Abstract
Heterologous polyclonal antibodies might represent an alternative to the use of convalescent plasma or monoclonal antibodies (mAbs) in coronavirus disease (COVID‐19) by targeting multiple antigen epitopes. However, heterologous antibodies trigger human natural xenogeneic antibody responses particularly directed against animal‐type carbohydrates, mainly the N‐glycolyl form of the neuraminic acid (Neu5Gc) and the α1,3‐galactose, potentially leading to serum sickness or allergy. Here, we immunized cytidine monophosphate‐N‐acetylneuraminic acid hydroxylase and α1,3‐galactosyl‐transferase (GGTA1) double KO pigs with the Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike receptor binding domain to produce glyco‐humanized polyclonal neutralizing antibodies lacking Neu5Gc and α1,3‐galactose epitopes. Animals rapidly developed a hyperimmune response with anti‐SARS‐CoV‐2 end‐titers binding dilutions over one to a million and end‐titers neutralizing dilutions of 1:10 000. The IgG fraction purified and formulated following clinical Good Manufacturing Practices, named XAV‐19, neutralized spike/angiotensin converting enzyme‐2 interaction at a concentration <1 μg/mL, and inhibited infection of human cells by SARS‐CoV‐2 in cytopathic assays. We also found that pig GH‐pAb Fc domains fail to interact with human Fc receptors, thereby avoiding macrophage‐dependent exacerbated inflammatory responses and a possible antibody‐dependent enhancement. These data and the accumulating safety advantages of using GH‐pAbs in humans warrant clinical assessment of XAV‐19 against COVID‐19.
Keywords: COVID‐19, pig, polyclonal antibodies, SARS‐CoV‐2, spike
GGTA1/CMAH double KO pigs produced high titers of XAV‐19, an anti‐SARS‐Cov‐2 RBD glyco‐humanized polyclonal antibody. XAV‐19 is presented with a high complement activation and a high neutralization potency in vitro and in cell‐based neutralization assays. XAV‐19 does not interact with human Fc gamma receptors, preventing Fc‐dependent enhancement or immune cells skewing.
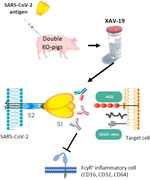
Introduction
Prevention and therapeutic strategies to fight against SARS‐CoV‐2 comprise, beside steroids and anticlotting agents, vaccination with stabilized mRNA, recombinant virus proteins or attenuated virus, the use of antiviral molecules, and antibodies targeting the S1 protein. The anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2) antibody passive administration may offer a rapid effect on the virus when administered early in the disease course or complement vaccination by providing an immediate humoral immunity to susceptible persons. This has been demonstrated in immunodeficient coronavirus disease (COVID‐19) patients where transfer of convalescent plasma (CP) with anti‐SARS‐CoV‐2 antibodies provided a clinical benefit within 48 h [1], and further confirmed in immunocompetent patients [2]. Interestingly, passive antibody therapy has been invented in the 19th century when infusing heterologous immunoglobulins was the only means of treating certain infectious diseases prior to the development of antibiotics or to treating cancers [3, 4]. Today, several passive heterologous immunotherapy products including polyclonal and monoclonal antibodies against infectious agents are on the market and have demonstrated robustness and efficacy to fight against bacterial infections, including tetanus, botulism, diphtheria, or viral infections such as hepatitis A and B, varicella [5], and rabies [6]. The anticipated mechanism of action by which antibodies would mediate protection against viruses is neutralization, that is, inhibition of viral particles interaction with their cognate receptor and, thereby, entry into human cells. However, other antiviral mechanisms involving antibodies may be possible, such as antibody‐dependent cellular cytotoxicity and/or phagocytosis. In the context of enveloped viruses, such as coronaviruses, direct complement‐mediated lysis might also participate in viral particles elimination since expression by viral particles of complement‐regulatory proteins belongs to the viral‐mediated immune escape mechanisms [7].
Beside potential benefits, however, administration of heterologous animal‐derived immunoglobulins may elicit serious adverse effects in humans such as serum sickness disease (SSD) almost always present in immunocompetent hosts [8] and may provide “xenosialitis,” a systemic inflammation still elusive in human but associated with cardiovascular diseases and cancer in animal models [9, 10, 11].
The description of serum sickness more than a century ago in humans perfused with animal sera [12] eventually led to the identification of a class of human antibodies directed against glycans bearing the common mammalian sialic acid, N‐glycolylneuraminic acid (Neu5Gc) [13, 14]. Humans lack Neu5Gc on glycosylated proteins or lipids due to a human‐lineage specific genetic mutation in the enzyme cytidine monophosphate‐N‐acetylneuraminic acid hydroxylase (CMAH). A direct consequence is that Neu5Gc epitopes are excluded from “self‐tolerance” and natural anti‐Neu5Gc antibodies are present, that is, antibodies found consistently in humans without intentional immunization. Furthermore, new antibodies can be easily elicited following infusion of Neu5Gc‐positive proteins of animal origin, including therapeutic immunoglobulins. Humans also lack the α1,3‐galactosyl‐transferase (GGTA1) enzyme, are not tolerant to α1,3‐galactose (αGal) epitopes, present various levels of natural anti‐αGal antibodies, and increase their level of anti‐αGal antibodies after infusion of animal‐derived products [15]. Rabbit anti‐lymphocyte serum treatment in diabetic patients, for example, resulted in highly significant increases of both anti‐αGal and anti‐Neu5Gc IgM and IgG, peaking at 1 month and still detectable 1‐year postinfusion, that were responsible for the induction of SSD, an immune‐complex disease, in almost all patients [16].
Initially developed for xenotransplantation purposes, pigs have been genetically engineered to knock out the CMAH and GGTA1 enzymes. Immunoglobulins from these animals are devoid of Neu5Gc and αGal carbohydrate epitopes and may not elicit SSD and possible deleterious anti‐xenoantigen antibodies when administered in human. The limited clinical experience gained so far with such glyco‐humanized swine polyclonals, lacking both Neu5Gc and αGal sugar moieties, used to confer immunosuppression in kidney transplant recipients suggests their good tolerance and efficacy (NCT 04431219).
Another feature of such swine glyco‐humanized polyclonals revealed here is their inability to bind human FcγR (CD16, CD32, CD64), which should protect recipients from antibody‐dependent enhancement (ADE), a process by which several viruses (among which dengue, zika, SARS‐CoV) have an increased capacity to enter into cells expressing FcγR (mainly macrophages) in the presence of virus‐specific antibodies or cross‐reactive antibodies [17, 18]. However, whether infection with SARS‐CoV‐2 might elicit ADE remains speculative in human [19]. Failure to bind human FcγR should also limit FcγR‐dependent recruitment/activation of macrophages described to contribute in skewing inflammation‐resolving response during SARS‐CoV infection [20]. Here, we described the immunization of CMAH/GGTA1 double KO (DKO) pigs with a recombinant receptor binding domain (RBD) domain from SARS‐CoV‐2 spike protein and the development of XAV‐19, a drug candidate manufactured from hyperimmune serum presenting a higher therapeutic index than CP.
