Abstract
Purpose
First, to investigate the added diagnostic value of chest computed tomography (CT) for evaluating COVID‐19 in symptomatic children by comparing chest CT findings with chest radiographic findings, and second, to identify the imaging signs and patterns on CT associated with COVID‐19 pneumonia in children.
Materials and Methods
From March 2020 to December 2020, 56 consecutive children (33 males and 23 girls; mean age ± SD, 14.8 ± 5.0 years; range, 9 months–18 years) with mild to moderate symptom and laboratory confirmed COVID‐19 (based on Centers for Disease Control criteria) underwent both chest radiography and chest CT on the same day within the first 2 days of initial presentation to the hospital. Two experienced radiologists independently evaluated chest radiographs and chest CT studies for thoracic abnormalities. The findings from chest radiography and chest CT were compared to evaluate the added diagnostic value of chest CT for affecting patient management. Interobserver agreement was measured with Cohen's κ statistics.
Results
Eleven (19.6%) of 56 patients had abnormal chest radiographic findings, including ground‐glass opacity (GGO) in 5/11 (45.4%) and combined GGO and consolidation in 6/11 (54.5%). On chest CT, 26 (46.4%) of 56 patients had abnormal CT findings, including combined GGO and consolidation in 19/26 (73.1%), GGO in 6/26 (23.1%), and consolidation in 1/26 (3.8%). Chest CT detected all thoracic abnormalities seen on chest radiography in 11/26 (42.3%) cases. In 15/26 (57.7%), chest CT detected lung abnormalities that were not observed on chest radiography, which included GGO and consolidation in 9/15 (60%), GGO in 5/15 (33.3%), and consolidation in 1/15 (6.6%) cases. These additional CT findings did not affect patient management. In addition, chest CT detected radiological signs and patterns, including the halo sign, reversed halo sign, crazy paving pattern, and tree‐in‐bud pattern. There was almost perfect interobserver agreement between the two reviewers for detecting findings on both chest radiographs (κ, 0.89, p = .001) and chest CT (κ, 0.96, p = .001) studies.
Conclusion
Chest CT detected lung abnormalities, including GGO and/or consolidation, that were not observed on chest radiography in more than half of symptomatic pediatric patients with COVID‐19 pneumonia. However, these additional CT findings did not affect patient management. Therefore, CT is not clinically indicated for the initial evaluation of mild to moderately symptomatic pediatric patients with COVID‐19 pneumonia.
Keywords: children, computed tomography, COVID-19 pneumonia, pediatric patients, radiography
1. INTRODUCTION
Since the first case of coronavirus disease 2019 (COVID‐19) was reported in China in November 2019, most COVID‐19 cases were epidemiologically linked to exposure to Wuhan's Huanan seafood market, where wild animals are traded. 1 , 2 This highly contagious, novel coronavirus infection is rapidly transmitted mainly via droplet inhalation, and has spread extensively among humans across many countries and continents throughout the world. 3 The World Health Organization (WHO) has declared COVID‐19 a public health emergency of international concern. As of February 7, 2021, there have been 106,673,989 confirmed cases globally with 2,326,773 deaths. 4
Initially, there was a misconception that COVID‐19 spares the pediatric population. However, emerging data have shown that children are indeed affected by this novel viral infection. In fact, there have been several reports describing COVID‐19 infection across all pediatric age groups, even affecting neonates. 5 , 6 , 7 , 8 Early and accurate diagnosis of pediatric COVID‐19 is paramount for optimal pediatric patient management and limiting the spread of disease, as it has been suggested that asymptomatic or mildly symptomatic children can be a reservoir of unrecognized COVID‐19 infection. 9
Chest imaging, mainly chest radiography and CT, are two main imaging modalities currently used for evaluating acute COVID‐19 infection in the pediatric population. 10 , 11 , 12 , 13 , 14 Although there have been several publications focusing on chest radiographic and CT findings of pediatric COVID‐19 pneumonia, 10 , 11 , 12 , 13 , 14 , 15 , 16 to our knowledge, there is no published information regarding the added diagnostic value of chest CT in comparison with chest radiography for detecting thoracic abnormalities related to COVID‐19 pneumonia in the pediatric population. In addition, there is limited information regarding the imaging signs and patterns seen on CT in symptomatic pediatric patients with COVID‐19 pneumonia. Therefore, the purpose of this study is twofold: (1) to investigate the added diagnostic value of chest CT for evaluating COVID‐19 in symptomatic children by comparing chest CT findings with chest radiographic findings and, (2) to identify the imaging signs and patterns on CT associated with COVID‐19 pneumonia in children.
2. MATERIALS AND METHODS
2.1. Institutional review board approval
The institutional review board approved this retrospective study for the review of chest radiographs, chest CT studies, and electronic medical records. The informed consent was waived. Patient confidentiality was maintained in accordance with Health Insurance Portability and Accountability Act (HIPAA) guidelines.
