Abstract
The disease spectrum of coronavirus disease 2019 (COVID‐19) varies from asymptomatic infection to critical illness and death. Identification of prognostic markers is vital for predicting progression and clinical practice. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) RNA, known as RNAemia, has been detected in the blood. However, the potential clinical value of SARS‐CoV‐2 RNAemia remains unknown. We, therefore, conducted a meta‐analysis using a random‐effects model to estimate the pooled prevalence of SARS‐CoV‐2 RNAemia as well as summary strength of RNAemia in association with disease severity and unfavorable clinical outcomes. A total of 21 studies involving 2181 patients were included. SARS‐CoV‐2 RNAemia in COVID‐19 patients varied from 9.4% to 74.1%, with a pooled estimate of 34% (95% confidene interval [CI]: 26%–43%). Overall, SARS‐CoV‐2 RNAemia was associated with COVID‐19 severity with odds ratio (OR) of 5.43 (95% CI: 3.46–8.53). In addition, SARS‐CoV‐2 RNAemia was a significant risk factor for unfavorable clinical outcomes (OR = 6.54, 95% CI: 3.82–11.21). The summary OR was 4.28 (95% CI: 2.20–8.33) for intensive care unit (ICU) admission, 11.07 (95% CI: 5.60–21.88) for mortality. Furthermore, RNAemia was also a significant risk factor for invasive mechanical ventilation and multiple organ failure. SARS‐CoV‐2 RNAemia is associated with disease severity, ICU admission, death in COVID‐19, and may serve as a clinical predictor. More prospective trials in evaluating the potential of SARS‐CoV‐2 RNAemia as a prognostic indicator are necessary.
Keywords: clinical severity, COVID‐19, SARS‐CoV‐2 RNAaemia, unfavorable outcome
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), originally reported from Wuhan, China, has spread globally in a very short period. 1 As of December 17, 2020, there were over 75 million confirmed cases and 1.6 million deaths. 2 Deep sequencing revealed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was responsible for the development of the COVID‐19 pandemic. 3 Although most patients present mild‐to‐moderate symptoms, including fever, dry cough, and fatigue, 5%–10% progress to a severe or critical disease characterized by pneumonia and respiratory failure, and death occurred in 2%–5% of the cases. 4
SARS‐CoV‐2 RNA can be detected in nasopharyngeal (NP)/throat swab, sputum, respiratory tract, peripheral blood, serum, feces, urine, and tear by polymerase chain reaction (PCR)‐based methods. 5 , 6 , 7 , 8 , 9 Viral load by sample type is indicative of virus replication and clearance and is routinely used to monitor disease progression, response to antiviral agents, and relapse. 10 Since the lungs are most often affected and viral RNA is commonly detected in NP swabs, several studies have investigated viral loads in samples from the upper respiratory tract as a biomarker for severity assessment. Zou et al.reported that the viral load was similar among asymptomatic patients and symptomatic patients. 11 Metagenomic sequencing of NP swabs from COVID‐19 patients with different severity indexes suggested that increased SARS‐CoV‐2 RNA detection is associated with the early stages rather than disease severity. 12 These results suggest that viral load in respiratory samples cannot be considered as a prognostic indicator for severe or critically ill cases. Currently, the relationship between viral load dynamics in samples from extrapulmonary sites (fecal, tear, and urinary samples) and disease severity remains unknown.
Recently, coagulopathy has been reported in severe COVID‐19 cases, 13 , 14 implying the spread of SARS‐CoV‐2 within extrapulmonary sites via blood flow. In addition to the difficulty of SARS‐CoV‐2 virus culture from the blood, 15 serum SARS‐CoV‐2 nucleic acid (RNAemia) represents a practical and powerful approach to evaluate the impact of viral load dynamics on disease severity in extrapulmonary sites. Increasing evidence addressing SARS‐CoV‐2 RNAemia and disease progression are available; a definite conclusion has not yet been reached. Therefore, we aimed to establish a comprehensive picture of the association between RNAemia and disease severity as well as unfavorable outcomes, including intensive care unit (ICU) admission, invasive mechanical ventilation (IMV), and death.
