COVID‐19 brought multiple societal challenges that became a catalyst for worsening health in people with Parkinson's disease (PwPD) 1 and brought disruption in their social activities. 2 It is hypothesized that the COVID‐19 confinement also impacted the functional mobility of PwPD. One of the building blocks of functional mobility is the ability to walk. It enables independence and participation in social activities and contributes to global health. Long‐term unsupervised assessments are crucial to quantify walking activity from ecologically valid and patient‐relevant environments and overcome the limitations of conventional clinical assessments. 3
The COVID‐19 pandemic caught us in the middle of a longitudinal study about the feasibility and usability of a CE‐marked medical device smartphone application (Kinetikos, Coimbra, Portugal) for long‐term unsupervised walking mobility quantification, remote assessment of motor signs, longitudinal (onetime or regular) assessments of self‐reported global health outcomes and medication management tested 24/7 over 8 months including 16 PwPD (mean ± SD age, 61.1 ± 11.8 years; 3 women [19%]; mean ± SD Hoehn and Yahr, 2.0 ± 0.5; 8 fluctuators [50%]; mean ± SD Movement Disorders Society–Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) total score, 51.3 ± 26.5; part I score, 9.8 ± 5.6; part II score, 10.6 ± 7.1; and part III score, 27.6 ± 16.7). All patients were asked to register in the application when they took their medication.
Data collected to date show that mobile‐based measures successfully captured differences between the preconfinement and the confinement periods. Figure 1 shows a marked reduction imposed by the COVID‐19 confinement in the mobility of PwPD (walking minutes/day), corroborating previous reports about the disrupted activities of surveyed PwPD. 2 In line with previous studies, 4 most PwPD failed to meet the recommended 30 minutes of activity per day (18.81 ± 14.13 min/day), being aggravated by 44% during the confinement. Interestingly, those who opted for an urban exodus (n = 3) saw their walking activity increased by 99% — identified by the oblique stripes pattern in Figure 1 — reaching 27.68 ± 25.83 min/day. At the end of the study and compared with baseline, MDS‐UPDRS part I changed by 0.9 ± 6.3, part II by −1.5 ± 3.1, and part III by −2.3 ± 14.6, supporting that there was no relevant disease disability aggravation. Similarly, therapeutic adherence did not differ between the preconfinement and the confinement periods. None of the participants suffered from COVID‐19 infection during the period of the study. These results support the conclusion that the impact on the activity of PwPD was mainly because of the confinement and not of aggravation in PD disability. However, these short‐term findings do not completely exclude the possibility of later complications.
FIG. 1.
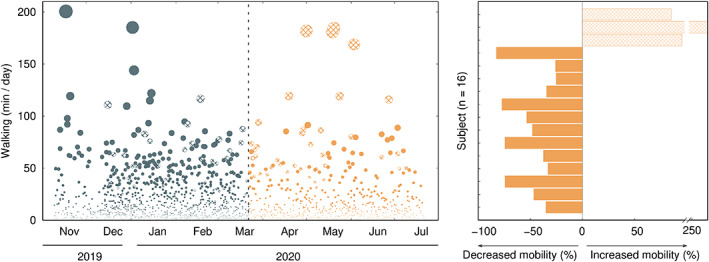
Left, daily accumulated walking minutes per individual. Right, percentage of change in walking activity associated with COVID‐19 confinement. Gray (orange) dots stand for the period before (during) confinement. Uniformly filled circles and bars stand for individuals with decreased walking activity because of COVID‐19, whereas the oblique stripe pattern corresponds to individuals with increased walking activity.
It is becoming evident how the pandemic is impacting the lives of PwPD even in a country like Portugal, where the magnitude of the first wave was relatively low. 5 A multicentric study would clarify the heterogeneity across countries. Unsupervised mobility quantification from daily living environments adds information to support a clinical decision, complements surveyed data, and serves as outcomes in clinical trials, overcoming the so‐far lagged confirmation of its potential. 6
Authors’ Roles
1. Research project: A. Conception, B. Organization, C. Execution;
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
3. Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
Diogo Vila Viçosa — 1B, 2A, 2B, 2C, 3A, 3B.