2.2. Patient population
Using our radiology department information system in the Al Ain Hospital, Al Ain, United Arab Emirates, we retrospectively identified all pediatric patients (≤18 years of age) diagnosed with COVID‐19 from March 2020 to December 2020. Included patients had to meet all three of the following inclusion criteria: (1) pediatric patients with COVID‐19 infection confirmed via quantitative reverse‐transcription polymerase chain reaction (RT‐PCR) testing of respiratory secretions obtained by use of a nasopharyngeal or oropharyngeal swab based on the Centers for Disease Control criteria 17 ; (2) symptomatic pediatric patients with COVID‐19 infection on the basis of clinical symptoms as determined by referring physicians; and (3) pediatric patients who underwent both chest radiography and chest CT studies on the same day within the first 2 days of initial presentation to the hospital.
2.3. Imaging technique
2.3.1. Chest radiography technique
Anteroposterior projection radiographs [n = 6 (10.7%)] were obtained with mobile radiography equipment (Digital Radiographic Mobile X‐ray System; Shimadzu) by using high voltage, tube current, and exposure times at a 100‐cm focus‐film distance. Posteroanterior projection radiographs [n = 50 (89.3%)] were obtained with non‐portable radiography equipment (Digital Diagnost; Philips Healthcare) by using high voltage, tube current, and exposure times at a 180‐cm focus‐film distance.
2.3.2. Chest CT technique
All chest CT studies were obtained with a 64‐slice helical CT scanner (Sensation 64; Siemens Healthcare) using a tube kilovoltage (kV), 100–120 kV; tube current (mAs), automatic exposure control; collimation, 2.0 mm; pitch, 1; reconstruction algorithm, iterative‐based reconstruction; reconstruction slice thickness, 0.5 mm; interslice gap, 0 mm and reformatted with lung (width, 1500 HU; level, −500 HU) and soft tissue (width, 350 HU; level, 50 HU) window settings. Intravenous contrast was not administered. With the patient in the supine position, CT images were obtained in a single breath‐hold at end‐inspiration. The scanned area included the entire lungs from the thoracic inlet to the level of the diaphragm.
2.4. Imaging study review
Two radiologists (K. M. D. and J. K.), each with more than 20 years of experience in interpreting pediatric chest radiographs and CT studies, independently evaluated all chest radiographs and CT images. Reviewers were blinded to all clinical information, prior imaging studies, and the original reports of chest radiographs and CT studies. In addition, the order of review was randomized. In the case of discrepant findings by two initial reviewers, a third pediatric radiology fellowship‐trained and subspecialty‐certified reviewer (E. Y. L., with 20 years of experience in interpreting pediatric chest radiography and CT study) served as an arbitrator, without knowledge of the findings and interpretations of the other two initial reviewers.
All chest radiographs and CT studies were reviewed in a picture archiving and communication system (Cedara I‐Read 5.2 P11; Cerner Image Devices). The chest CT images were evaluated by using standard lung (width, 1500 HU; level, −500 HU) and soft‐tissue (width, 350 HU; level, 50 HU) window settings. For both chest radiographs and CT studies, the reviewers could manually zoom into areas of interest. For chest CT studies, the reviewers routinely used multiplanar reformats (e.g., coronal and sagittal) and manually adjusted window level and width settings for evaluation of thoracic abnormalities.
2.4.1. Chest radiographic evaluation
2.4.1.1. Chest radiography image quality evaluation
Chest radiographs were first evaluated for image quality, including anatomic coverage, the presence of motion, and visualization of the chest radiographic findings. Suboptimal image quality chest radiographs, defined as incomplete anatomic coverage of chest, substantial motion, and limited visualization of thoracic anatomy, were excluded.
2.4.1.2. Chest radiographic review
The reviewers then reviewed chest radiographs for abnormalities in the thoracic structures including: (1) lung parenchyma and airway (ground‐glass opacity [GGO], consolidation, peribronchial thickening); (2) pleura (pleural effusion), and (3) mediastinum (lymphadenopathy) based on the established criteria from the Fleischner Society's glossary of terms for thoracic imaging in following section. 18 , 19 , 20
In addition, the reviewers were also instructed to record any other thoracic findings that were not included in the aforementioned diagnostic categories.
2.4.2. Chest CT evaluation
To decrease potential bias, chest CT evaluation was performed 2 weeks after chest radiographic evaluation, and chest CT studies were also re‐randomized before review.
2.4.2.1. Chest CT image quality evaluation
Chest CT images were first evaluated for image quality, including anatomic coverage, the presence of motion, and visualization of the chest CT findings. Suboptimal image quality chest CT studies, defined as incomplete anatomic coverage of chest, substantial motion, and limited visualization of thoracic anatomy, were excluded.