2. MATERIALS AND METHODS
2.1. Identification and eligibility of relevant studies
RNAemia was defined as the presence of viral RNA, above the technical limits of detection of PCR‐based assays, in the blood, serum, or plasma. To identify studies addressing SARS‐CoV‐2 RNAemia and outcome in patients with COVID‐19, a systematic literature search from December 1, 2019 to December 24, 2020 was conducted in PubMed, MedRxiv, BioRxiv, Embase, Cochrane Library, and Chinese National Knowledge Infrastructure without any restriction. The search term included keywords relevant to SARS‐CoV‐2 RNAemia (e.g., “SARS‐CoV‐2 RNAemia,” “RNAemia,” and “viral load”) in combination with words related to the clinical outcome (e.g., “severity,” “mild,” “moderate,” “severe,” “critical,” “IMV,” “Invasive mechanical ventilation,” “ICU,” “intensive care unit,” “death,” and “mortality”) and the blood (“whole blood,” “serum,” and “plasma”). The titles and abstracts from retrieved articles were screened to determine their relevance and the references from included studies were scrutinized and hand‐searched for additional eligible studies.
Eligible studies were required to meet the following criteria: (1) clinical study evaluated the association between SARS‐CoV‐2 RNAemia and COVID‐19 severity or outcomes; (2) original articles reported independent data; (3) reported relative risks with 95% confidence intervals (CIs) or sufficient information for effect size calculation. Studies with fewer than 10 patients; studies only examined samples other than the blood (e.g., digestive tract, feces, and respiratory samples) were excluded.
2.2. Data extraction
Information with regard to authorship, publication year, country, study design, numbers of patients, age, gender distribution, SARS‐CoV‐2 RNAemia rate, unfavorable outcomes (ICU admission and/or death), severity (mild, moderate, severe, and critical illness), IMV, multiple organ failure (organ ≥ 2), viral RNAemia, SARS‐CoV‐2 detection method, variables adjusted for in the multivariable analysis, and risk estimates with corresponding 95% CIs was summarized independently by two reviewers according to a fixed protocol. Inconsistency from data reports was resolved by further discussion among all authors through consensus.
2.3. Statistical analysis
The strength of the association between the presence of SARS‐CoV‐2 RNAemia and clinical severity or outcomes was estimated using odds ratios (ORs), with the corresponding 95% CIs. Hazard ratio (HR), relative risk (RR), and rate ratio were treated as equivalent estimates of OR since the unfavorable outcome of patients with COVID‐19 is relatively rare. 16 The association of SARS‐CoV‐2 RNAemia was first compared between different clinical severity (mild/moderate vs. severe/critical). Then we examined the association between SARS‐CoV‐2 RNAemia and adverse clinical outcomes (ICU admission and/or death). Stratified meta‐analyses based on country (East Asian vs. Western country) and study design (prospective vs. retrospective) were also performed. We calculated the prevalence of SARS‐CoV‐2 RNAemia from individual studies and then pooled to estimate the overall prevalence. Random effects model which takes into account the variation between studies was adopted to calculate pooled effect estimates. 17 Cochran's Q test and I 2 index were calculated to explore heterogeneity across studies. 18 To assess the stability of the results, a sensitivity analysis was performed by removing each individual study in turn from the total and reanalyzing the remaining studies. Egger's tests and Begg's tests were used to identify potential publication bias and small studies effect. 19 , 20 Type I error rate was set at 0.05 for two‐sided analysis. All statistical analyses were done using the STATA software (version 11.0).
3. RESULTS
3.1. Study characteristics
The search identified 2837 publications, and 21 studies involving 2182 patients were finally included 5 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 (Figure 1). A retrospective study design was used in the majority of included studies (17/21). As for method used for SARS‐CoV‐2 RNA detection, 17 of included studies employed real‐time reverse transcriptional polymerase chain reaction (RT–PCR) and 4 studies employed droplet digital PCR (ddPCR). Nine studies were conducted in East Asian and 12 in Western countries. Characteristics of the included studies are shown in Table 1.
Figure 1.
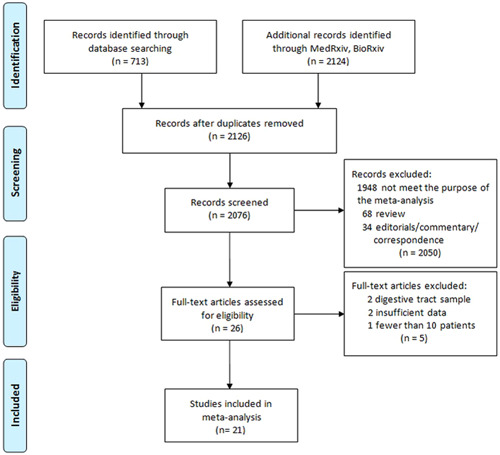
Flowchart of study selection
Table 1.
