et al. Biochemical characterization of SARS‐CoV‐2 nucleocapsid protein. Biochem Biophys Res Commun. 2020;527:618‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26:845‐848. [DOI] [PubMed] [Google Scholar]
- 11. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat Med. 2020;26:1033‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okba NMA, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2‐specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. [DOI] [PubMed] [Google Scholar]
- 14. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. medRxiv. 2020. 10.1101/2020.03.30.20047365 [DOI] [Google Scholar]
- 15. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS‐CoV‐2). Science. 2020;368:489‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grzelak L, Temmam S, Planchais C, et al. A comparison of four serological assays for detecting anti‐SARS‐CoV‐2 antibodies in human serum samples from different populations. Sci Transl Med. 2020;12(559). 10.1101/2020.04.21.20068858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493‐497. [Google Scholar]
- 18. Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS‐associated coronavirus after recovery. N Engl J Med. 2007;357:1162‐1163. [DOI] [PubMed] [Google Scholar]
- 19. Chang SC, Wang JT, Huang LM, et al. Longitudinal analysis of Severe Acute Respiratory Syndrome (SARS) coronavirus‐specific antibody in SARS patients. Clin Diagn Lab Immunol. 2005;12:1455‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of Anti‐SARS‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med. 2020;383:1085‐1087NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]


