Abstract
Background
COVID‐19 disease can lead to severe functional impairments after discharge. We assessed the quality of life of invasively ventilated COVID‐19 ARDS survivors.
Methods
We carried out a prospective follow‐up study of the patients admitted to the Intensive Care Units (ICUs) of a teaching hospital. Patients affected by COVID‐19 ARDS who required invasive ventilation and were successfully discharged home were assessed through the telephone administration of validated tests. We explored survival, functional outcomes, return to work, quality of life, cognitive and psychological sequelae. The main variables of interest were the following: demographics, severity scores, laboratory values, comorbidities, schooling, working status, treatments received during ICU stay, complications, and psychological, cognitive, functional outcomes.
Results
Out of 116 consecutive invasively ventilated patients, overall survival was 65/116 (56%) with no death occurring after hospital discharge. Forty‐two patients were already discharged home with a median follow‐up time of 61 (51‐71) days after ICU discharge and 39 of them accepted to be interviewed. Only one patient (1/39) experienced cognitive decline. The vast majority of patients reported no difficulty in walking (32/35:82%), self‐care (33/39:85%), and usual activities (30/39:78%). All patients were either malnourished (15/39:38%) or at risk for malnutrition (24/39:62%). Exertional dyspnea was present in 20/39 (51%) patients. 19/39 (49%) reported alterations in senses of smell and/or taste either before or after hospitalization.
Conclusions
Invasively ventilated COVID‐19 ARDS survivors have an overall good recovery at a 2‐months follow‐up which is better than what was previously reported in non‐COVID‐19 ARDS patients.
Keywords: Acute respiratory distress syndrome, COVID‐19, critically ill patients, follow‐up, intensive care, quality‐of‐life
Editorial Comment.
In this prospective follow‐up of survivors after severe COVID‐19 ARDS, 39 of 42 patients discharged to their homes were assessed. There was an overall good recovery 2 months after discharge, with reduced body weight and exertional dyspnea being the main complaints.
1. INTRODUCTION
The Coronavirus Disease 2019 (COVID‐19) pandemic led to a dramatic number of Intensive Care Unit (ICU) admissions. In Italy, as of November 7th, 2020, 902,490 people were diagnosed with SARS‐CoV‐2 infection, 1 and 41,063 died.
COVID‐19 is in most cases a self‐limited lower respiratory tract illness, but in some patients, it may cause acute respiratory distress syndrome (ARDS), shock, and multi‐organ failure. 2 , 3 , 4 Long‐term clinical outcomes of ARDS survivors is a topic of high interest 5 ; over the years, ARDS mortality declined but its incidence increased, and a growing number of ARDS survivors present functional, psychological, and cognitive consequences persisting for years. 6 Long‐term follow‐up of survivors of Severe Acute Respiratory Syndrome (SARS‐CoV‐1) and Middle East Respiratory Syndrome (MERS) showed a high prevalence of post‐traumatic stress disorder (PTSD) (39%), depression (33%), anxiety (30%), and reduced quality of life. 7
Up to one‐third of general ICU patients develop the so‐called Post‐Intensive Care Syndrome (PICS), 8 which includes cognitive, physical, and psychological sequelae, occurring independently of the reason for ICU admission. PICS leads to significant burden and costs for patients, caregivers, and society. It reduces patients’ quality of life, due to an impaired physical and cognitive functioning and a delay or inability to return to work. Follow‐up ICU of patients can facilitate prompt recognition and treatment of PICS and improve long‐term physical, psychological, and cognitive outcomes. 9
The short‐term mortality of invasively ventilated COVID‐19 ARDS patients is extremely high, in the range of 80%‐90%, 10 , 11 and the middle‐term outcome and quality of life of survivors is unknown. COVID‐19 is severe and multifactorial, and it involves several organs and systems. 12 In the hypothesis that COVID‐19 disease can lead to severe functional impairments after discharge, the primary aim of this study was to assess the quality of life of invasively ventilated COVID‐19 ARDS survivors.
2. METHODS
2.1. Study design and setting
This study is part of the COVID‐BioB study, an observational investigation performed at San Raffaele Scientific Institute—a 1,350‐bed university hospital in Milan, Italy. The study was approved by the hospital Ethics Committee (protocol No. 34/int/2020) and was registered on ClinicalTrials.gov (NCT04318366). All the authors reviewed the manuscript and vouch for the accuracy and completeness of the data and adherence to the study protocol.