Characteristics of the included studies
| Study | Sample size | Country | Sample source | Detection method (primers) | Study design | RNAemia positive rate (%) |
|---|---|---|---|---|---|---|
| Bermejo‐Martin et al. 21 | 250 | Spain | Plasma | ddPCR (N) | Cross‐sectional | 42.8 |
| Berastegui‐Cabrera et al. 22 | 72 | Spain | Plasma | RT‐PCR (ORF, E, N) | Prospective cohort | 15.3 |
| Hogan et al. 23 | 85 | USA | Plasma | RT‐PCR (E) | Cross‐sectional | 32.9 |
| Xu et al. 24 | 85 | China | Plasma | RT‐PCR (ORF1ab/N) | Retrospective cohort | 37.6 |
| Veyer et al. 25 | 58 | France | Plasma | ddPCR (ORF1ab/N) | Cross‐sectional | 74.1 |
| Andersson et al. 26 | 212 | UK | Plasma | RT‐PCR (ORF1b/N) | Retrospective cohort | 12.7 |
| Prebensen et al. 27 | 123 | Norway | Plasma | RT‐PCR (E) | Prospective cohort | 39.0 |
| Chen et al. 28 | 48 | China | Serum | RT‐PCR (ORF1ab/N) | Retrospective | 10.4 |
| Hagman et al. 29 | 167 | Sweden | Serum | RT‐PCR (ORF1,E, RdRp) | Retrospective cohort | 36.5 |
| Huang et al. 30 | 41 | China | Plasma | RT‐PCR (E) | Prospective cohort | 14.6 |
| Chen et al. 31 | 97 | China | Plasma | RT‐PCR (ORF, E, N) | Retrospective cohort | 34.0 |
| Fang et al. 32 | 32 | China | Blood | RT‐PCR (CDC primers) | Retrospective | 71.9 |
| Chen et al. 33 | 57 | China | Blood | RT‐PCR (ORF1ab/N) | Retrospective cohort | 10.5 |
| Zheng et al. 5 | 96 | China | Serum | RT‐PCR (ORF1ab) | Retrospective cohort | 41.1 |
| Mancuso et al. 34 | 22 | Italy | Serum | ddPCR (CDC primers) | Retrospective | 36.4 |
| Eberhardt et al. 35 | 32 | Germany | Serum | RT‐PCR (ORF1ab/E) | Retrospective cohort | 43.7 |
| Kawasuji et al. 36 | 56 | Japan | Serum | RT‐PCR (N) | Retrospective cohort | 19.6 |
| van Riel et al. 37 | 20 | Netherlands | Serum | RT‐PCR (E) | Retrospective | 70.0 |
| Ram‐Mohan et al. 38 | 191 | USA | Plasma | ddPCR, RT‐PCR (N) | Prospective | 23.0 |
| Ramírez et al. 39 | 203 | Spain | Serum | RT‐PCR (ORF1ab/E) | Retrospective cohort | 62.6 |
| Lei et al. 40 | 234 | China | Blood | RT‐PCR (ORF1ab/N) | Retrospective | 9.4 |
Abbreviations: ddPCR, droplet digital polymerase chain reaction; RT‐PCR, real‐time polymerase chain reaction.
3.2. Association between SARS‐CoV‐2 RNAemia and disease severity
The positive rate of serum SARS‐CoV‐2 viral RNA varied from 9.4% to 74.1%, with a pooled estimate of 34% (95% CI: 26%–43%, Figure 2). Significant heterogeneity (p < 10−5, I 2 = 95.6%) was detected among included studies, indicating a significant difference across different studies. Overall, SARS‐CoV‐2 RNAemia was associated with COVID‐19 severity with OR of 5.43 (95% CI: 3.46–8.53, p < 10−5; Figure 3). The data on age distribution were reported in four studies; individuals with RNAemia were significantly older than those with undetectable SARS‐CoV‐2 RNA in a combined analysis (p < .01). When stratified by study country and study design, significant results were maintained (Table 2).
Figure 2.
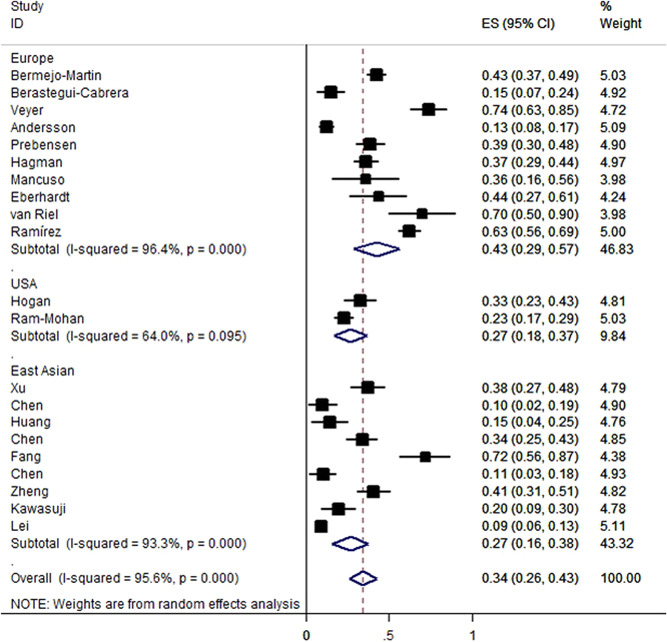
Prevalence of SARS‐CoV‐2 RNAemia in COVID‐19 patients in different countries. Each box represents the prevalence point estimate, and its area is proportional to the weight of individual study. COVID‐19, coronavirus disease 2019; SARS‐CoV‐2 RNAemia, severe acute respiratory syndrome coronavirus 2 RNA
Figure 3.