Results
Absence of αGal and Neu5Gc carbohydrate on immunoglobulins from CMAH/GGTA1 DKO swine
We have already shown that IgG from CMAH and GGTA1 DKO pigs were deprived of Neu5GC and αGal carbohydrates [21]. To confirm the absence of these carbohydrates on IgG from animals immunized with the SARS‐CoV‐2 spike RBD domain to produce XAV‐19, the clinical batch of anti‐CoV IgG, N‐glycan profiling was carried out as previously described [22, 23], and compared with WT pig IgG. The absence of N‐glycan structure exhibiting Neu5Gc was clearly confirmed according to two independent methods: N‐glycan profiling by ultraperformance liquid chromatography and ESI‐MS detection (UPLC‐FLD/ESI MS) after enzymatic deglycosylation by PNGase F, on the one hand, and quantitative analysis of sialic acid by HPLC after DMB labeling on the other hand. Instead, the human‐type acetylated form of neuraminic acid Neu5Ac was observed (Fig. 1). Limited by the sensitivity of the method, no N‐glycan with αGal moieties was observed and detected on glyco‐humanized pig IgG (data not shown).
Figure 1.
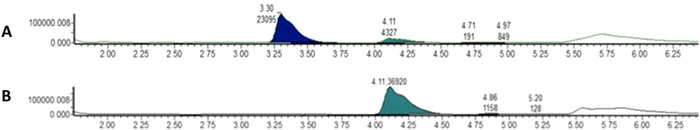
RP‐UPLC chromatograms of pig IgG and the clinical batch of glyco‐humanized pig IgG showing absence of Neu5Gc residues on glyco‐humanized pig IgG. Sialic acids quantification was performed by DMB‐labeled released sialic acid analysis by RP UPLC‐FLD/ESI‐MS. Labeled monosaccharides were separated by UPLC using a BEH C18 column and the quantification was performed with a fluorescent detector coupled to the chromatography. The procedure was performed following standard procedures from Waters (Application Note UPLC/FLR/QT of MS Analysis of Procainamide‐Labeled N‐glycans). Panel A: WT pig IgG with Neu5GC (left filled histogram) and Neu5AC (right filled histogram) peaks. Panel B: Glyco‐humanized pig IgG with one Neu5AC peak. Filled histograms height denotes the relative amount of each N‐glycan in the sample. Data are from a single analysis from one representative sample of each condition.
Reduced recognition of glyco‐humanized swine IgG by human natural antibodies
Formation of immune complexes between pre‐existing or elicited anti‐Neu5Gc antibodies and therapeutic immunoglobulins of animal origin has been identified as a major trigger of serum sickness after serotherapy [13, 14, 16, 24, 25] . To explore the actual impact of removal of αGal and Neu5Gc epitopes on recognition of swine IgG by pre‐existing human natural antibodies, human serums from healthy volunteers were assessed in a binding assay against WT or glyco‐humanized swine IgG, or against rabbit IgG used as a comparator. The data revealed an overall lower reactivity of human natural antibodies against pig IgG than against rabbit IgG. Reactivity against glyco‐humanized swine IgG was considerably reduced compared with reactivity against WT IgG (p < 0.001). Interestingly, this difference was due to the lack of Neu5Gc and not to the lack of αGal, since recognition of IgG from WT or GGTA1 single KO animals was similar (Fig. 2), fitting to the previously reported [21] low αGal loading of pig IgG on mass spectrometry pattern. The importance of the two terminal sugar epitopes in elicited responses against foreign IgG has been also well documented elsewhere [16, 24, 25].
Figure 2.
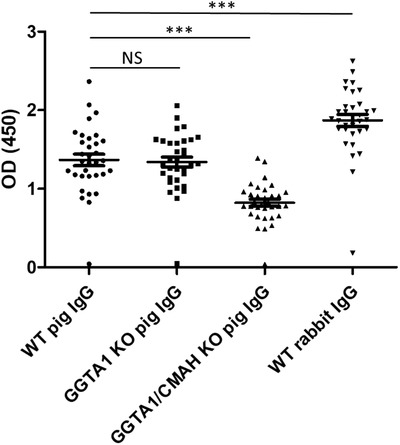
Reactivity of human IgG against rabbit, pig, and CMAH/GGTA1 DKO pig IgG. Serum from healthy volunteers (n = 59; 100‐fold dilutions) was incubated on ELISA plates coated with WT pig IgG, GGTA1 KO pig IgG, CMAH/GGTA1 DKO pig IgG, or rabbit IgG. Binding of human IgG was revealed with a labeled secondary antibody against human IgG. Symbols represent individual sera, and error bar represents mean ± SEM. Data are from three independent experiments. Nonparametric statistics were used for pairwise comparisons using Kruskal–Wallis tests. NS: not significant. ***p < 0.001.
IgG from CMAH/GGTA1 DKO pigs show high complement activation capacity but no binding to human FcγR
We observed that the IgG fraction of CMAH/GGTA1 DKO pigs presented an overall higher complement‐activating capacity when compared with rabbit immunoglobulins. This could be evidenced by immunizing CMAH/GGTA1 DKO pigs with human T cells and assessing complement‐mediated cytotoxicity of the IgG fraction, in comparison with a rabbit IgG anti‐T‐cell immunoglobulin (Thymoglobulin). We used adjusted concentration of pig and rabbit IgG preparations to obtain identical binding intensity to target cells by flow cytometry (Fig. 3A). The data showed a complement‐mediated cytotoxicity activity of Neu5Gc/αGal‐negative pig IgG higher than with rabbit IgG (Fig. 3B). To further investigate whether Neu5Gc/αGal‐negative pig IgG inherently activate more complement than rabbit IgG, independently of their antigen target recognition capacities, we directly assessed complement component 1q (C1q) binding intensity to immobilized IgG. This experiment confirmed higher recruitment of complement C1q by Neu5Gc/αGal‐negative pig IgG than by rabbit IgG (Supporting Information Fig. S1A and B). To understand whether this high complement‐activation activity was a consequence of the altered glycosylation of Neu5Gc/αGal‐negative pig IgG (glycosylation being required for efficient C1 activation [26]), we also compared C1q binding to immobilized WT and Neu5Gc/αGal‐negative pig IgG. The data revealed similar C1q recruitment (Supporting Information Fig. S1C and D), suggesting absence of intrinsic difference due to conversion of Neu5Gc to Neu5Ac type of N‐glycosylation.
Figure 3.
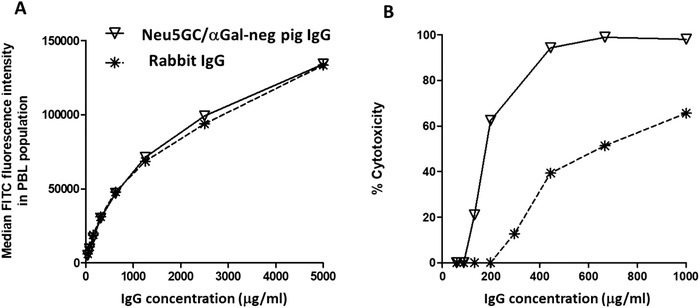
High complement activation with Neu5Gc and αGal‐negative pig IgG. GGTA1/CMAH double KO pigs (n = 4) were immunized with human T cells and the hyperimmune IgG fraction purified from pooled sera by Protein A chromatography. Rabbit anti‐human T cells used here was Thymoglobulin. The two IgG preparations were differentially diluted in nonimmune IgG of the respective species to obtain similar binding titer and intensity on target human T cells by flow cytometry. A fluorescent Protein‐G reagent has been used instead of species‐specific secondary antibodies to ensure similar, reagent‐independent revelation (A). Target human T cells were used in a complement‐mediated cytotoxicity (CDC) assay where rabbit IgG and GGTA1/CMAH double KO pig IgG directed against human T cells were compared (B). Results shown here are one representative experiment of three independent assays.