Our hospital was immediately involved in the management of the COVID‐19 surge. Since the beginning, a reorganization of large areas of the hospital took place, in order to admit COVID‐19 patients, and elective surgical activity was rapidly reduced and then stopped. 2 , 3 , 13 The emergency department admitted simultaneously up to 70 patients requiring oxygen therapy or non‐invasive ventilation (NIV). In a few days, we had a total of 279 general ward beds dedicated to COVID‐19 patients; moreover, the ICU beds were also increased from 28 to 72 (54 of them dedicated to critical COVID‐19 patients). Healthcare staff was rapidly trained in order to use personal protective equipment and deliver care to critically ill patients. We were able to have a nurse ratio of at least 1:3 (one nurse for three patients) in our ICUs, therefore, ensuring high standards of care.
2.2. Inclusion and exclusion criteria
We included all adult patients with COVID‐19 ARDS admitted to an ICU of San Raffaele Scientific Institute during the study period (February 25th, 2020 – April 27th, 2020), who received at least one day of invasive ventilation, and were already discharged home on June 3rd. Patients aged 18 years or over admitted to an ICU at San Raffaele Scientific Institute, affected by confirmed SARS‐CoV‐2 infection (defined as positive real‐time reverse‐transcriptase polymerase chain reaction from a nasal and/or throat swab together with signs, symptoms, and radiological findings suggestive of COVID‐19 pneumonia), were included in the study.
2.3. Patients management
Anesthesiologists and intensivists managed patients in the ICUs, while internal medicine and infectious diseases specialists managed the general wards, supported by intensivists for deteriorating patients.
General ward patients could receive non‐invasive ventilation, usually continuous positive airway pressure (CPAP) and, in selected cases, some were treated with prone positioning while receiving non‐invasive ventilation (NIV). Prone position in the main ward was suggested in case of poor response to NIV, and if the first hour of treatment showed improvement it was continued. 14 , 15 We were fully aware of the theoretical risk of aerosolization during NIV, exposing staff and patients to an increased risk of infection, but during such a pandemic, the number of ICU beds for mechanical ventilation through tracheal intubation could rapidly become insufficient, whereas NIV can be offered also outside the ICU. 16
A management protocol for patients with COVID‐19 respiratory failure was implemented in our hospital. 2 If the partial pressure of arterial oxygen (PaO2) was less than 8 kPa (60 mm Hg) or saturation of peripheral oxygen (SpO2) was less than 90%, while breathing room air, physicians would increase the fraction of inspired oxygen (FiO2) up to 70‐80% via non‐rebreathing mask with an O2 flow up to 15 L/min, and the target SpO2 would be >94%. If SpO2 was stable above 94%, the indication was to continue the treatment and monitor for deterioration. If SpO2 < 94% despite 15 L/min O2 via nonrebreathing mask, the physicians would start CPAP (initial parameters FiO2 0.5, PEEP 7.5cmH2O), with target SpO2 > 94% and recommended blood gas analysis after 1 hour, with the possibility to increase the PEEP up to 12 cmH2O if SpO2 < 94%. Intubation was considered if SpO2 < 94% and/or PaO2/FiO2 < 26.7 kPa (200 mm Hg) and respiratory rate (RR) > 25‐30 after 1 hour. For mechanically ventilated patients in the ICU, we adopted current recommendations for mechanical ventilation in patients with ARDS.
2.4. Data collection
Study methodology has been previously described. 2 We prospectively collected data on medical history, comorbidities, the Simplified Acute Physiology Score II (SAPS II), 17 ARDS severity according to the Berlin Definition, 18 major organ support, and outcome.
To assess mid‐term follow‐up, discharged patients were contacted by phone by a trained investigator after a median of 61 (51‐71) days from ICU discharge. The follow‐up questionnaire is described in detail in the Supplemental Digital Content. Data were progressively recorded in a dedicated database during the phone interview. For this study, we present the follow‐up data as of June 3rd, 2020.
2.5. Study outcomes and follow‐up protocol
We evaluated multidimensional outcomes through the phone administration of various tests.