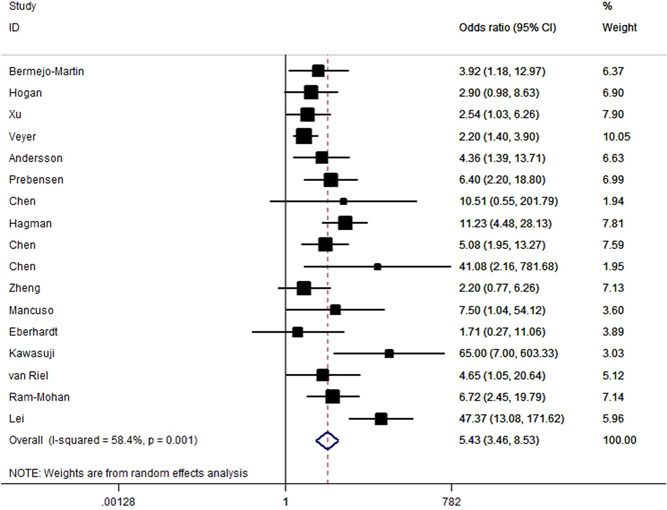
Risk for disease severity in patients with SARS‐CoV‐2 RNAemia versus patients without detectable SARS‐CoV‐2 RNA. Each box represents the OR point estimate, and its area is proportional to the weight of the individual study. OR, odds ratio; SARS‐CoV‐2 RNAemia, severe acute respiratory syndrome coronavirus 2 RNA
Table 2.
Overall and stratified analyses of RNAemia and COVID‐19 severity and clinical outcomes
| Overall and subgroup analysis | Disease severity | Unfavorable outcome | ICU admission | Mortality | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies/patients | OR (95% CI) | p | P(Q) | I 2 | No. of studies/patients | OR (95% CI) | p | P(Q) | I 2 | No. of studies/ICU patients | OR (95% CI) | p | P(Q) | I 2 | No. of studies/deaths | OR (95% CI) | p | P(Q) | I 2 | |
| Overall | 17/1833 | 5.43 (3.46–8.53) | <10−5 | 0.001 | 58 | 13/1252 | 6.54 (3.82–11.21) | <10−5 | 0.03 | 46 | 7/215 | 4.28 (2.20–8.33) | <10−4 | 0.07 | 49 | 8/100 | 11.07 (5.60–21.88) | <10−5 | 0.81 | 0 |
| Country | ||||||||||||||||||||
| East Asian | 7/673 | 9.30 (3.21–26.96) | <10−5 | 0.001 | 74 | 5/262 | 7.79 (2.11–28.78) | 0.002 | 0.05 | 57 | 3/33 | 5.60 (0.58–54.43) | 0.14 | 0.02 | 76 | 3/19 | 10.03 (3.33–30.23) | <10−4 | 0.68 | 0 |
| Western country | 10/1160 | 4.32 (2.84–6.56) | <10−5 | 0.15 | 32 | 8/990 | 5.94 (3.36–10.51) | <10−5 | 0.10 | 42 | 4/182 | 3.37 (2.16–5.27) | <10−5 | 0.47 | 0 | 5/81 | 11.76 (4.94–27.98) | <10−5 | 0.57 | 0 |
| Study design | ||||||||||||||||||||
| RE | 15/1519 | 5.35 (3.20–8.96) | <10−5 | 0.001 | 62 | 10/1016 | 7.26 (3.77–13.99) | <10−5 | 0.03 | 51 | 5/192 | 4.47 (2.10–9.52) | <10−4 | 0.07 | 53 | 7/93 | 11.07 (5.26–23.32) | <10−5 | 0.72 | 0 |
| PR | 2/314 | 6.56 (3.10–13.87) | 0.001 | 0.95 | 0 | 3/236 | 5.01 (1.56–16.10) | 0.007 | 0.13 | 51 | 2/23 | 3.42 (0.42–27.89) | 0.25 | 0.07 | 68 | 1/7 | 11.05 (2.04–59.86) | 0.005 | NA | NA |
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; NA, not available; PR, prospective; RE, retrospective.