In parallel, using either wild or the DKO pig‐derived IgG preparations against human T cells, we noticed the absence of antibody‐dependent cell‐mediated cytotoxicity using human effector cells, when compared with rabbit IgG directed against the same target cells (Supporting Information Fig. S2). This led us to investigate interaction between DKO pig IgG and human FcγR (CD16, CD32, and CD64). Surface plasmon resonance and ELISA experiments revealed absence of interaction of pig IgG, either DKO or WT, with human CD16, CD32, or CD64, whereas binding of IgG from the rabbit, cow, horse, donkey, goat, and human all presented reactivity against at least one Fc receptor (Table 1 and Supporting Information Fig. S3).
Table 1.
Interaction of polyclonal IgG from the indicated species with human FcγR
| FcγRIII (CD16) | FcγRIIa (CD32a) | FcγRIIb (CD32b) | FcγRI (CD64) | |
|---|---|---|---|---|
| Pig | − | − | − | − |
| GH‐pig | − | − | − | − |
| Rabbit | + | + | + | + |
| Bovine | ND | + | + | + |
| Horse | ND | − | − | + |
| Donkey | ND | − | − | + |
| Goat | ND | + | + | + |
| Human | + | + | + | + |
ND: not done.
Anti‐SARS‐CoV‐2 hyperimmune serum titers
CMAH/GGTA1 KO pigs were immunized by intramuscular administrations of SARS‐CoV‐2 RBD spike antigens in mineral adjuvant. The RBD sequence was selected based on recent demonstration that it is instrumental in the binding of the spike protein to angiotensin converting enzyme‐2 (ACE‐2) [27] and that antibodies to RBD consequently inhibit SARS‐CoV‐2 entry into ACE‐2‐positive cells [28, 29]. Immune serum presented shortly after immunization end binding titers above 1:40 000 (Fig. 4A). After several immunizations, end titers in individual animals ranged between 1:100 000 and >1:106 (Fig. 4B). IgG extracted from sera by Protein A chromatography presented an initial EC50 in ELISA of 2.5 μg/mL after two immunization and below 1 μg/mL after three or more immunizations (Fig. 4C and D).
Figure 4.
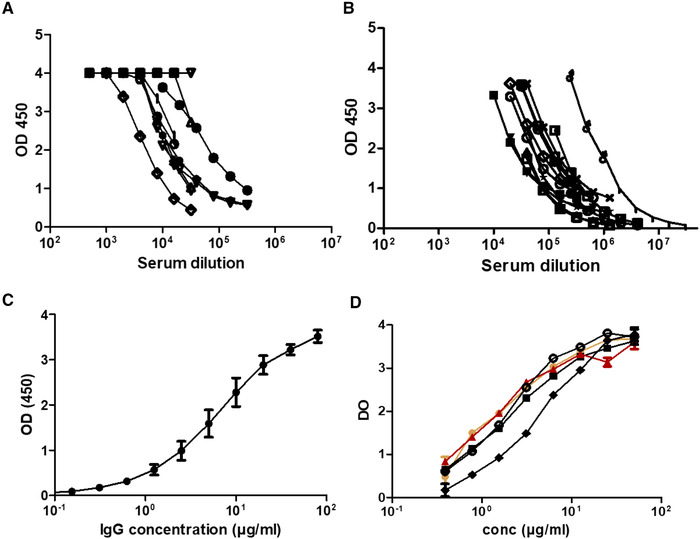
SARS‐CoV‐2 spike binding ELISA. GGTA1/CMAH double KO pigs were immunized with SARS‐CoV‐2 spike RBD recombinant proteins and blood was collected after two (A and C) or three to five (B and D) immunizations. Serum (A and B) and the IgG fraction (C and D) were tested by ELISA in a single experiment for binding to recombinant SARS‐CoV‐2 spike molecules. In (A) (n = 10), (B) (n = 12), and D (n = 4), curves represent data from individual animals (one animal per experiment). In (C), IgGs were extracted from a serum pool from animals depicted in (A) (means of triplicates ± SD, assessed by ELISA in a single experiment). In (D), the orange curve corresponds to one of the best responding animals and the red one to the pool of sera selected for further GMP processing and clinical usage (n = 10).
Inhibition of spike/ACE‐2 interaction
Anti‐SARS‐CoV‐2 hyperimmune sera were also tested in a spike/ACE‐2 binding competition assay. Hyperimmune sera demonstrated an inhibitory capacity with an end titer of 1:4000 soon after immunization (Fig. 5A) and IgG extracted from sera by protein A chromatography presented an inhibitory concentration 50 (IC50) of 2.5 μg/mL (Fig. 5B). After a few repeated immunizations, serum presented inhibitory capacities at much higher dilutions and IgG from these sera could be diluted down to <0.1 μg/mL (Fig. 5C and D).
Figure 5.
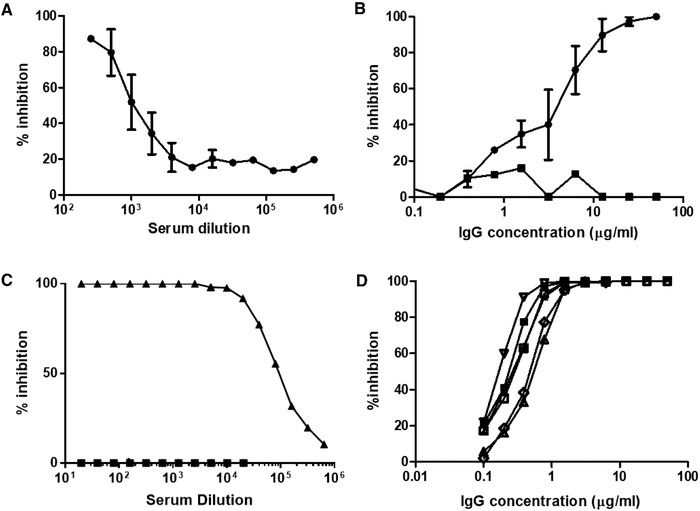
Assessment of inhibition of SARS‐CoV‐2 spike/ACE‐2 interaction. Samples analyzed in Fig. 4 were tested in an ELISA interaction assay where ACE‐2 was immobilized on plastic and Spike‐Fc ligand binding to ACE‐2 was revealed with a secondary antibody against Fc. 100% inhibition represents absence of spike/ACE‐2 interaction. (A) Mean ± SD of four individual sera collected after two immunizations, assessed in a single ELISA. (B) Mean (n = 4) ± SD of individual IgG fractions shown in (A). Black square symbols: IgG extracted from a pool of pre‐immune serum (n = 4). (C) Triangles represent data from a serum pool from six animals obtained after three to five immunizations. Square symbols: nonimmune serum. (D) Individual profiles (means of duplicate measures for each point) of six individual IgG fractions from the six animals shown in (C).