Functional outcomes were explored via the Glasgow Outcome Scale extended (GOSe) which assesses physical recovery and disability, 19 , 20 the Functional Ambulation Classification (FAC) which evaluates the autonomy in walking, 21 the Borg Category Ratio 10 (CR‐10) scale for self‐evaluation of dyspnea 22 , 23 (either at rest and during an effort such as two floors of stairs), and the Mini Nutritional Assessment – Short Form (MNA‐SF) which evaluates nutritional status. 24 Quality of life was assessed through the Euro Quality 5 Dimensions 3 Levels (EQ5D‐3L), 25 , 26 which includes an overall score self‐evaluated by the patient, the Visual Analogue Scale (VAS). Psychological outcomes were evaluated with the Hospital Anxiety and Depression Scale (HADS), 27 , 28 the PTSD Checklist for DSM‐5 (PCL‐5 ‐ which assesses PTSD), 29 , 30 , 31 and the Insomnia Severity Index (ISI). 32 Cognitive status was assessed through the Italian Telephonic version of the Mini‐Mental State Examination (Itel‐MMSE). 33
We also explored patients’ smoking habit, basal working status and return to work, alterations in senses of smell and taste. We also asked the patients to report any form of discrimination that they (or their families) may have endured because of the disease.
Baseline data of consecutive COVID‐19 patients who died during or after ICU stay were collected as well.
2.6. Statistical analysis
Statistical analysis was performed using Stata 16 (StataCorp. 2016. Stata Statistical Software: Release 16. College Station, TX: StataCorp LP). Data were presented as medians with interquartile range (IQR: 25th – 75th percentiles) or as means with standard deviation (SD). Means and SD were used with normally distributed variables, while medians and IQR were used with non‐normally distributed variables. Categorical and dichotomous variables were presented as absolute number and percentages (%). No data imputation for missing data was performed.
3. RESULTS
3.1. Baseline characteristics of patients and ICU course
Among invasively ventilated COVID‐19 ARDS patients admitted to our ICUs in the study period, all the first 42 discharged home were contacted after a median follow‐up of 61 (51‐71) days after ICU discharge (Figure 1): 39 accepted to reply (adherence rate 93%: one patient was abroad; one had a psychiatric illness; and one was confirmed alive by the general practitioner but did not answer).
FIGURE 1.
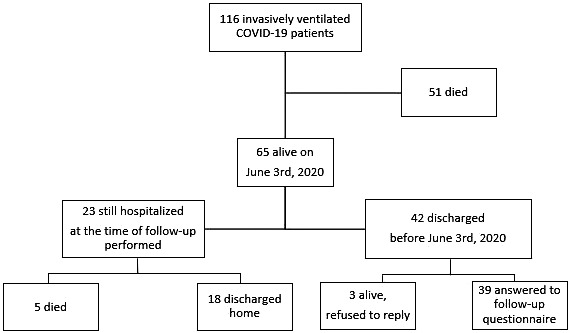
Flowchart
The mean age of our cohort of patients was 56 ± 10.5 years at ICU admission (six were >70 y), and 35 (90%) were males. Twenty‐eight (72%) had a job, two patients (5.1%) were current smokers, and the most frequent comorbidity was hypertension (49%). Mean SAPS II score was 31 ± 8.7, while the PaO2/FiO2 mean ratio was 16.7 ± 8.2 kPa (125 ± 61.8 mm Hg). Patients were on mechanical ventilation for a median of 9 (6‐14) days. At the time of evaluations for the start of mechanical ventilation, according to the Berlin criteria, 18 15 out of 39 patients were affected by severe ARDS, 18 out of 39 by moderate ARDS, and 4 out of 39 by mild ARDS, with two missing data. Then, during their ICU stay, all but one fulfilled the criteria for severe ARDS. Also, at the time of ICU admission, 38 out of 39 patients were already intubated.
The vast majority of patients required inotropic support (33 patients, 87%), neuromuscular blocking agents (31 patients, 82%), and prone positioning (28 patients, 74%). Two patients (5.3%) received extra‐corporeal membrane oxygenation (ECMO), and three patients (8.1%) received continuous renal replacement therapy.
The median length of ICU stay was 10 (7‐16) days, while the overall hospital stay was 30 (23‐44) days. Overall survival was 65/116 (56%) with no death occurring after hospital discharge; of the 51 patients who died in hospital, 46 died while still in the ICU, and 5 died in the general ward, after ICU discharge.
Characteristics of the patients at baseline and during ICU course are presented in Table 1 and Table S1, and results of mid‐term follow‐up questionnaires are presented in Table 2 and Table 3.
TABLE 1.