3.3. Association between SARS‐CoV‐2 RNAemia and clinical outcomes
We found that unfavorable clinical outcomes are significantly more likely to occur in patients with SARS‐CoV‐2 RNAemia compared with those with undetectable SARS‐CoV‐2 RNA (OR = 6.54, 95% CI: 3.82–11.21, p < 10−5; Figure 4). ORs for SARS‐CoV‐2 RNAemia positivity were 4.28 (95% CI: 2.20–8.33; Figure 5) for ICU admission and 11.07 (95% CI: 5.60–21.88; Figure 6) for all‐cause mortality. When analyzed according to country and study design, significant associations were detected almost in all comparisons (Table 2). IMV was used significantly more frequently in individuals with RNAemia compared with those with undetectable SARS‐CoV‐2 RNA (OR = 8.25, 95% CI: 2.98–22.80, p < 10−4; P heterogeneity = 0.68, I 2 = 0%, Figure S1). By combining 2 available studies, a significantly increased risk of multiple organ failure was observed among patients with RNAemia (OR = 7.33, 95% CI: 2.46–21.88, p < 10−4; P heterogeneity = 0.57, I 2 = 0%).
Figure 4.
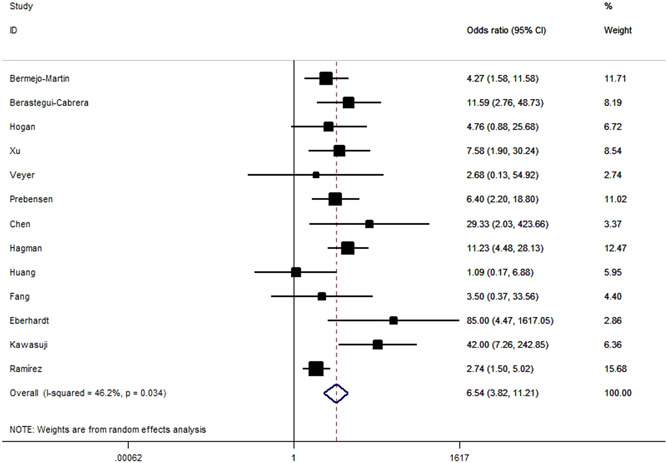
Risk for unfavorable outcomes in patients with SARS‐CoV‐2 RNAemia versus patients without detectable SARS‐CoV‐2 RNA. SARS‐CoV‐2 RNA, severe acute respiratory syndrome coronavirus 2 RNA
Figure 5.
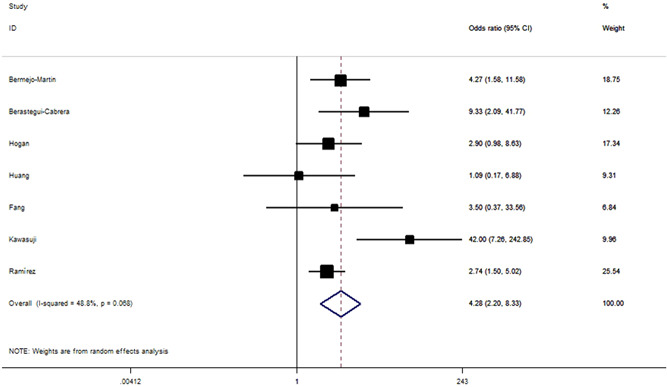
Odds ratios for ICU admission between the patient with and without SARS‐CoV‐2 RNAemia. ICU, intensive care unit; SARS‐CoV‐2 RNAemia, severe acute respiratory syndrome coronavirus 2 RNA
Figure 6.
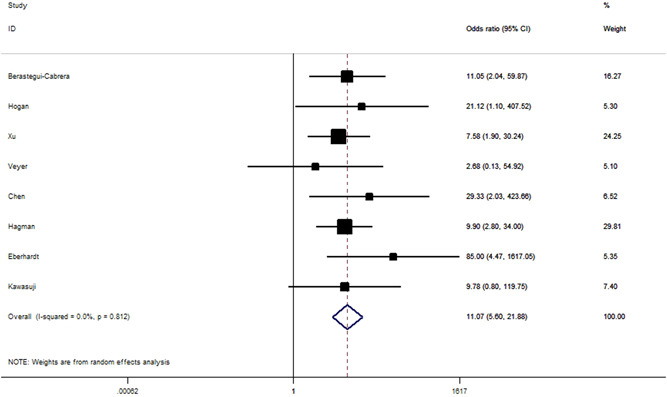
Odds ratios for all‐cause mortality between the patient with and without SARS‐CoV‐2 RNAemia. SARS‐CoV‐2 RNA, severe acute respiratory syndrome coronavirus 2 RNA
3.4. Assessment of heterogeneity
There was statistical heterogeneity in comparison (Table 2) and the study by Lei et al. was identified as the main source of between‐studies heterogeneity in sensitivity analyses. As for limited data, meta‐regression could not be performed to assess the correlation between SARS‐CoV‐2 RNAemia and disease severity or clinical outcomes.