Neutralization of SARS‐CoV‐2 by glyco‐humanized swine IgG
The neutralizing effect of the hyperimmune serum and IgG fractions against live SARS‐CoV‐2 virus (G614 form) was determined by cytopathic effect (CPE) reduction assay. CPE100 (maximal dilution to reach 100% of neutralization) with pooled hyperimmune sera used to prepare therapeutic IgG reached 1:1600 and the concentration of the IgG preparation required to reach CPE100 ranged between 15 and 25 μg/mL for the samples collected after two immunizations. CPE100 was reached at 3.125 μg/mL when using IgG preparations collected after three immunizations or more (Table 2).
Table 2.
Neutralizing activity by cytopathic effect (CPE) assay and plaque reduction neutralizing test (PRNT). Data are from two independent experiments and are means of duplicates
| Serum pool* | Purified IgG from serum pool | |
|---|---|---|
| End titer or concentration (μg/mL) to abrogate CPE (100% efficacy) against SARS‐CoV‐2—samples collected after two immunizations | 1:1600 | 15–25 μg/mL |
| Concentration (μg/mL) to abrogate CPE (100% efficacy) against SARS‐CoV‐2—samples collected after three immunizations or more | ND | 3.125 μg/mL |
| End titer or concentration (μg/mL) to abrogate PRNT (90% efficacy) against SARS‐CoV‐2—samples collected after two immunizations | >1:320 | ND |
| End titer or concentration (μg/mL) to abrogate PRNT (90% efficacy) against SARS‐CoV—samples collected after two immunizations | 1:10** | ND |
Corresponding to the sample tested in Fig. 5A (pool of four individual sera collected after two immunizations).
Another sample drawn after several immunizations presented a PRNT90 at the dilution of 1:80 against SARS‐CoV.
ND: not done.
The neutralizing effect of the hyperimmune serum was also determined in parallel by plaque reduction neutralization test (PRNT) using another SARS‐CoV‐2 strain (presenting the D614 form), as previously described [30]. Serum samples collected from three pigs immunized twice showed PRNT90 at 1:160, 1:80, and >1:320 against SARS‐CoV‐2, and the serum collected from the pig displaying the highest titer by PRNT90 (>1:320) also cross‐neutralized SARS‐CoV at PRNT90 1:10. The first pool of serum obtained after repeated immunizations, from which IgG have been extracted to prepare the initial clinical batch of neutralizing immunoglobulins, presented a PRNT90 titer of >1:320 and cross‐neutralized SARS‐CoV at PRNT90 1:80 (Table 2).
Discussion
The ongoing COVID‐19 pandemic requires prompt availability of prophylactic and therapeutic interventions. Preliminary data on transfer of CP from COVID‐19 recovering patients to critically ill patients demonstrated efficacy to reduce viral titers and to improve clinical symptoms when provided early in the disease course [1, 31]. The effect has been attributed to the presence of neutralizing antibodies against the virus spike RBD sequence, since antibodies uniquely directed against RBD do protect mice in preclinical studies [32]. Yet CP show highly variable neutralization half‐titers of anti‐SARS‐CoV‐2 antibodies, ranging from less than 1:50 in one‐third of patients, between 1:50 and 1:1000 in the majority of them, with only rare patients showing titers above 1:5000 [33].
In the past clinical experience with CP from SARS‐convalescent patients in 2003 (80 patients), the average CP volume was 279 mL and the neutralizing titer was 1:160 [34]. Today, in critically ill patients with COVID‐19, Duan et al. [35] reported using one dose of 200 mL of CP presenting a neutralizing titer above 1:160. Shen et al. [36] reported efficacy after two consecutive transfusions with 200 mL of CP with end point dilution titers greater than 1:40, corresponding to a SARS‐CoV‐2–specific antibody (IgG) binding titer greater than 1:1000. In this report, the ratio between binding and neutralization titers is therefore of 25‐fold on average. Recent guidelines from the European Commission on the use of CP revised the desired neutralizing CP titers and suggested that “neutralizing antibody titers should optimally be greater than 1:320, but lower thresholds might also be effective” (https://ec.europa.eu/health/blood_tissues_organs/covid‐19_en). Thus, altogether, estimating that transfusion of 200 mL CP corresponds to the administration of approximately 25 mg/kg IgG (considering that plasma contains 10 mg/mL IgG and considering 80 kg as an average patient's weight), available data suggest that 25 mg/kg of an IgG preparation with a neutralizing titer of at least 1:40 meets the clinically required efficacy.
A few weeks after immunization of CMAH/GGTA1 KO pigs with SARS‐CoV‐2 RBD proteins, binding and neutralization serum antibody titers rapidly reached 1:100,000 and 1:4,000 (end titer dilutions in the ELISA neutralization assay), respectively, corresponding to the same 25‐fold ratio described in humans. The neutralization titer in a cytopathogenic effect assay was of 1:1600 but corresponds to the dilution required to reach 100% inhibition (as opposed to end‐titer dilution). The neutralizing capacity was confirmed in a PRNT90 assay with another, independent SARS‐Cov‐2 strain (showing PRNT90 > 1:320). This neutralizing titer is at least 40‐fold higher (depending on the assay being considered) as compared to COVID‐19 CP presenting a clinical interest according to the literature, and at least 12.5‐fold higher than the European Commission recommended. IgG purified from pig hyperimmune serum could have therefore the potential to be used successfully at doses 12.5‐ to 40‐fold lower than CP, that is, at doses starting at 0.6 mg/kg.
Glyco‐humanization refers to removal of the αGal epitopes and conversion of animal‐type Neu5Gc/Ac forms of sialic acid into human single type Neu5Ac. The procedure has been initially developed to circumvent problems related to the occurrence of high titers of anti‐αGal and anti‐Neu5Gc antibodies following administration of anti‐T lymphocytes rabbit immunoglobulins, which is potentially causing allergies [37], serum sickness [24], and a possible “xenosialitis,” a systemic inflammation so far only demonstrated in animal models [9, 10]. Although our experiments do not directly address the clinical importance of avoiding anti‐αGal/Neu5Gc antibody responses, our data show that “natural” anti‐pig antibodies found in human serum are essentially directed against Neu5Gc and that reactivity against rabbit IgG is higher than against pig IgG (Fig. 2). These data are of importance in a context where hyperimmune pig immunoglobulins are being used therapeutically to treat aplastic anemia [37, 38] and are being developed as an anti‐lymphocyte induction treatment in kidney transplantation (NCT 04431219). Glyco‐humanized pig immunoglobulins would potentially present, therefore, a safety advantage over rabbit polyclonal antibodies or over immunoglobulins from other species currently in clinical use, such as bovine (e.g., SAB‐301 from SAB biotherapeutics, Sioux Falls, USA), equine (e.g., Fabenflu from Fab'entech, Lyon, France), and ovine (e.g., CroFab, Protherics, Salt Lake City, USA), that among others have been successfully applied to rabies, envenomation, and intoxication (reviewed by Dixit et al. [6]). Another potential advantage of using glyco‐humanized antibodies from pigs is related to its unique mechanism of action in humans. Because these modified pig IgGs fail to interact with human Fc receptors and to recruit human effectors (Table 1 and Supporting Information Figs. S2 and S3), likely due to divergent molecular evolution, they are not susceptible to promote inflammatory‐related ill‐effects associated with IgG Fc/ FcγR interactions. One such interaction demonstrated with diverse viral families including Coronaviridae is ADE [39, 40, 41]. However, the importance of ADE phenomenon, whereby a previous immune response could render an individual more susceptible to a Cov‐2 reinfection, remains undetermined [19, 42]. Another potential unwanted outcome associated with anti‐SARS‐CoV‐2 antibodies is severe acute lung injury mediated by IgG Fc‐dependent macrophage recruitment in productively infected lungs, thereby skewing inflammation‐resolving response [20]. Another expected benefit is to blunt the key mechanisms of the SSD, which is an IC complex disease known to be mostly FcγR dependent. This risk of additional local inflammation should thus not be carried by pig IgG. Although unable to elicit antibody‐dependent cell‐mediated cytotoxicity, pig IgG are nevertheless endowed with a preserved complement‐activation capacity. As enveloped viruses might be sensitive to direct complement‐mediated lysis, administration of anti‐SARS‐CoV‐2 pig antibodies might therefore lead to viral neutralization without targeting viral particles to inflammatory cells. Another potential risk of using animal‐derived immunoglobulins in humans is related to the presence of viral particles in the starting material (pig hyperimmune serum), mainly retroviruses. The risk is well controlled by the application of the ICH Q5A guidelines addressing viral safety biotechnology products of animal origin, ensuring less than one viral particle per 6 million doses.