2.4.2.2. Chest CT review
The reviewers then reviewed chest CT studies for abnormalities in the thoracic structures including: (1) lung parenchyma and airway (GGO, consolidation, peribronchial thickening); (2) pleura (effusion), and (3) mediastinum (lymphadenopathy) based on the established criteria from the Fleischner Society's glossary of terms for thoracic imaging in following section. 18 , 19 , 20
In addition, the presence of radiological signs or patterns (“halo” sign, “reversed halo” sign, crazy paving pattern, and tree‐in‐bud pattern) was also evaluated. The “halo” sign was diagnosed when there was a GGO surrounding a pulmonary nodule or consolidation. The “reversed halo” sign was diagnosed when there was a focal rounded area of GGO surrounded by a more or less complete ring of consolidation. 18 Crazy‐paving pattern was defined as the presence of thickened interlobular septa superimposed on a background of GGO. 18 Tree‐in‐bud pattern was diagnosed when multiple areas of centrilobular nodules connected with a linear branching pattern were visualized. 18
In addition, the reviewers were also instructed to record any other thoracic findings that were not included in the aforementioned diagnostic categories.
2.5. Added diagnostic value of chest CT
One of the investigators (K. M. D.) in this study reviewed the patient's medical records to assess whether additional findings on CT affected patient management, specifically medication choice, hospital and intensive care unit (ICU) admission decision, and discharge decision. Then, the investigator recorded the findings.
2.6. Statistical analysis
Normally distributed continuous variables (e.g., age and the average days of clinical symptoms before image studies) were expressed as the mean ± standard deviation and range. The frequency and percentage of the presence of abnormalities seen in the lung parenchyma and airway, pleura, and mediastinum on the chest radiography and chest CT studies were calculated. The interobserver agreement was determined using Cohen's κ statistics. The strength of agreement interpretation beyond chance level is based on the Landis and Koch benchmarks. 21 , 22 κ values were interpreted as: 0–0.20 = slight agreement, 0.21–0.40 = fair agreement, 0.41–0.60 = moderate agreement, 0.61–0.80 = substantial agreement, and 0.81–1.00 = almost perfect agreement. Statistical software, STATA/SE version 14.2 (StataCorp LP), was used for the analysis.
3. RESULTS
3.1. Patient cohort
The total number of patients admitted and seen at our institution over the study period was 187 consecutive pediatric patients (95 males and 92 females) and all of them underwent chest radiography. Among these 187 patients, 56 patients underwent CT on the same day as chest radiography based on symptoms, as determined by the referring physicians. Therefore, the final patient population consisted of 56 consecutive children (33 males and 23 females; mean age ± SD, 14.8 ± 5.0 years; range, 9 months–18 years).
All patients had positive RT‐PCR test for COVID‐19 and had either mild or moderate symptoms. None of them required supplemental oxygen. All patients were subsequently admitted to the hospital, but none of them required ICU admission. The duration of their hospital admission ranged from 1 to 11 days (mean = 2.6 days). Upon discharge from the hospital, all patients were detained in a quarantine center for a total period of 15 days, including days in the hospital.
The details of the initial clinical symptoms are summarized in Table 1. Fever (28/56 (50%)) and cough [28/56 (50%)] were two most common initial clinical symptoms, followed by rhinorrhea [14/56 (25%)], dyspnea [13/56 (23.2%)], chest pain [12/56 (21.4%)], loss of smell [3/56 (5.4%)], myalgia [2/56(3.6%)], sore throat [2/56(3.6%)], headache [1/56 (1.8%)], and diarrhea [1/56 (1.8%)]. The average days of clinical symptoms before image studies were 2.6 days (±SD, ±2.3 days and range, 1–11 days).
Table 1.
Initial clinical symptoms in 56 pediatric patients with COVID‐19
| Types of symptoms | Number (percentage) of patients |
|---|---|
| Fever | 28 (50) |
| Cough | 28 (50) |
| Rhinorrhea | 14 (25) |
| Dyspnea | 13 (23.3) |
| Chest pain | 12 (21.4) |
| Loss of smell | 3 (5.4) |
| Myalgia | 2 (3.6) |
| Sore throat | 2 (3.6) |
| Headache | 1 (1.8) |
| Diarrhea | 1 (1.8) |
3.2. Imaging findings
3.2.1. Chest radiographic findings
The chest radiographic findings are summarized in Table 2. Among 56 patients, 45 patients (80.4%) had normal chest radiograph. The remaining eleven patients (19.6%) had abnormal chest radiographic findings, including GGO and consolidation in 6/11 (54.6%) and (GGO) in 5/11 (45.4%). No peribronchial thickening was seen. No pleural or mediastinal abnormality was seen. In addition, there were no other thoracic findings that were not included in the aforementioned diagnostic categories.
Table 2.
Chest radiographic findings in 56 symptomatic pediatric patients with COVID‐19
| Chest radiographic findings | Number (percentage) of studies |
|---|---|
| Normal CXR | 45 (80.4) |
| Abnormal CXR | 11 (19.6) |
| Type of abnormal CXR findings | |
| Peribronchial thickenings | 0 (0) |
| GGO | 5 (45.4) |
| Consolidation | 0 (0) |
| Peribronchial thickenings and GGO | 0 (0) |
| Peribronchial thickenings and consolidation | 0 (0) |
| GGO and consolidation | 6 (54.6) |
| Peribronchial thickenings, GGO, and consolidation | 0 (0) |
Abbreviations: CXR, chest radiography; GGO, ground‐glass opacity.