Characteristics of the 39 invasively ventilated COVID‐19 ARDS ICU patients who were discharged home and replied to the follow‐up questionnaire
| Baseline | Value | Missing data |
|---|---|---|
| Age (years), mean ± SD | 56 ± 10.5 | ‐ |
| Male sex, no. (%) | 35 (90%) | ‐ |
| BMI (kg∙m‐2), mean ± SD | 29 ± 5.1 | 11 |
| SAPS II (points) | 31 ± 8.7 | 2 |
| PaO2/FiO2 at ICU admission, mean ± SD (kPa) | 16.7 ± 8.2 | 2 |
| (mmHg) | 125 ± 61.8 | |
| ARDS severity at evaluation (according to Berlin criteria) | 2 | |
|
4 (11%) 18 (48%) 15 (40%) |
|
| Comorbidities | ‐ | |
|
18 (46%) | |
|
16 (41%) | |
|
4 (10%) | |
|
1 (2.6%) | |
| Schooling | 2 | |
|
4 (11%) | |
|
12 (32%) | |
|
14 (38%) | |
|
3 (8.1%) | |
|
4 (11%) | |
| Working status | ‐ | |
|
28 (72%) | |
|
11 (28%) | |
| Smoking status | ‐ | |
|
17 (44%) | |
|
2 (5.1%) | |
|
20 (51%) | |
| During ICU stay | ||
| Worst grade of ARDS severity (according to Berlin criteria) | 1 | |
|
‐ | |
|
‐ | |
|
38 (100%) | |
| CRRT, no. (%) | 3 (8.1%) | 2 |
| Tracheostomy, no. (%) | 7 (18%) | ‐ |
| Prone positioning, no. (%) | 28 (74%) | 1 |
| Inotropic support/vasopressors, no. (%) | 33 (87%) | 1 |
| Pneumothorax, no. (%) | 3 (7.9%) | 1 |
| ECMO, no. (%) | 2 (5.3%) | 1 |
| Neuromuscular Blocking agents, no. (%) | 31 (82%) | 1 |
| Days in hospital before ICU admission, median (IQR) | 1 (0‐4) | ‐ |
| Length of ICU stay, median (IQR) | 10 (7‐16) | ‐ |
| Length of Overall hospital stay, median (IQR) | 30 (23‐44) | ‐ |
| Days from ICU discharge to follow‐up, median (IQR) | 61 (51‐71) | ‐ |
ARDS, acute respiratory distress syndrome; COVID‐19, coronavirus disease 2019; ICU, intensive care unit; SD, standard deviation; BMI, body mass index; SAPS II, simplified acute physiology score II; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; CRRT, continuous renal replacement therapy; ECMO, extra‐corporeal membrane Oxygenation; IQR, interquartile range; FU, follow‐up.
Percentage may not total 100 because of rounding.
TABLE 2.
Quality of life, psychological, cognitive, and miscellaneous outcomes of 39 invasively ventilated COVID‐19 ARDS ICU patients already discharged home
| Items | Value | Missing data |
|---|---|---|
| Euro Quality 5 Dimensions 3 Levels (EQ5D3L) ‐mobility | ‐ | |
|
32 (82%) | |
|
6 (15%) | |
|
1 (2.6%) | |
| Euro Quality 5 Dimensions 3 Levels (EQ5D3L) – self‐care | ‐ | |
|
33 (85%) | |
|
6 (15%) | |
| Euro Quality 5 Dimensions 3 Levels (EQ5D3L) – usual activities | ‐ | |
|
30 (78%) | |
|
8 (20%) | |
|
1 (2.6%) | |
| Euro Quality 5 Dimensions 3 Levels (EQ5D3L) – pain or discomfort | ‐ | |
|
21 (54%) | |
|
2 (5.1%) | |
|
16 (41%) | |
| Euro Quality 5 Dimensions 3 Levels (EQ5D3L) – anxiety and depression | ‐ | |
|
31 (79%) | |
|
8 (21%) | |
|
0 (0%) | |
| Visual Analogue Scale (VAS) for self‐perceived health state, mean ± SD | 74 ± 16 | ‐ |
| Hospital Anxiety and Depression Scale (HADS) – anxiety, median (IQR) | 2 (0‐3) | 2 |
| Hospital Anxiety and Depression Scale (HADS) – depression, median (IQR) | 1 (0‐3) | 2 |
| Post‐Traumatic Stress Disorder Checklist for DSM‐5 (PCL‐5), median (IQR) | 7 (4‐16) | 2 |
| Insomnia Severity Index (ISI), median (IQR) | 1 (0‐5) | 1 |
| Italian telephone Mini Mental State Examination (I‐tel MMSE), median (IQR) | 22 (21‐22) | 2 |
| Working status | ‐ | |
|
8 (21%) | |
|
11 (28%) | |
|
1 (2.6%) | |
|
19 (49%) | |
| Alteration in smell Before ICU, no. (%) | 6 (15%) | ‐ |
| Persisting after Hospital discharge, no. (%) | 5 (13%) | ‐ |
| Alteration in taste Before ICU, no. (%) | 12 (31%) | ‐ |
| Persisting after Hospital discharge, no. (%) | 8 (21%) | ‐ |
| Discrimination due to the disease – Personal – at least one episode, no (%) | 3 (7.7%) | ‐ |
| None, no. (%) | 36 (92%) | ‐ |
| Discrimination due to the disease – Family – at least one episode, no. (%) | 2 (5.1%) | ‐ |
| None, no. (%) | 37 (95%) | ‐ |
| Denied access to non‐urgent care, no. (%) | 0 (0%) | ‐ |
ARDS, acute respiratory distress syndrome; COVID‐19, coronavirus disease 2019; ICU, intensive care unit; SD, standard deviation; IQR, interquartile range.