Our first attempt to use polyclonal glyco‐humanized pig IgG against an emergent infectious disease was in an in vivo EBOLA virus model [43]. Despite low levels of neutralizing antibodies had been obtained, we reported significant effect on the viral load and on animal survival demonstrating the high efficacy of the polyclonal approach. More recently, horse polyclonal IgG against SARS‐CoV‐2 spike RBD have been developed and demonstrated potent in vitro neutralizing capacity [44]. Horse IgG have to be used in their F(ab’)2 form to reduce the risk of serum sickness, that is, without Fc fragments, a problem addressed in the current study by use of swine glyco‐humanized antibodies, that cannot be recognized by anti‐carbohydrate antibodies known to particularly evoke serum sickness, and also Fc‐dependent macrophage activation [20]. Yet using glyco‐humanized swine IgG antibodies has the advantage over F(ab’)2 fragments to present an extended in vivo half‐life comparable to that of other heterologous IgG immunoglobulins (unpublished data from our preclinical and clinical investigations). However, due to the allogeneic nature of peptides released from pig antibodies, it is expected that anti‐drug antibodies will be generated that might preclude chronic or repeated administrations.
Our data indicate that pig hyperimmune glyco‐humanized IgG against SARS‐CoV‐2 may be of clinical benefit, relying on the potency criteria recently set forth by the U.S. Center for Drug Evaluation and Research (CDER) of the FDA (COVID‐19: Potency Assay Considerations for Monoclonal Antibodies and Other Therapeutic Proteins Targeting SARS‐CoV‐2 Infectivity, Guidance for Industry, January 2021). In addition, the foreseen therapeutic doses are predicted to show efficacy at neutralizing virus while decreasing the level of evoked immune complexes and of serum sickness associated with the production of anti‐Neu5Gc and anti‐Gal antibodies. Although conclusions drawn in the current study would benefit from a future in vivo assessment in a preclinical SARS‐CoV‐2 infection model, data from previous experiments with CP transfer and from preclinical investigations in mice and primates [32, 45] as well as preliminary data in the clinical arena [2, 31] show that antibodies against RBD can indeed provide an immediate clinical benefit. Altogether, this information warrants clinical evaluation of the administration of pig glyco‐humanized IgG against SARS‐CoV‐2 RBD in COVID‐19 patients. Owing to the high antibody titers produced by CMAH/GGTA1 KO animals in a short period of time and to the significantly shorter, cheaper and easier pharmaceutical development of polyclonal antibodies as compared to mAbs or mAb cocktails [46], glyco‐humanized anti‐SARS‐CoV‐2 pig immunoglobulins have been among the first treatments specific to COVID‐19 reaching the clinic (NCT04453384).
Methods
Animals and reagents
Pigs double knocked out for GGTA1 and CMAH encoding genes [23] were obtained by cloning procedures described in Lagutina et al. [47]. All procedures involving the use of animals in this study were carried out in accordance with the Italian Law (D. Lgs 26/2014) and EU directive 2010/63/EU regulating animal experimentation after authorization by relevant authorities (Italy, project n 959/2017‐PR; Belgium 2010/63/EU and AR 29/05/2013). The invalidating mutations in genes encoding the CMAH and GGTA1 were confirmed by direct amplification and sequencing of the gene sequences at the animal facility to ensure the stability of the genetic background. The phenotype has been checked by mass spectrometry to confirm the absence of αGal and Neu5Gc residues on gamma immunoglobulins. Recombinant proteins from SARS‐CoV‐2 have been manufactured from Hek‐293 cells and were purchased from Interchim, Monluçon, France.
LC‐MS analysis of glycosylation
Assessment of N‐glycan Neu5Gc and αGal expression on DKO and WT porcine IgGs has been performed by deglycosylation, N‐glycan procainamide labeling and treatment with α‐galactosidase, as previously described [48].
Analysis of human anti‐pig IgG reactivity
Pig WT IgG, IgG from GGTA1 KO, IgG from CMAH/GGTA1 DKO pigs, or rabbit IgG (WT) have been coated on Maxisorp (NUNC) ELISA plates at 10 μg/mL overnight at 4°C in carbonate buffer at pH 9.5. After washing with PBS‐Tween 20 (0.05%), free binding sites were blocked with 1% chicken egg albumin. Anonymously coded human serum samples from healthy volunteers (from the Etablissement Français du Sang, Nantes, France) were incubated at a 1:100 dilution, for 2 h at 37°C. After washing, bound IgG molecules were revealed with peroxidase conjugated goat anti‐human IgG antibodies (Jackson ImmunoResearch, 1/10 000 in 1% chicken egg albumin) and tetramethylbenzidine (TMB) reagent. Optical density was read at 450 nm.
Complement‐mediated cytotoxicity
Rabbit anti‐T‐cell globulins (Thymoglobulin) and glyco‐humanized pig anti‐T‐cell globulins were differentially diluted in nonimmune IgG solutions to obtain preparations presenting similar binding titers to human T cells. Then, serial dilutions were added to 250 000 human PBMC at 4°C for 30 min. After washing, human serum used as a source of complement diluted three times in cold RPMI medium was added, on ice, on each well. Reaction was then initiated by placing tubes at 37°C, and maintained for 30 min. After this step, the pellets were placed on ice and resuspended with PI, which is a viability marker of cells and the cytotoxic activity of antibodies was determined by flow cytometry, after gating on lymphocytes, based on their morphology.