3.2.2. Chest CT findings
3.2.2.1. Findings of chest CT studies
The chest CT findings are summarized in Table 3. Among 56 patients, 30 patients (53.6%) had normal chest CT studies. The remaining 26 (46.4%) of 56 patients had abnormal CT findings, including GGO and consolidation in 19/26 (73.1%), GGO in 6/26 (23.1%), and consolidation in 1/26 (3.8%). No peribronchial thickening was seen. No pleural or mediastinal abnormality was seen. In addition, there were no other thoracic findings that were not included in the aforementioned diagnostic categories.
Table 3.
Chest CT findings in 56 symptomatic pediatric patients with COVID‐19
| Chest CT findings | Number (percentage) of studies |
|---|---|
| Normal chest CT | 30 (53.6) |
| Abnormal chest CT | 26 (46.4) |
| Type of abnormal chest CT findings | |
| Peribronchial thickenings | 0 (0) |
| GGO | 6 (23.1) |
| Consolidation | 1 (3.8) |
| Peribronchial thickenings and GGO | 0 (0) |
| Peribronchial thickenings and consolidation | 0 (0) |
| GGO and consolidation | 19 (73.1) |
| Peribronchial thickenings, GGO, and consolidation | 0 (0) |
| Signs and patterns of chest CT findings | |
| Halo sign | 11 (42.3) |
| Reversed halo sign | 5 (19.2) |
| Crazy‐paving pattern | 2 (7.6) |
| Tree‐in‐bud pattern | 1 (3.8) |
Abbreviations: CT, computed tomography; GGO, ground‐glass opacity.
3.2.2.2. Findings of radiological signs and pattern
On CT, the most frequently seen radiological signs or patterns were: “halo” sign (11/56, 42.3%), followed by “reversed halo” sign (5/56, 19.2%), crazy paving pattern (2/56, 7.6%), and tree‐in‐bud pattern (1/56, 3.8%).
3.2.3. Comparison of chest radiographic and chest CT Findings
The comparison of chest radiographic and chest CT findings is summarized in Table 4.
Table 4.
Comparison of chest radiographic and chest CT findings in 56 symptomatic pediatric patients with COVID‐19 pneumonia
| Chest imaging findings | Number (percentage) of studies |
|---|---|
| Abnormal CXR and abnormal chest CT | 11 (19.6) |
| Normal CXR and normal chest CT | 30 (53.6) |
| Normal CXR and abnormal chest CT | 15 (26.8) |
| Type of abnormal chest CT findings that were not seen on CXR (n = 15) | |
| Peribronchial thickenings | 0 (0) |
| GGO | 5 (33.3) |
| Consolidation | 1 (6.7) |
| Peribronchial thickenings and GGO | 0 (0) |
| Peribronchial thickenings and consolidation | 0 (0) |
| GGO and consolidation | 9 (60.0) |
| Peribronchial thickenings, GGO, and consolidation | 0 (0) |
Abbreviations: CT, computed tomography; CXR, chest radiography; GGO, ground‐glass opacity.
Chest CT studies detected all thoracic abnormalities seen on chest radiographs in 11/26 (42.3%) cases, including combined GGO and consolidation in 6/11 (54.6%) and GGO in 5/11 (45.4%) (Figure 1). In 15/26 (57.7%), chest CT detected lung abnormalities that were not observed on chest radiography, which included combined GGO and consolidation in 9/15 (60%), GGO in 5/15 (33.3%), and consolidation in 1/15 (6.7%) cases (Figure 2).
Figure 1.
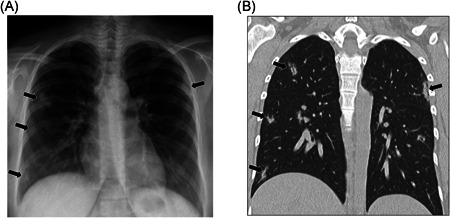
A 16‐year‐old female with positive COVID‐19 RT‐PCR test who presented with fever and cough. (A) Frontal chest radiograph shows multifocal ground‐glass opacities and consolidations (arrows) in both lungs. (B) Coronal lung window CT image demonstrates bilateral multifocal ground‐glass opacities and consolidation (arrows) corresponding with findings seen on chest radiograph (A). CT, computed tomography; RT‐PCR, reverse‐transcription polymerase chain reaction
Figure 2.
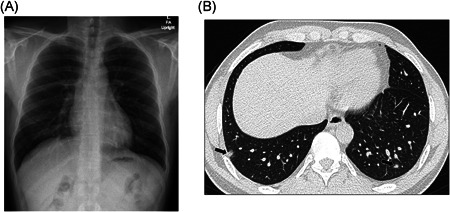
A 15‐year‐old male with positive COVID‐19 RT‐PCR test who presented with fever and chest pain. (A) Frontal chest radiograph shows clear lungs without radiographic abnormality. (B) Axial lung window CT image shows focal small areas of consolidation and subtle ground‐glass opacities (arrows) in the bilateral lower lobes, which were not detected on chest radiography. CT, computed tomography; RT‐PCR, reverse‐transcription polymerase chain reaction
In addition, chest CT detected radiological signs and patterns, such as “halo” sign [4/15 (26.7%)] (Figure 3), “reversed halo” sign [2/15 (13.3%)] (Figure 4), crazy paving pattern [2/15 (13.3%)] (Figure 5), and tree‐in‐bud pattern [1/15 (6.7%)] (Figure 6) that were not observed on chest radiographs.