Percentage may not total 100 because of rounding.
TABLE 3.
Functional outcomes of 39 invasively ventilated COVID‐19 ARDS ICU patients already discharged home
| Items | Value | Missing data |
|---|---|---|
| Glasgow Outcome Scale extended (GOSe) | ‐ | |
|
10 (26%) | |
|
16 (41%) | |
|
7 (18%) | |
|
5 (13%) | |
|
1 (2.6%) | |
|
0 (0.0%) | |
| Dyspnea at rest (Borg Category Ratio 10 scale) | ‐ | |
|
38 (97%) | |
|
1 (2.6%) | |
| Exertional Dyspnea (Borg Category Ratio 10 scale) | ‐ | |
|
19 (49%) | |
|
1 (2.6%) | |
|
4 (10%) | |
|
10 (26%) | |
|
3 (7.7%) | |
|
1 (2.6%) | |
|
1 (2.6%) | |
| Mini Nutritional Assessment – Short Form (MNA‐SF) | ‐ | |
|
15 (38%) | |
|
24 (62%) | |
|
0 (0%) | |
| Functional Ambulation Classification (FAC) | ‐ | |
|
32 (82%) | |
|
4 (10%) | |
|
1 (2.6%) | |
|
2 (5.1%) |
ARDS, acute respiratory distress syndrome; COVID‐19, coronavirus disease 2019; ICU, intensive care unit.
Percentage may not total 100 because of rounding.
3.2. Cognitive outcomes
After a median of 61 (51‐71) days after ICU discharge, only one patient (2.6%) had cognitive impairment at the Itel‐MMSE scale.
3.3. Quality of life
The overall quality of life explored through the administration of the EQ5D‐3L test showed no difficulty in walking (32/39:82%), self‐care (33/39:85%), and usual activities (30/39:78%), with only eight (21%) patients reporting moderate anxiety or depression.
3.4. Psychological outcomes
Psychological tests confirmed low rates of anxiety, depression, PTSD, and insomnia.
3.5. Working status
Before the onset of the disease, 28 out of 39 patients (72%) were working. At 2 months after discharge, despite a good recovery, only eight patients (21%) had returned to their usual job, while one patient (2.6%) returned with different tasks due to the disease. Eleven out of 39 patients (28%) were unemployed or retired as before the COVID‐19 disease, but 19 patients (49%) were not working because of COVID‐19 disease‐dependent reasons.
3.6. Other outcomes
When investigating the subjective perception of patients and relatives after the discharge, asking if they ever felt discriminated in any field of their life because of the disease, upon returning to their everyday life, very few patients reported personal (3/39:7.7%) or family (2/39:5.1%) discrimination, in their everyday life, due to their COVID‐19 illness. No patient reported a lack of access to non‐urgent care (ie, outpatient clinics) because of the disease.
A total of 6 out of 39 patients (15%) reported alterations in smell before the disease, and only in one of them, this situation persisted after hospital discharge. Four further patients reported alterations in smell only after hospital discharge. Alteration in taste was reported by 12 out of 39 patients (31%) before the disease, and in 3 patients this situation persisted after hospital discharge. Five further patients reported alteration in taste only after hospital discharge. Overall, 19/39 (49%) reported either alteration in senses of smell or taste either before or after hospitalization.