3.5. Sensitivity analyses and publication bias
A single study involved in the meta‐analysis was deleted each time to reflect the influence of the individual data set to the pooled ORs, and the corresponding pooled ORs were not qualitatively altered. No publication bias (p > .05 for all) was observed for this overall meta‐analysis (Figures S2 and S3).
4. DISCUSSION
To our knowledge, this is the first meta‐analysis to explore the association of SARS‐CoV‐2 RNAemia and disease progression of COVID‐19. Patients with positive SARS‐CoV‐2 RNAemia have a greater risk for COVID‐19 severity, especially for ICU admission and mortality. These findings are also in line with SARS and Middle East respiratory syndrome (MERS), in which detection of the virus in serum correlated with adverse clinical outcome. 41 , 42
There is inconsistency in the positive rate of SARS‐CoV‐2 RNAemia among published data, whereas some authors reported 0%–1% of RNAemia. 1 , 11 Here are several explanations to interpret the abovementioned phenomenon. First, clinical characteristics (e.g., the number of patients with severe/critically illness, and age) may attribute to these different results since SARS‐CoV‐2 RNAemia occurred more frequently in older age and severe cases. Second, differences in sample collection protocols (e.g., at hospital admission, after confirmed COVID‐19 cases, several days before or after the NP collection or symptom) may also have affected the results, as an optimal period for blood sampling remain unknown. Third, differences in the sensitivity and/or specificity of the analytical techniques (ddPCR or RT‐PCR target to N, S, E genes or ORF1ab region), or sample size and preparation (plasma, serum, and whole blood) may also have affected the results. 39
In our pooled analysis, we found that RNAemia is associated with a higher risk for ICU admission, IMV, and death in COVID‐19 patients. This would be clinically important because insufficient ICU resource and IMV facility are currently available worldwide, besides the limited manufacturing capacity during pandemic in a short period. Risk factors, including age, smoking, and comorbidities, have been identified, while these factors seemed unable to reflect the severity or progression of the disease or replication level of the virus. 43 , 44 , 45 Indeed, there is an urgent need for a robust COVID‐19 clinical scoring system, and RNAemia reflecting the potential capability of the virus may be considered as a prognostic indicator for the early identification of individuals likely to develop severe/critical COVID‐19. 23
Bouadma et al. reported that a patient with positive SARS‐CoV‐2 RNAemia developed multiorgan failure and died. 46 Our meta‐analysis also observed RNAemia in COVID‐19 patients with an increased risk of multiple organ failure. These findings are in line with the multiorgan involvement in patients with the critical disease and autopsy data reporting viral infection in several organs, indicating the hematogenic spread of the virus. 47 , 48 Of note, the subgroup analysis considering the interaction between SARS‐CoV‐2 RNAemia and multiple organ failure was performed on the basis of limited data currently available; statistical power for the analyses is limited. Further follow‐up is needed to determine the impact on long‐term organ damage in patients with positive or negative for SARS‐CoV‐2 RNAemia after discharge. Furthermore, whether the detected RNAemia belongs to circulating viral particles and is a marker for an infectious systemic spread of SARS‐CoV‐2 should be examined. 25
Detailed immunopathologic mechanisms underlying the association between RNAemia and adverse outcomes remain a matter of speculation. RNAemia patients presented with endothelial dysfunction (Angiopoien‐2), hypercitokinemia (CCL2, IL‐6), 21 , 27 coagulation activation (d‐Dimer), systemic inflammatory response (CRP, Ferrin), neutrophil degranulation (Myeloperoxidase) as well as tissue damage (LDH) indicates dysregulated host response to infection and thus lead to high mortality rates. 49 , 50 Furthermore, detection of RNAemia in severe COVID‐19 suggests that viral replication is more robust in severe cases and shows the incapability of critically ill patients in viral replication control.