Ana Clemente — 1B, 1C, 3B.
Filipa Pona‐Ferreira — 1B, 1C, 3B.
Mariana Leitão — 1B, 1C, 3B.
Raquel Bouça‐Machado — 1B, 1C, 3B.
Linda Azevedo Kauppila — 1B, 1C, 3B.
Rui M. Costa — 2C, 3B.
Ricardo Matias — 1A, 1B, 2C, 3A, 3B.
Joaquim J. Ferreira — 1A, 1B, 2C, 3B.
Financial Disclosures for Previous 12 Months
Diogo Vila Viçosa reports no additional disclosures.
Ana Clemente reports no additional disclosures.
Filipa Pona‐Ferreira reports no additional disclosures.
Mariana Leitão reports no additional disclosures.
Raquel Bouça‐Machado reports no additional disclosures.
Linda Azevedo Kauppila reports no additional disclosures.
Rui M. Costa reports no additional disclosures; has been on the advisory boards of Medicane; has intellectual property rights with MindReach; and is employed by Zuckerman Mind Brain Behavior Institute, Columbia University, New York, New York.
Ricardo Matias reports no additional disclosures.
Joaquim J. Ferreira has no conflicts of interest to report; is a consultant for Ipsen, GlaxoSmithKline, Novartis, Teva, Lundbeck, Solvay, Abbott, AbbVie, BIAL, Merck‐Serono, Merz, Sunovion, Affiris, and Zambon; is on the advisory boards of BIAL amd Lundbeck; has received honoraria from AbbVie, BIAL, Sunovion, Medtronic, and Zambon; has received grants from GlaxoSmithKline, Grunenthal, Teva, and Fundação MSD; has given expert testimony for Novartis; and is employed by Faculdade de Medicina da Universidade de Lisboa, CNS – Campus Neurológico Sénior.
Ethics Statement
The studies involving human participants were reviewed and approved by the CNS Ethics Committee (Ref. 06–2019). The patients/participants provided their written informed consent to participate in this study.
Relevant conflicts of interest/financial disclosures: R.M.C. as a shareholder position at Kinetikos. R.M. is a shareholder of a Kinetikos’ affiliated company. D.V.V. and A.C. are employees at Kinetikos. The other authors have no conflicts of interest to declare.
Funding agencies: No specific funding was received for this work.
References
- 1. Schirinzi T, Cerroni R, Di Lazzaro G, et al. Self‐reported needs of patients with Parkinson's disease during COVID‐19 emergency in Italy. Neurol Sci 2020;3:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown E, Chahine L, Goldman S, et al. The effect of the COVID‐19 pandemic on people with Parkinson's disease. J Parkinsons Dis 2020;10(4):1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warmerdam E, Hausdorff J, Atrsaei S, et al. Long‐term unsupervised mobility assessment in movement disorders. Lancet Neurol 2020;19(5):462–470. [DOI] [PubMed] [Google Scholar]
- 4. van Nimwegen M, Speelman AD, Overeem S, et al. Promotion of physical activity and fitness in sedentary patients with Parkinson's disease: randomised controlled trial. BMJ 2013;346:f576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kontis V, Bennett JE, Rashid T, et al. Magnitude, demographics and dynamics of the effect of the first wave of the COVID‐19 pandemic on all‐cause mortality in 21 industrialized countries. Nat Med 2020;26(12):1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Artusi CA, Imbalzano G, Sturchio A, et al. Implementation of Mobile health Technologies in Clinical Trials of movement disorders: underutilized potential. Neurotherapeutics 2020. 10.1007/s13311-020-00901-x [DOI] [PMC free article] [PubMed] [Google Scholar]