3.7. Functional outcomes
67% of patients (26 out of 39) reported good recovery, according to the GOSe. Only one patient (2.6%) complained about dyspnea at rest, while almost half of the patients (20 out of 39) reported exertional dyspnea (varying from “very light” to “very strong”). We found a good level of autonomy in walking (82% of the patients—32 out of 39—could walk independently anywhere). The mean nutritional status, explored with the MNA‐SF, showed that 15 patients (38%) were malnourished and 34 (62%) at risk for malnutrition.
4. DISCUSSION
4.1. Key findings
This report of outcomes and quality of life of invasively ventilated COVID‐19 ARDS survivors shows that, at a median follow‐up of 2 months, overall survival was 56% with no death occurring after hospital discharge. The vast majority of patients reported no cognitive decline, no limitation in daily activities, and no psychological impairment or PTSD. On the other side, all patients were at least at risk for malnutrition and half of them had exertional dyspnea.
4.2. Relationship to previous studies
Only two studies investigated the quality of life of COVID‐19 ICU patients so far, and the majority of them focused on the need for a post‐ICU follow‐up of COVID‐19 critical patients, due to the well‐known PICS. 34 , 35 , 36 , 37
Valent A. et al, 38 explored the health‐related quality of life (HRQOL) of COVID‐19 ICU French survivors at a 3 months evaluation: 89% of patients described pain or discomfort; 47% worsened mobility; 42% worsened usual activities; 42% worsened anxiety/depression; and 10% worsened self‐care. These results are different from those reported by our study: 45% of our patients experienced pain or discomfort to some extent; 18% worsened mobility; 22% worsened usual activities; 21% anxiety/depression; and 15% worsened self‐care. This difference might be explained by a different follow‐up period, and by small study populations. Moreover, different ICU managements (eg, neuromuscular blocking, inotropic support) can modify HRQOL scores.
Garrigues E. et al 39 explored post‐discharge persistent symptoms and HRQOL in another cohort of COVID‐19 French patients. They compared patients managed in hospital wards without need for intensive care, with those who were transferred to the ICU, and found that most patients requiring hospitalization for COVID‐19 still have persistent symptoms, especially fatigue and dyspnea. They also found that HRQOL was quite satisfactory, and except for pain and discomfort, there was no significant difference regarding persistent symptoms and HRQOL between ward and ICU patients. These findings are not comparable with ours since only 14 patients were mechanically ventilated among the ICU patients, and their outcomes were grouped together with patients admitted to ICU who only required NIV, while all our patients required invasive mechanical ventilation.
Our patients were relatively young, a vast majority were males and overweight with few comorbidities, consistent with literature data reported so far. 40 We found that the number of active smokers was extremely low. This finding is surprising and counterintuitive. We had already noticed this finding when presenting the short‐term outcome of our patients, 2 , 41 but at that time there was the possibility of poor medical history recording during the pandemic outbreak. Nonetheless, the protective effect of active smoking on the progression toward severe COVID‐19 disease has now been confirmed by several publications in high‐impact journals which have recently been systematically reviewed. 41
Previous studies assessed long‐term sequelae of similar coronavirus diseases (MERS and SARS). 7 These studies collectively showed a high prevalence of post‐traumatic stress disorder (PTSD) (39%), depression (33%), anxiety (30%), and reduced quality of life. Previous studies on general ICU population 42 and non‐COVID‐19 ARDS 5 , 43 patients also showed that at least one‐third of patients are affected by PTSD. The difference with our study could be explained by a different baseline severity or a different length of follow‐up. In fact, patients could show a delayed onset of psychological disorders, as reported in previous studies. 44 , 45
Interestingly, although patients reported a good recovery, few of them returned to work. This is consistent with what has been previously reported for non‐COVID‐19 ARDS. 5 , 46 Indeed, previous data showed that it can take up to 5 years for critical patients to get back to their previous work. In these terms, our data show that COVID‐19 ARDS is not different from other types of critical illness.
Of note, our mid‐term mortality was 44%. This is in the lower range of short‐term mortality data from other groups in February‐March 2020, 47 , 48 but far more encouraging when compared to early reports of short‐term mortality which was in the range of 80%‐90% for invasively ventilated patients with COVID‐19 ARDS. 10 , 11 Interestingly, mortality in our cohort was higher‐than‐expected as calculated by the SAPS II score, but in line with ARDS predicted mortality based on PaO2/FiO2 ratio.