Figure 3.
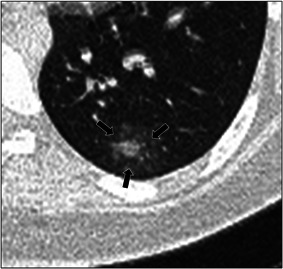
A 12‐year‐old male with positive COVID‐19 RT‐PCR test who presented with cough. Axial lung window CT image shows a ground‐glass opacity (arrows) surrounding a central area of consolidation, in keeping with the “halo” sign. CT, computed tomography; RT‐PCR, reverse‐transcription polymerase chain reaction
Figure 4.
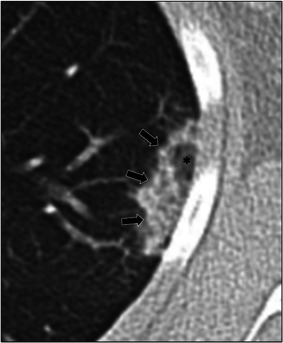
A 16‐year‐old female with positive COVID‐19 RT‐PCR test who presented with fever and rhinorrhea. Axial lung window CT image shows presence of central ground‐glass opacity (asterisk) surrounded by ring of denser consolidation (arrows), also known as the “reversed halo” sign. CT, computed tomography; RT‐PCR, reverse‐transcription polymerase chain reaction
Figure 5.
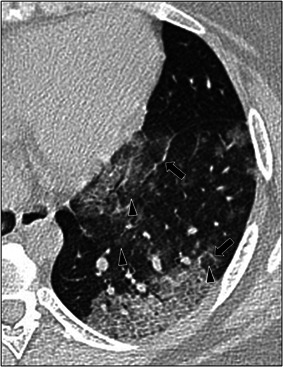
A 17‐year‐old female with positive COVID‐19 RT‐PCR test who presented with fever and cough. Axial lung window CT image shows areas of thickened interlobular septa and intralobular lines (arrows) superimposed on a background of ground‐glass opacity (arrowheads) in the left lower lobe, also known as crazy‐paving pattern. CT, computed tomography; RT‐PCR, reverse‐transcription polymerase chain reaction
Figure 6.
A 15‐year‐old male with positive COVID‐19 RT‐PCR test who presented with cough and dyspnea. Axial lung window CT image shows multiple small centrilobular nodules with connection to opacified or thickened branching structure representing the dilated and opacified bronchioles or inflamed arterioles, also known as tree‐in‐bud pattern. CT, computed tomography; RT‐PCR, reverse‐transcription polymerase chain reaction
3.2.4. Interobserver agreement
Among all 224 evaluations (112 evaluations for the chest radiography and 112 evaluations for CT studies, respectively) between two reviewers, discrepancies were noted regarding peribronchial thickening versus normal on two chest radiographs and GGO versus consolidation on one chest CT study. The arbitrator assessed these cases independently and determined that two chest radiographs were normal and chest CT showed consolidation. There was almost perfect interobserver agreement between two reviewers for detecting findings on chest radiographs (κ, 0.89, p = .001) and chest CT studies (κ, 0.96, p = .001).
3.3. Added diagnostic value of chest CT
Chest CT detected lung abnormalities, including GGO and/or consolidation, that were not observed on chest radiography in 15/26 (57.7%) in symptomatic pediatric patients with COVID‐19 pneumonia. However, these additional CT findings did not affect patient management in all 15 pediatric patients (100%).
4. DISCUSSION
The results of our study showed that chest CT detected all thoracic abnormalities, including GGO and consolidation, seen on chest radiography in mild to moderately symptomatic pediatric patients with COVID‐19 infection. In addition, in more than half of cases (57.7%), chest CT detected lung abnormalities, such as GGO and consolidation, that were not observed on chest radiography. However, these additional CT findings did not affect patient management. Therefore, in mild to moderately symptomatic pediatric patients with COVID‐19 pneumonia and normal chest radiography, CT is not clinically indicated as part of the initial evaluation.
Although there have been a few studies focusing on the thoracic imaging findings of pediatric COVID‐19 infection, 10 , 11 , 12 , 13 , 14 , 15 , 16 , 23 , 24 to our knowledge, there has been no published study that specifically investigated the added diagnostic value of chest CT in comparison with chest radiography for detecting thoracic abnormalities related to COVID‐19 infection in the pediatric population. Although chest radiographs are not as sensitive as CT for detecting early or subtle pulmonary parenchymal manifestations of pediatric COVID‐19 pneumonia, our study showed that additional, radiographically occult CT findings of COVID‐19 pneumonia did not significantly alter patient management in mild‐moderately symptomatic children at initial presentation. Further studies evaluating the role of CT (and its increased sensitivity for the findings of pediatric COVID‐19 pneumonia) in severely ill children and/or during the course of their hospitalization may be helpful.