Our study has several limitations. First, our results are based on a number of retrospective literature with different study protocols (e.g., a criterion for ICU admission and IMV, sampling time), and the recall and selection bias might exist. Additional prospective trials are warranted to further evaluate the clinical potential of SARS‐CoV‐2 RNAemia as a prognostic marker for predicting progression and outcomes of COVID‐19. Second, our results were mainly based on unadjusted estimates. Ideally, we would like to pool individual‐level data and conduct a more precise analysis, which could be adjusted for other covariants, such as age, obesity, and other common comorbidities, including cancer, hypertension, diabetes, and cardiovascular diseases. Third, subgroup meta‐analyses were performed on the basis of a fraction of all the possible data, so selection bias may have occurred.
5. CONCLUSIONS
In summary, the findings of this study revealed that SARS‐CoV‐2 RNAemia is a significant risk factor for disease severity and adverse outcomes in COVID‐19, particularly for ICU admission and death. These results suggest that RNAemia may serve as a clinical scoring component for risk stratification, and SARS‐CoV‐2 RNAemia evaluation would be crucial for COVID‐19 patients. To confirm these findings, future studies should involve a prospective design, strict selection of cases, testing protocols, and larger studies of diverse populations.
6. AUTHOR CONTRIBUTIONS
Kefu Tang conceived and designed the study. Kefu Tang, Lei Wu, and Ying Luo contributed to the literature search. Kefu Tang, Lei Wu, Ying Luo, and Bo Gong contributed to data collection. Kefu Tang, Lei Wu, and Ying Luo contributed to data analysis. Kefu Tang and Ying Luo contributed to data interpretation. Kefu Tang, Lei Wu, Ying Luo, and Bo Gong contributed to the figures. Kefu Tang contributed to the writing of the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information.
Tang K, Wu L, Luo Y, Gong B. Quantitative assessment of SARS‐CoV‐2 RNAemia and outcome in patients with coronavirus disease 2019. J Med Virol. 2021;93:3165–3175. 10.1002/jmv.26876
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26876
DATA AVAILABILITY STATEMENT
Data analyzed in this study are openly available in the references 21 , 40 , 45
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronavirus Resource Center. COVID‑19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html
- 3. Chen L, Liu W, Zhang Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 2020;9:313‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS‑CoV‑2 in Zhejiang province, China, January‑March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kashi AH, De la Rosette J, Amini E, Abdi H, Fallah‐Karkan M, Vaezjalali M. Urinary viral shedding of COVID‐19 and its clinical associations: a systematic review and meta‐analysis of observational studies. Urol J. 2020;17(5):433‐441. [DOI] [PubMed] [Google Scholar]
- 7. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta‐analysis. Gastroenterology. 2020;159(1):81‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID‐19) in Hubei Province China. JAMA Ophthalmol. 2020;138(5):575‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814‐1820. [DOI] [PubMed] [Google Scholar]
- 11. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mostafa HH, Fissel JA, Fanelli B, et al. Metagenomic next‐generation sequencing of nasopharyngeal specimens collected from confirmed and suspect COVID‐19 patients. mBio. 2020;11(6):e01969‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med. 2020;58(7):1116‐1120. [DOI] [PubMed] [Google Scholar]
- 14. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang W, Du RH, Li B, Zheng, X‑S , et al. Molecular and serological investigation of 2019‑nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aune D, Chan DS, Greenwood DC, et al. Dietary fiber and breast cancer risk: a systematic review and meta‐analysis of prospective studies. Ann Oncol. 2012;23:1394‐1402. [DOI] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ. AltmanDG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088‐1101. [PubMed] [Google Scholar]
- 21. Bermejo‐Martin JF, González‐Rivera M, Almansa R, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID‐19. Crit Care. 2020;24(1):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berastegui‐Cabrera J, Salto‐Alejandre S, Valerio M, et al. SARS‐CoV‐2 RNAemia is associated with severe chronic underlying diseases but not with nasopharyngeal viral load. J Infect. 2020:S0163‐4453(20):30719‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hogan CA, Stevens BA, Sahoo MK, et al. High frequency of SARS‐CoV‐2 RNAemia and association with severe disease. Clin Infect Dis. 2020:ciaa1054. 10.1093/cid/ciaa1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu D, Zhou F, Sun W, et al. Relationship between serum SARS‐CoV‐2 nucleic acid (RNAemia) and organ damage in COVID‐19 patients: a cohort study. Clin Infect Dis. 2020:ciaa1085. 10.1093/cid/ciaa1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veyer D, Kernéis S, Poulet G, et al. Highly sensitive quantification of plasma severe acute respiratory syndrome coronavirus 2 RNA sheds light on its potential clinical value. Clin Infect Dis. 2020:ciaa1196. 