ICU triage is challenging and controversial during pandemics, when demand exceeds resources. A “first come, first served” basis could possibly be not the best choice while dealing with a pandemic surge. The Society of Critical Care Medicine stated that “The foremost consideration in triage decisions is the expected outcome of the patient in terms of survival and function, which turns on the medical status of the patient. In general, patients with good prognoses for recovery have priority over patients with poor prognoses.” 49 More recently, a triage consensus statement unanimously agreed on the need for explicit guidelines that would facilitate the fair use of scarce resources and the importance of triage guidelines. 50
Literature data regarding COVID‐19 critical patients receiving invasive mechanical ventilation confirmed that the elderly have poor outcomes: a recent systematic review and meta‐analysis of 57,420 adult patients in 69 studies, reported a case fatality rate greater than 70% among patients aged above 60 years of age. 51 Data from our group, although preliminary, confirmed that age is a strong predictor of survival in COVID‐19 ARDS critical patients. 2 Attempts have been made to develop objective triage scores, but none is currently being used. 52 Therefore, during the pandemic surge, patients were declared eligible or non‐eligible for intubation for age and/or comorbidities by expert intensive care physicians’ collegial evaluation. 15
4.3. Significance of study findings and what this study adds to our knowledge
Due to improvement in short‐term survival, there is now increasing awareness toward long‐term sequelae of critical illness survivors. Ensuring good long‐term quality of life, rather than simply survive an acute event, is now becoming the major goal of intensive care medicine. Our findings are important to raise awareness about the need for follow‐up of COVID‐19 ICU patients, being SARS‐CoV‐2 a novel and still little‐known disease. For this reason, our Hospital set up a multidisciplinary COVID‐19 follow‐up outpatient clinic that was operative since the beginning of April, and in 2 months (April 7th – June 5th), 453 patients were evaluated. The aim of the follow‐up clinic is to identify and address the clinical needs of COVID‐19 survivors. 53 From 2016, our hospital also offers a Post‐ICU Outpatient Clinic, conducted by an intensivist, an ICU nurse, and a psychologist, with the aim to identify patients affected or at risk for PICS. This outpatient clinic was interrupted during the first phase of the pandemic, since all the intensivists were involved in the acute care of patients, but it started again in July 2020 and COVID‐19 ICU survivors are offered the possibility to participate. Furthermore, our data, although exploratory, will be of help to provide baseline data in order to plan future studies on the long‐term outcome of COVID‐19 ARDS. Importantly, our data suggest that, despite disease severity, COVID‐19 ARDS is associated with a high probability of long‐term physical and psychological recovery, if the patient survives the acute illness.
4.4. Strengths and limitations of the study
The strengths of our study are the well‐defined and detailed characterization of the cohort of COVID‐19 survivors and our high‐rate of response to follow‐up. Even for the three patients that did not complete the interview, we were able to assess survival status by other strategies. We had very few missing data in the questionnaire, mainly due to language issues (2 patients were foreigners, although Italian speaking) in the administration of slightly more complex tests (HADS, PCL‐5, ISI, Itel‐MMSE). Outcomes are deeply influenced by age and frailty as testified by the baseline data of the first 39 invasively ventilated COVID‐19 ARDS ICU patients who died (Table S1).
A limitation of the study is the limited sample size and the short follow‐up time. Moreover, it is proved that the depth of patient's insights is strongly influenced by means of communication: questionnaires administered by telephone do not have the same degree of reliability as tests administered face to face. Another limitation is the different rehabilitation interventions received by patients, which may influence the reported outcomes. All patients were offered a period of rehabilitation, and almost all had an in‐hospital rehabilitation, while very few were the ones that showed a level of recovery good enough to be discharged home immediately after their general ward stay. Our data are limited to ICU survivors from a single‐center, and may not be generalizable to all COVID‐19 patients.
4.5. Future studies and prospects
In consideration of the exercise limitations reported by our patients, future studies should involve objective measures of pulmonary functioning through the administration of tests such as the 6‐minutes walking test (6MWT), Spirometry, and CT‐scan. Also, the role of current or previous smoking in the course of the disease should be addressed in the future. Pain is a field that should be as well adequately explored: nearly 50% of our COVID‐19 ARDS survivors report pain to some extent, and further studies should investigate its quality and characteristics.
5. CONCLUSION
In summary, in a cohort of consecutive COVID‐19 invasively ventilated ARDS patients we found a 56% survival rate at 2 months after ICU discharge. The overall quality of life in survivors was good, and cognitive and psychological outcomes showed no impairment at the 2 months follow‐up, suggesting that recovery in COVID‐19 patients with ARDS could be better than previously published in non‐COVID‐19 patients.