Binding to human FcγR
The binding of IgG from DKO swine to the FcγRI, FcγRIIa, and FcγRIIb human receptors was tested using an ELISA assay and BIAcore surface plasmon resonance. IgG from different species were purified by protein A affinity and coated on Maxisorp™ ELISA plates overnight at 4°C (100 μL/well, 20 μg/mL in carbonate buffer pH 9.5). After washing with PBS‐Tween 20 0.05%, free binding sites were saturated with 2% BSA. His‐Tagged FcγRI, FcγRIIa, and FcγRIIb receptors (CD64/32a/32b, R&D Systems; 100 μL, 2.4 μg/mL in 2% BSA) were then added and incubated for 2 h at room temperature (RT). After washing, bound FcγR molecules were revealed with peroxidase conjugated anti‐tag antibody (Miltenyi, 120‐003‐811; 1/5000 in 2% BSA) and TMB reagent. Optical density was read at 450 nm and 630 nm. Specific OD is the value read at 450 nm minus that read at 630 nm. The binding of pig and rabbit (Thymoglobulin®, Sanofi) IgG to human FcγRIIIa (CD16a; R&D Systems, 4325‐FC) receptor was also measured by surface plasmon resonance. IgGs were immobilized on a CM5 sensor chip by amine coupling to a level of 2000RU. Free reactive sites were then inactivated with ethanolamine 1 M pH 8.5. Different concentrations (5, 2.5, 1.25, 0.625, 0.312, and 0.156 μM) of human FcγRIIIa were injected on the chip, with association time intervals of 180 s and dissociation time of 600 s. Regeneration between cycles were performed by 100 mM NaOH treatment for 45 s.
Anti‐SARS‐CoV‐2 binding ELISA
The target antigen (SARS‐CoV‐2 Spike RBD protein; Interchim) was immobilized on Maxisorp plates at 1 μg/mL in carbonate/bicarbonate buffer at 4°C overnight). After washing, saturation was performed with PBS‐Tween‐BSA for 2 h at RT. Samples were diluted into PBS‐Tween and added into the plate in duplicate, incubated 2 h at RT and washed three times. Bound pig IgG were revealed with a secondary anti‐pig‐HRP‐conjugated antibody (Bethyl Laboratories, USA) diluted in washing buffer, at 1:1000, incubated 1 h at RT and washed three times. TMB reagent was added in the plate, incubated up to 20 min in the dark and stopped with H2SO4. Reading was performed at 450 nm.
Inhibition of spike/ACE‐2 interaction
An assay was developed to assess the properties of swine antibodies to inhibit binding of a recombinant SARS‐CoV‐2 spike S1 molecule to immobilized recombinant ACE‐2 molecules.
The target antigen (human ACE‐2; Interchim) was immobilized on Maxisorp plates at 1 μg/mL in carbonate/bicarbonate buffer at 4°C overnight. The plates were washed in PBS‐Tween‐0.05% and saturated with PBS‐Tween‐0.05‐2% skimmed milk for 2 h at RT. Ligand Spike S1‐hFc tag (Interchim) at 125 ng/mL in PBS‐Tween‐0.05–1% skimmed milk was then added into another plate in duplicate, and pre‐incubated 30 min at RT in the presence of test samples diluted in PBS‐Tween‐0.05–1% skimmed milk powder (range between 50 and 0.39 μg/mL). Then the mixture was added to the plate for a 2‐h incubation at RT. The human Fc tag was then revealed with a specific HRP‐conjugated anti‐human IgG secondary antibody (diluted in PBS‐Tween‐0.05–1% skimmed milk powder at 1:1000, incubated 1 h at RT and washed three times). TMB reagent was added in the plate, incubated up to 30 min in the dark and stopped with H2SO4. The plate was read at 450 nm.
Plaque reduction neutralization testing
The PRNT was performed in duplicate using 24‐well tissue culture plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) in a biosafety level 3 facility. Swine serum dilutions were mixed with equal volumes of SARS‐CoV‐2 (BetaCoV/Hong Kong/VM20001061/2020 [KH1], corresponding to the D614 variant) or SARS‐CoV (strain HK39849, SCoV) at a dose of 200 plaque‐forming units determined by Vero E6 cells. Briefly, after 1 h of incubation at 37°C, the virus‐serum mixture was added to Vero E6 cell monolayers in 24‐well tissue culture plates. After 1 h of adsorption, the virus‐serum mixture was removed, and plates were overlaid with 1% agarose with cell culture medium. Plates were then incubated for 3 days at 37°C in 5% CO2 in a humidified incubator before fixation with 4% paraformaldehyde and staining crystal violet solution. The highest plasma dilution that resulted in 90% reduction of plaque numbers was denoted as PRNT90. A virus back‐titration of the input virus was included in each batch of tests.
CPE assay
To further analyze the neutralization potency and to confirm data with another independent virus strain, a CPE assay was carried out at VibioSphen, Labège, France. The purpose of this study was to assess the ability of the serum pool, XAV‐19 R&D, and XAV‐19 batches BMG170‐B01 to BMG170‐B04 to inhibit entry and cytopathic impact of SARS‐CoV‐2 on sensitive cells (Vero E6 Cells). All experimental protocols involving live SARS‐CoV‐2 followed the approved standard operating procedures of the Biosafety Level 3 facility. SARS‐CoV‐2 was isolated from a patient with laboratory‐confirmed COVID‐19 at VibioSphen, corresponding to the G614 variant. The viral isolate was amplified by one additional passage in VeroE6 cells to make working stocks of the virus. Vero E6 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% v/v fetal bovine serum, and 1% v/v penicillin‐streptomycin supplemented with 1%v/v sodium pyruvate at 1 × 105 cells per well in 12‐well tissue culture plates. At 100% confluence (2 days postseeding), the cells were washed twice with PBS and six serial dilutions of the virus (1/10 each time) will added to the cells. Following infection with 0.3 mL per well of each dilution, plates were incubated at 37°C for 1 h, and the cells were washed with PBS before the addition of 2% w/v agar containing 1 μg/mL‐5 tosyl phenylalanyl chloromethyl ketone‐trypsin (Sigma‐Aldrich) to the cell surface. Plates were left at RT for 20–30 min to allow for the overlay to set, and were then incubated at 37°C for 72 h. Cells were fixed with 4% v/v paraformaldehyde before both the fixative and agar will be removed and the cells were stained with 0.1% w/v Crystal Violet (Fisher) in 20% v/v ethanol. Plaque titers were determined as plaque forming units per milliliter.
CPE reduction assay was performed as follows: Vero E6 cells were seeded in 96‐well clusters at a density of 5000 cells/well 2 days before infection. Twofold serial dilutions, starting from 200 or 250 μg/mL or from a 1/25 dilution of the serum, were then mixed with an equal volume of viral solution containing 100 TCID50 of SARS‐CoV‐2. The serum‐virus mixture was incubated for 1 h at 37°C in a humidified atmosphere with 5% CO2. After incubation, 100 μL of the mixture at each dilution was added in triplicate to a cell plate containing a semiconfluent VERO E6 monolayer. The plates were then incubated for 3 days at 37°C in a humidified atmosphere with 5% CO2. Cell controls were infected with SARS‐CoV‐2 at a MOI of 0.01. The incubation period was 72 h. Uninfected cells were included as controls to exclude potential cytotoxic/cytostatic effects of the compound treatment. All samples were tested in duplicate.