The chest radiographic findings of GGO and/or consolidation seen in our study correlate well with the findings of other studies of thoracic abnormalities in pediatric patients with COVID‐19 on chest radiography. 10 , 15 , 24 Previously published studies of COVID‐19 infection in pediatric patients found that unilateral or bilateral GGO and/or consolidation were the most common chest radiographic findings. 10 , 15 , 23 Lack of pleural or mediastinal abnormalities on chest radiography in pediatric patients with COVID‐19 in our study is also concordant with chest radiographic findings from prior studies in the pediatric population. 10 , 15 , 23 Interestingly, the previously described radiographic finding of peribronchial thickening in pediatric patients with COVID‐19 pneumonia and other viral infections was not seen in our patient population. 19 , 25 Such peribronchial thickening is often seen in asymptomatic or mildly symptomatic pediatric patients during early phase of viral infection. It is possible that peribronchial thickening, which is an early viral infectious manifestation on chest radiography, may have been already progressed to the alveolar level, as GGO or consolidation, in our study, which consisted only of symptomatic pediatric patients.
The results of our study also showed that, although the pulmonary parenchymal abnormalities seen on chest CT (e.g., GGO and consolidation) in the pediatric patients with COVID‐19 pneumonia are similar to previously published studies that investigated chest CT findings in pediatric patients with COVID‐19, 11 , 12 , 16 , 23 , 24 our study found the frequency (46.4%) of chest CT with positive findings to be higher than the previously published frequency (23%) of chest CT with positive findings reported by Steinberger et al. in the pediatric patients with COVID‐19 pneumonia. 11 This is most likely due to the difference in patient population between our study and their study. While our study only included symptomatic pediatric patients, Steinberger et al.'s study included both symptomatic (70%) and asymptomatic (30%) pediatric patients, where all asymptomatic patients had negative CT findings. 11 In our study, it is understandable that the majority (93.3%) of findings, that were not detected on chest radiographs but only observed on chest CT, had a component of GGO, which is often difficult to appreciate on chest radiography. Previously published study in adult patients focusing on the relationship of chest imaging findings and duration of infection showed that GGO is typically observed during early phase of COVID‐19 infection, and ultimately progresses to more conspicuous consolidation in later phases of infection. 26
Timely and accurate recognition of radiological signs and patterns typically associated with COVID‐19 can lead to optimal pediatric COVID‐19 patient management. Our study showed that the “halo” sign, “reversed halo” sign, crazy‐paving pattern, and tree‐in‐bud pattern were detected on chest CT studies in symptomatic pediatric patients with COVID‐19 infection. Among them, the most frequently seen radiological signs or patterns was “halo” sign (42.3%), followed by “reversed halo” sign (19.2%), crazy paving pattern (7.6%), and tree‐in‐bud pattern (3.8%). We believe that the “halo” sign, which is currently considered to be present during early phase of COVID‐19 infection in the pediatric population, 10 , 15 , 16 is particularly important because it is unique to pediatric patients with COVID‐19 infection in comparison with adults with COVID‐19 infection. As pediatric COVID‐19 pneumonia progresses, crazy‐paving pattern (likely due to underlying alveolar edema coupled with interstitial inflammation) and “reversed halo” sign (likely representing the healing phase of lung infection) are more frequently seen. 10 , 11 , 15 , 27 Our findings of crazy‐paving pattern and “reversed halo” sign on chest CT studies of symptomatic pediatric patients with COVID‐19 correlate well with findings from previously published studies in the pediatric population. 10 , 11 , 15 Interestingly, one patient (3.8%) in our study showed tree‐in‐bud pattern on chest CT study, which has not been previously reported in pediatric patients, however has been rarely described in adult COVID‐19 infection. 28 We believe that the tree‐in‐bud pattern on chest CT study is not typical for COVID‐19 infection and is usually indicative of other underlying disease (e.g., aspiration bronchopneumonia) or co‐infection (e.g., pulmonary tuberculosis). Future investigation on chest CT findings of tree‐in‐bud pattern in pediatric patients with COVID‐19 infection in the larger patient population will be needed for clarification.