10.1093/cid/ciaa1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersson MI, Arancibia‐Carcamo CV, Auckland K, et al. SARS‐CoV‐2 RNA detected in blood products from patients with COVID‐19 is not associated with infectious virus. Wellcome Open Res. 2020;5: 181 10.12688/wellcomeopenres.16002.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prebensen C, Myhre PL, Jonassen C, et al. SARS‐CoV‐2 RNA in plasma is associated with ICU admission and mortality in patients hospitalized with COVID‐19. Clin Infect Dis. 2020:ciaa1338. 10.1093/cid/ciaa1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen X, Zhao B, Qu Y, et al. Detectable serum SARS‐CoV‐2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL‐6) level in critically ill COVID‐19 patients. Clin Infect Dis. 2020;71(8):1937‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hagman K, Hedenstierna M, Gille‐Johnson P, et al. SARS‐CoV‐2 RNA in serum as predictor of severe outcome in COVID‐19: a retrospective cohort study. Clin Infect Dis. 2020:ciaa1285. 10.1093/cid/ciaa1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen M, Tu C, Tan C, et alKey to successful treatment of COVID‐19: accurate identification of severe risks and early intervention of disease progression medRxiv. 2020:04.06.20054890. 10.1101/2020.04.06.20054890 [DOI] [Google Scholar]
- 32. Fang Z, Zhang Y, Hang C, Ai J, Li S, Zhang W. Comparisons of viral shedding time of SARS‐CoV‐2 of different samples in ICU and non‐ICU patients. J Infect. 2020;81(1):147‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen W, Lan Y, Yuan X, et al. Detectable 2019‐nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mancuso P, Gidaro A, Gregato G, et al. Viable circulating endothelial cells and their progenitors are increased in Covid‐19 patients. medRxiv. 2020:04.29.20085878. 10.1101/2020.04.29.20085878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eberhardt KA, Meyer‐Schwickerath C, Heger E, et al. RNAemia corresponds to disease severity and antibody response in hospitalized COVID‐19 patients. Viruses. 2020;12(9):1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawasuji H, Morinaga Y, Tani H, et al. SARS‐CoV‐2 RNAemia with higher nasopharyngeal viral load is strongly associated with severity and mortality in patients with COVID‐19. medRxiv. 2020:12.17.20248388. 10.1101/2020.12.17.20248388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riel D, Embregts C, Sips GJ, et al. Temporal kinetics of RNAemia and associated systemic cytokines in hospitalized COVID‐19 patients. bioRxiv. 2020:12.17.423376. 10.1101/2020.12.17.423376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ram‐Mohan N, Kim D, Zudock EJ, et al. SARS‐CoV‐2 RNAaemia predicts clinical deterioration and extrapulmonary complications from COVID‐19. medRxiv. 2020. 12.19.20248561 10.1101/2020.12.19.20248561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martín Ramírez A, Zurita Cruz ND, Gutiérrez‐Cobos A, et al. Evaluation of two RT‐PCR techniques for SARS‐CoV‐2 RNA detection in serum for microbiological diagnosis. medRxiv. 2020:11.15.20231795. 10.1101/2020.11.15.20231795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lei C, Lin W, Deng X, et al. Factors associated with clinical outcomes in patients with coronavirus disease 2019 in Guangzhou, China. J Clin Virol. 2020;133:104661. 10.1016/j.jcv.2020.104661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wong CK, Lam CWK, Wu AKL, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oh M, Park WB, Choe PG, et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med. 2016;375:1303‐1305. [DOI] [PubMed] [Google Scholar]
- 43. Zhang JJY, Lee KS, Ang LW, Leo YS, Young BE. Risk factors for severe disease and efficacy of treatment in patients infected with COVID‐19: a systematic review, meta‐analysis, and meta‐regression analysis. Clin Infect Dis. 2020;71(16):2199‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID‐19‐infected cancer patients: a systematic review and meta‐analysis. J Natl Cancer Inst. 2020:djaa168. 10.1093/jnci/djaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bae S, Kim SR, Kim MN, Shim WJ, Park SM. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID‐19 according to age: a systematic review and meta‐analysis. Heart. 2020:heartjnl‐2020‐317901. 10.1136/heartjnl-2020-317901 [DOI] [PubMed] [Google Scholar]
- 46. Bouadma L, Wiedemann A, Patrier J, et al. Immune alterations during SARS‐CoV‐2‐relatedacute respiratory distress syndrome. J Clin Immunol. 2020;40(8):1082‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020;383:590‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173:268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID‐19: a rapid systematic review, meta‐analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laing AG, Lorenc A, Barrio DMD, et al. A dynamic COVID‐19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623‐1635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data analyzed in this study are openly available in the references 21 , 40 , 45