Statistical analysis
Continuous variables are expressed as mean ± SEM, unless otherwise indicated, and compared with the nonparametric Mann–Whitney two‐sided test or Kruskal–Wallis tests with Dunn's ad hoc pairwise comparisons for more than two groups. p Values of <0.05 were considered to be statistically significant. All statistical analyses were performed on GraphPad Software (GraphPad Software, San Diego, CA).
Author Contributions
Conceived the study: OD, BV, JPS, and EC. Designed and supervised some experiments: OD, BV, CM, RB, SB, RTS, JMB, NG, MP, and JPS. Performed the experiments: JR, GE, PJR, RD, CG, PD, ML, and CM. Analyzed data: BV, JR, EL, and JPS.
Conflict of interests
JR, PJR, CC, GE, EL, and BV are employees of Xenothera, a company developing glycol‐humanized polyclonal antibodies as those described in this manuscript, and OD, JPS, JMB, CG are cofounders of Xenothera. All other authors of this manuscript have no commercial or financial conflicts of interest.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202049072.
Abbreviations
- ACE‐2
angiotensin converting enzyme‐2
- ADE
antibody‐dependent enhancement
- αGal
α1,3‐galactose
- CMAH
cytidine monophosphate N‐acetyl hydroxylase
- CP
convalescent plasma
- CPE
cytopathic effect
- DKO
double KO
- GGTA1
α1,3‐galactosyltransferase
- Neu5Gc
N‐glycolylneuraminic acid
- PRNT
plaque reduction neutralization testing
- RBD
receptor binding domain
- RT
room temperature
- TMB
tetramethylbenzidine
Supporting information
Supporting Information
Acknowledgements
This work was supported by Xenothera and by grants from the Société d'Accélération et Transfert de Technologie Ouest Valorisation, French Region Pays de la Loire, and Bpifrance.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Hueso, T. , Pouderoux, C. , Pere, H. , Beaumont, A. L. , Raillon, L. A. , Ader, F. , Chatenoud, L. et al., Convalescent plasma therapy for B‐cell‐depleted patients with protracted COVID‐19. Blood 2020. 136: 2290–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Libster, R. , Perez Marc, G. , Wappner, D. , Coviello, S. , Bianchi, A. , Braem, V. , Esteban, I. et al., Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl. J. Med. 2021. 384: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casadevall, A. , Dadachova, E. and Pirofski, L. A. , Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004. 2: 695–703. [DOI] [PubMed] [Google Scholar]
- 4. Lahaie, Y. M. and Watier, H. , Contribution of physiologists to the identification of the humoral component of immunity in the 19th century. MAbs 2017. 9: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsu, J. L. and Safdar, N. , Polyclonal immunoglobulins and hyperimmune globulins in prevention and management of infectious diseases. Infect. Dis. Clin. North Am. 2011. 25: 773–788. [DOI] [PubMed] [Google Scholar]
- 6. Dixit, R. , Herz, J. , Dalton, R. and Booy, R. , Benefits of using heterologous polyclonal antibodies and potential applications to new and undertreated infectious pathogens. Vaccine 2016. 34: 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li, Y. , Johnson, J. B. and Parks, G. D. , Parainfluenza virus 5 upregulates CD55 expression to produce virions with enhanced resistance to complement‐mediated neutralization. Virology 2016. 497: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gitelman, S. E. , Gottlieb, P. A. , Rigby, M. R. , Felner, E. I. , Willi, S. M. , Fisher, L. K. , Moran, A. et al., Antithymocyte globulin treatment for patients with recent‐onset type 1 diabetes: 12‐month results of a randomised, placebo‐controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013. 1: 306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawanishi, K. , Dhar, C. , Do, R. , Varki, N. , Gordts, P. and Varki, A. , Human species‐specific loss of CMP‐N‐acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms. Proc. Natl. Acad. Sci. USA 2019. 116: 16036–16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhar, C. , Sasmal, A. and Varki, A. , From “serum sickness” to “xenosialitis”: past, present, and future significance of the non‐human sialic acid Neu5Gc. Front. Immunol. 2019. 10: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tector, A. J. , Mosser, M. , Tector, M. and Bach, J. M. , The possible role of anti‐Neu5Gc as an obstacle in xenotransplantation. Front. Immunol. 2020. 11: 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kasukawa, R. , Kano, K. , Bloom, M. L. and Milgrom, F. , Heterophile antibodies in pathologic human sera resembling antibodies stimulated by foreign species sera. Clin. Exp. Immunol. 1976. 25: 122–132. [PMC free article] [PubMed] [Google Scholar]
- 13. Higashi, H. , Naiki, M. , Matuo, S. and Okouchi, K. , Antigen of “serum sickness” type of heterophile antibodies in human sera: indentification as gangliosides with N‐glycolylneuraminic acid. Biochem. Biophys. Res. Commun. 1977. 79: 388–395. [DOI] [PubMed] [Google Scholar]
- 14. Merrick, J. M. , Zadarlik, K. and Milgrom, F. , Characterization of the Hanganutziu‐Deicher (serum‐sickness) antigen as gangliosides containing n‐glycolylneuraminic acid. Int. Arch. Allergy Appl. Immunol. 1978. 57: 477–480. [DOI] [PubMed] [Google Scholar]
- 15. Berg, E. A. , Platts‐Mills, T. A. and Commins, S. P. , Drug allergens and food—the cetuximab and galactose‐alpha‐1,3‐galactose story. Ann. Allergy Asthma Immunol. 2014. 112: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salama, A. , Evanno, G. , Lim, N. , Rousse, J. , Le Berre, L. , Nicot, A. , Bach, J. M. et al., Anti‐Gal and anti‐Neu5Gc responses in nonimmunosuppressed patients after treatment with rabbit antithymocyte polyclonal IgGs. Transplantation 2017. 101: 2501–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaume, M. , Yip, M. S. , Cheung, C. Y. , Leung, H. L. , Li, P. H. , Kien, F. , Dutry, I. et al., Anti‐severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH‐ and cysteine protease‐independent FcgammaR pathway. J. Virol. 2011. 85: 10582–10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ulrich, H. , Pillat, M. M. and Tarnok, A. , Dengue fever, COVID‐19 (SARS‐CoV‐2), and antibody‐dependent enhancement (ADE): a perspective. Cytometry A 2020. 97: 662‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ledford, H. , Coronavirus reinfections: three questions scientists are asking. Nature 2020. 585: 168–169. [DOI] [PubMed] [Google Scholar]
- 20. Liu, L. , Wei, Q. , Lin, Q. , Fang, J. , Wang, H. , Kwok, H. , Tang, H. et al., Anti‐spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS‐CoV infection. JCI Insight 2019. 4: e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopez, P. G. , Girard, L. , Buist, M. , de Oliveira, A. G. , Bodnar, E. , Salama, A. , Soulillou, J. P. et al., Characterization of N‐glycosylation and amino acid sequence features of immunoglobulins from swine. Glycoconj. J. 2016. 33: 79–91. [DOI] [PubMed] [Google Scholar]