Currently, the American College of Radiology and international expert consensus statement on chest imaging in pediatric COVID‐19 patient management assert that CT should not be used as the initial diagnostic test particularly in asymptomatic pediatric patients with COVID‐19 acknowledging that the PCR test for COVID‐19 is more reliable than CT. 29 , 30 Based on the results of our study, although chest CT can detect early lung abnormalities, such as GGO, as well as specific radiological signs and patterns, CT is not clinically indicated for initial evaluation of symptomatic pediatric patients with COVID‐19 pneumonia because it does not affect the patient management, even in pediatric patients with mild or moderate symptoms. Perhaps, the role of CT may be reserved just for evaluating COVID‐19‐related complications in pediatric patients with severe and progressively worsening symptoms despite treatment. However, we would like to emphasize that, if chest CT is considered in symptomatic pediatric patients with COVID‐19 for specific clinical indications, such as evaluating COVID‐19‐related complications, radiation dose should be tailored following as low as reasonably achievable (ALARA) principle due to the potentially harmful effect of radiation especially in the pediatric patient population. 31 , 32 , 33
There are several limitations of the present study. First, this study consisted of a relatively small patient population. However, we emphasize that obtaining more than fifty consecutive symptomatic pediatric patients with laboratory‐confirmed COVID‐19 infection, with both chest radiograph and chest CT performed on the same day, within the first 2 days of initial presentation to the hospital is challenging, and to current knowledge, our study is the first one to present such data. Second, our patient population was mainly from the Middle East region, and consequently, our data may be region‐specific. Future studies with pediatric patients from different regions around the world that can confirm the results of our study globally will be helpful. Third, to decrease the overall radiation exposure, only frontal chest radiographs were obtained in our study population. Although we acknowledge that addition of lateral chest radiographs may have increased the detection of subtle retrocardiac abnormalities and/or small pleural effusions on lateral radiographs, we believe that this did not have a substantial effect on our findings. Lastly, our study only focused on chest imaging findings of pediatric patients with COVID‐19 infection at its initial presentation. Future studies aimed at identifying the long‐term sequelae of chest imaging findings in pediatric patients with COVID‐19 on follow‐up imaging evaluation is needed to elucidate the potential residual or permanent lung findings related to pediatric COVID‐19 infection.
In conclusion, our investigation was the first study to evaluate the added diagnostic value of chest CT by directly correlating chest radiographic and chest CT findings of consecutive symptomatic pediatric patients with COVID‐19. Our findings showed that although chest CT detected lung abnormalities, including GGO and/or consolidation, that were not observed on chest radiography in more than half of symptomatic pediatric patients with COVID‐19 pneumonia as well as imaging signs and patterns associated with COVID‐19 pneumonia, these additional CT findings did not affect patient management. Therefore, CT is not clinically indicated for initial evaluation of mild to moderately symptomatic pediatric patients with COVID‐19 pneumonia. Future studies focusing on the role of CT for evaluation of pediatric COVID‐19 patients with severe symptoms, development of potential complications, and late sequelae are needed to refine the appropriateness of chest CT in the evaluation of pediatric patients with COVID‐19.
AUTHOR CONTRIBUTIONS
Karuna M. Das: conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); writing original draft (equal); writing review & editing (equal). Jamal A. Alkoteesh: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Jumaa Al Kaabi: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Taleb Al Mansoori: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Abbey J. Winant: investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Rajvir Singh: conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Rajesh Paraswani: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Rizwan Syed: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Elsadeg M. Sharif: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Ghazala B. Balhaj: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal). Edward Y. Lee: conceptualization (equal); investigation (equal); methodology (equal); writing original draft (equal); writing review & editing (equal).
Das KM, Alkoteesh JA, Al Kaabi J, et al. Comparison of chest radiography and chest CT for evaluation of pediatric COVID‐19 pneumonia: Does CT add diagnostic value? Pediatric Pulmonology. 2021;56:1409–1418. 10.1002/ppul.25313
REFERENCES
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Eng J Med. 2020;392(12):1199‐1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)20183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foust AM, Johnston PR, Kasznia‐Brown J, et al. Perceived impact of COVID‐19 on pediatric radiology departments around the world: WFPI COVID‐19 Task Force survey results from 6 continents [published online ahead of print September 17, 2020]. Radiology Cardiothorac Imaging. 10.1148/ryct.2020200422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Worldometer . COVID‐19 CORONAVIRUS PANDEMIC; 2021. https://www.worldometers.info/coronavirus/. Accessed February 7, 2021.