- 22. Miyagawa, S. , Matsunari, H. , Watanabe, M. , Nakano, K. , Umeyama, K. , Sakai, R. , Takayanagi, S. , et al., Generation of alpha 1, 3‐galactosyltransferase and cytidine monophospho‐N‐acetylneuraminic acid hydroxylase gene double‐knockout pigs. J. Reprod. Dev. 2015. 61: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salama, A. , Mosser, M. , Leveque, X. , Perota, A. , Judor, J. P. , Danna, C. , Pogu, S. et al., Neu5Gc and alpha1‐3 GAL xenoantigen knockout does not affect glycemia homeostasis and insulin secretion in pigs. Diabetes 2017. 66: 987–993. [DOI] [PubMed] [Google Scholar]
- 24. Couvrat‐Desvergnes, G. , Salama, A. , Le Berre, L. , Evanno, G. , Viklicky, O. , Hruba, P. , Vesely, P. et al., Rabbit antithymocyte globulin‐induced serum sickness disease and human kidney graft survival. J. Clin. Invest. 2015. 125: 4655–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amon, R. , Ben‐Arye, S. L. , Engler, L. , Yu, H. , Lim, N. , Berre, L. L. , Harris, K. M. , et al., Glycan microarray reveal induced IgGs repertoire shift against a dietary carbohydrate in response to rabbit anti‐human thymocyte therapy. Oncotarget 2017. 8: 112236–112244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jefferis, R. , Isotype and glycoform selection for antibody therapeutics. Arch. Biochem. Biophys. 2012. 526: 159–166. [DOI] [PubMed] [Google Scholar]
- 27. Lan, J. , Ge, J. , Yu, J. , Shan, S., Zhou, H. , Fan, S. , Zhang, Q. et al., Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature 2020. 581: 215–220. [DOI] [PubMed] [Google Scholar]
- 28. Yuan, M. , Wu, N. C. , Zhu, X. , Lee, C. D. , So, R. T. Y. , Lv, H. , Mok, C. K. P. et al., A highly conserved cryptic epitope in the receptor binding domains of SARS‐CoV‐2 and SARS‐CoV. Science 2020. 368: 630–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ju, B. , Zhang, Q. , Ge, J. , Wang, R. , Sun, J. , Ge, X. , Yu, J. et al., Human neutralizing antibodies elicited by SARS‐CoV‐2 infection. Nature 2020. [DOI] [PubMed] [Google Scholar]
- 30. Perera, R. A. , Mok, C. K. , Tsang, O. T. , Lv, H. , Ko, R. L. , Wu, N. C. , Yuan, M. et al., Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), March 2020. Euro Surveill. 2020. 25 : 2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Focosi, D. , Anderson, A. O. , Tang, J. W. and Tuccori, M. , Convalescent plasma therapy for COVID‐19: state of the art. Clin Microbiol Rev 2020. 33. https∖∖:doi.org∖10.1128/CMR.00072‐20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alsoussi, W. B. , Turner, J. S. , Case, J. B. , Zhao, H. , Schmitz, A. J. , Zhou, J. Q. , Chen, R. E. et al., A potently neutralizing antibody protects mice against SARS‐CoV‐2 infection. J Immunol 2020. 205: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robbiani, D. F. , Gaebler, C. , Muecksch, F. , Lorenzi, J. C. C. , Wang, Z. , Cho, A. , Agudelo, M. et al., Convergent antibody responses to SARS‐CoV‐2 in convalescent individuals. Nature 2020. 584: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng, Y. , Wong, R. , Soo, Y. O. , Wong, W. S. , Lee, C. K. , Ng, M. H. L. , Chan, P. et al., Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005. 24: 44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duan, K. , Liu, B. , Li, C. , Zhang, H. , Yu, T. , Qu, J. , Zhou, M. et al., Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc. Natl. Acad. Sci. USA 2020. 117: 9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen, C. , Wang, Z. , Zhao, F. , Yang, Y. , Li, J. , Yuan, J. , Wang, F. et al., Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA 2020. 323: 1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen, M. , Liu, C. , Zhuang, J. , Zou, N. , Xu, Y. , Zhang, W. , Li, J. et al., Long‐term follow‐up study of porcine anti‐human thymocyte immunoglobulin therapy combined with cyclosporine for severe aplastic anemia. Eur. J. Haematol. 2016. 96: 291–296. [DOI] [PubMed] [Google Scholar]
- 38. Huang, Z. , Guo, J. , Zhang, Y. , Wang, C. , Zhu, X. and Zhang, Y. , Porcine antilymphocyte globulin (p‐ALG) plus cyclosporine A (CsA) treatment in acquired severe aplastic anemia: a retrospective multicenter analysis. Ann. Hematol. 2015. 94: 955–962. [DOI] [PubMed] [Google Scholar]
- 39. Taylor, A. , Foo, S. S. , Bruzzone, R. , Dinh, L. V. , King, N. J. and Mahalingam, S. , Fc receptors in antibody‐dependent enhancement of viral infections. Immunol. Rev. 2015. 268: 340–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arvin, A. M. , Fink, K. , Schmid, M. A., Cathcart, A. , Spreafico, R. , Havenar‐Daughton, C. , Lanzavecchia, A. et al., A perspective on potential antibody‐dependent enhancement of SARS‐CoV‐2. Nature 2020. 584: 353–363. [DOI] [PubMed] [Google Scholar]
- 41. Yip, M. S. , Leung, N. H. , Cheung, C. Y. , Li, P. H. , Lee, H. H. , Daeron, M. , Peiris, J. S. et al., Antibody‐dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol. J. 2014. 11: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coish, J. M. and MacNeil, A. J. , Out of the frying pan and into the fire? Due diligence warranted for ADE in COVID‐19. Microbes Infect 2020. 22: 405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reynard, O. , Jacquot, F. , Evanno, G. , Mai, H. L. , Salama, A. , Martinet, B. , Duvaux, O. et al., Anti‐EBOV GP IgGs lacking alpha1‐3‐galactose and Neu5Gc prolong survival and decrease blood viral load in EBOV‐infected guinea pigs. PLoS One 2016. 11: e0156775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zylberman, V. , Sanguineti, S. , Pontoriero, A. V. , Higa, S. V. , Cerutti, M. L. , Morrone Seijo, S. M. , Pardo, R. et al., Development of a hyperimmune equine serum therapy for COVID‐19 in Argentina. Medicina (B Aires) 2020. 80: 1–6. [PubMed] [Google Scholar]
- 45. Yu, J. , Tostanoski, L. H. , Peter, L. , Mercado, N. B. , McMahan, K. , Mahrokhian, S. H. , Nkolola, J. P. et al., DNA vaccine protection against SARS‐CoV‐2 in rhesus macaques. Science 2020. 369: 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Casadevall, A. and Pirofski, L. A. , The convalescent sera option for containing COVID‐19. J. Clin. Invest. 2020. 130: 1545–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lagutina, I. , Lazzari, G. and Galli, C. , Birth of cloned pigs from zona‐free nuclear transfer blastocysts developed in vitro before transfer. Cloning Stem Cells 2006. 8: 283–293. [DOI] [PubMed] [Google Scholar]
- 48. Komatsu, E. , Buist, M. , Roy, R. , Gomes de Oliveira, A. G. , Bodnar, E. , Salama, A. , Soulillou, J. P. et al., Characterization of immunoglobulins through analysis of N‐glycopeptides by MALDI‐TOF MS. Methods 2016. 104: 170–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