- 5. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 6. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689‐696. 10.1016/S1473-3099(20)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wenliang S, Li J, Zou N, Guan W, Pan J, Xu W. Clinical features of pediatric patients with coronavirus disease (COVID‐19). J Clin Virol. 2020;127:104377. 10.1016/j.jcv.2020.104377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoang A, Chorath K, Moreira A, et al. COVID‐19 in 7780 pediatric patients: a systemic review [published online ahead of print June 26, 2020]. EClinicalMedicine. 10.1016/j.eclinm.2020.100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zimmerman P, Curtis N. Coronavirus infections in children including COVID‐19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;20(20):1‐14. 10.1097/INF.0000000000002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foust AM, McAdam AJ, Chu WC, et al. Practical guide for pediatric pulmonologists on imaging management of pediatric patients with COVID‐19. Pediatr Pulmonol. 2020;55(9):2213–2224. 10.1002/ppul.24870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steinberger S, Lin B, Bernheim A, et al. CT features of coronavirus disease (COVID‐19) in 30 pediatric patients. AJR Am J Roentgenol. 2020;21:1‐9. 10.2214/AJR.20.23145 [DOI] [PubMed] [Google Scholar]
- 12. Duan Y‐N, Zhu Y‐Q, Tang L‐L, Qin J. CT features of novel coronavirus pneumonia (COVID‐19) in children. Eur Radiol. 2020;30(8):4427‐4433. 10.1007/s00330-020-06860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foust AM, Winant AJ, Restrepo R, Liszewski MC, Plut D, Lee EY. Private tour guide to pediatric coronavirus disease of 2019 and multisystem inflammatory syndrome in children in 10 minutes: what thoracic radiologists need to know. J Thorac Imaging. 2021;36(1):24‐30. 10.1097/RTI.0000000000000565 [DOI] [PubMed] [Google Scholar]
- 14. Winant AJ, Blumfield E, Liszewski MC, Kurian J, Foust AM, Lee EY. Thoracic imaging findings of multisystem inflammatory syndrome in children (MIS‐C) associated with COVID‐19: what radiologists need to know now [published online ahead of print July 30, 2020]. Radiol Cardiothorac Imaging. 10.1148/ryct.2020200346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foust AM, Winant AJ, Chu WC, Das KM, Philips GS, Lee EY. Pediatric SARS, H1N1, MERS, EVLAI, and now coronavirus disease (COVID‐19) pneumonia: what radiologists need to know. AJR Am J Roentgenol. 2020;30:1‐9. 10.2214/AJR.20.23267 [DOI] [PubMed] [Google Scholar]
- 16. Xia w, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169‐1174. 10.1002/ppul.24718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID‐19). Overview of testing for SARS‐CoV‐2; 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed February 7, 2021.
- 18. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697‐722. 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 19. Lee EY, McAdam AJ, Chaudry G, Fishman MP, Zurakowski D, Boiselle PM. Swine‐origin influenza a (H1N1) viral infection in children: initial chest radiographic findings. Radiology. 2010;254(3):934‐941. 10.1148/radiol.09092083 [DOI] [PubMed] [Google Scholar]
- 20. Lynch DA, Brasch RC, Hardy KA, Webb WR. Pediatric pulmonary disease: assessment with high‐resolution ultrafast CT. Radiology. 1990;176(1):243‐248. 10.1148/radiology.176.12353097 [DOI] [PubMed] [Google Scholar]
- 21. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228(2):303‐308. 10.1148/radiol.2282011860 [DOI] [PubMed] [Google Scholar]
- 22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159‐174. [PubMed] [Google Scholar]
- 23. Chen A, Huang J, Liao Y, et al. Differences in clinical and imaging presentation of pediatric patients with COVID‐19 in comparison with adults [published online ahead of print April 6, 2020]. Radiol Cardiothorac Imaging. 10.1148/ryct.2020200117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Z, Fan H, Cai J, et al. High‐resolution computed tomography manifestations of COVID‐19 infections in patients of different ages. Eur J Radiol. 2020;126:108972. 10.1016/j.ejrad.2020.108972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bramson RT, Griscom NT, Cleveland RH. Interpretation of chest radiographs in infants with cough and fever. Radiology. 2005;236(1):22‐29. 10.1148/radiol.2361041279 [DOI] [PubMed] [Google Scholar]
- 26. Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease‐19 (COVID‐19): relationship to duration of infection. Radiology. 2020;295(3):200463. 10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carotti M, Salaffi F, Sarzi‐Puttini P, et al. Chest CT features of coronavirus disease 2019 (COVID‐19) pneumonia: key points for radiologists. Radiol Med. 2020;125(7):636‐646. 10.1007/s11547-020-01237-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng Z, Lu Y, Cao Q, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID‐19) in a single‐center study in Shanghai, China. AJR Am J Roentgenol. 2020;215(1):121‐126. 10.2214/AJR.20.22959 [DOI] [PubMed] [Google Scholar]
- 29. American College of Radiology . ACR Recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID‐19 infection; 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed February 7, 2021.
- 30. Foust AM, Phillips GS, Chu WC, et al. International expert consensus statement on chest imaging in pediatric COVID‐19 patient management: imaging study reporting and imaging study recommendations [published online ahead of print April 23, 2020]. Radiol Cardiothorac Imaging. 10.1148/ryct.2020200214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sodhi KS, Lee EY. What all physicians should know about the potential radiation risk that computed tomography poses for paediatric patients. Acta Paediatr. 2014;103(8):807‐811. 10.1111/apa.12644 [DOI] [PubMed] [Google Scholar]
- 32. Sodhi KS, Krishna S, Saxena AK, Sinha A, Khandelwal N, Lee EY. Clinical application of “justification” and “optimization” principle of ALARA in pediatric CT imaging: “how many children can be protected from unnecessary radiation?”. Eur J Radiol. 2015;84(9):1752‐1757. 10.1016/j.ejrad.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 33. Macdougall RD, Strauss KJ, Lee EY. Managing radiation dose from thoracic multidetector computed tomography in pediatric patients: background, current issues, and recommendations. Radiol Clin North Am. 2013;51(4):743‐760. 10.1016/j.rcl.2013.04.007 [DOI] [PubMed] [Google Scholar]