6. CONFLICTS OF INTEREST AND SOURCE OF FUNDING
All the authors have disclosed that they do NOT have any conflicts of interest or source of funding.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank all healthcare personnel of our Institute for the care provided to COVID‐19 patients during the pandemic. All the authors equally contributed to the manuscript. All the authors approved the final version of the paper for publication. All the authors have disclosed that they do NOT have any conflicts of interest or source of funding.
Monti G, Leggieri C, Fominskiy E, et al. Two‐months quality of life of COVID‐19 invasively ventilated survivors; an Italian single‐center study. Acta Anaesthesiol Scand. 2021;65:912–920. 10.1111/aas.13812
REFERENCES
- 1. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1 (accessed on November 8th, 2020).
- 2. Zangrillo A, Beretta L, Scandroglio AM, for the COVID‐BioB Study Group . Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID‐19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22(3):200‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ciceri F, Castagna A, Rovere‐Querini P, et al. Early predictors of clinical outcomes of COVID‐19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically Ill patients with COVID‐19 in Washington State. JAMA. 2020;323(16):1612‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293‐1304. [DOI] [PubMed] [Google Scholar]
- 6. Zambon M, Monti G, Landoni G. Outcome of patients with acute respiratory distress syndrome: Causes of death, survival rates and long‐term implications. In: Annual Update in Intensive Care and Emergency Medicine 2014. Vincent JL (ed). Springer Science & Business Media, pp 245‐253. [Google Scholar]
- 7. Ahmed H, Patel K, Greenwood D, et al. Long‐term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta‐analysis. J Rehabil Med. 2020;52(5):jrm00063. [DOI] [PubMed] [Google Scholar]
- 8. Needham DM, Davidson J, Cohen H, et al. Improving long‐term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502‐509. [DOI] [PubMed] [Google Scholar]
- 9. Modrykamien A, The ICU. Follow‐up clinic: A new paradigm for intensivists. Respir Care. 2012;57(5):764‐772. [DOI] [PubMed] [Google Scholar]
- 10. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID‐19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: Facing the COVID‐19 pandemic emergency. Crit Care Resusc. 2020;22(2):91‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID‐19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323(22):2338‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramirez GA, Bozzolo EP, Castelli E, et al.; for the Covid‐19 BioB Study Group. Continuous positive airway pressure and pronation outside the intensive care unit in COVID 19 ARDS. Minerva Med. 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16. Cabrini L, Landoni G, Zangrillo A. Minimise nosocomial spread of 2019‐nCoV when treating acute respiratory failure. Lancet. 2020;395(10225):685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le Gall JR, Lemeshow S, Saulnier F, et al. A new simplified acute physiology score (SAPS II) based on a European/North American Multicenter Study. JAMA. 1993;270(24):2957‐2963. [DOI] [PubMed] [Google Scholar]
- 18. The ARDS Definition Task Force . Acute respiratory distress syndrome. The Berlin Definition. JAMA. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 19. Wilson JT, Pettigrew LE, Teasdale GM, et al. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573‐585. [DOI] [PubMed] [Google Scholar]
- 20. Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44(4):285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holden MK, Gill KM, Magliozzi MR, et al. Clinical Gait Assessment in the Neurologically Impaired, Reliability and Meaningfulness. Phys Ther. 1984;64(1):35‐40. [DOI] [PubMed] [Google Scholar]
- 22. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377‐381. [PubMed] [Google Scholar]
- 23. Morishita S, Yamauchi S, Fujisawa C, et al. Rating of perceived exertion for quantification of the intensity of resistance exercise. Int J Phys Med Rehabil. 2013;1:9. [Google Scholar]
- 24. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the mini nutritional assessment short‐form (MNA‐SF): A practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782‐788. [DOI] [PubMed] [Google Scholar]
- 25. Health Policy. EuroQoL group. EuroQol–a new facility for the measurement of health‐related quality of life. 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
- 26. Scalone L, Cortesi PA, Ciampichini R, et al. Italian population‐based values of EQ‐5D health states. Value Health. 2013;16(5):814‐822. [DOI] [PubMed] [Google Scholar]
- 27. Jutte JE, Needham DM, Pfoh ER, et al. Psychometric evaluation of the Hospital Anxiety and Depression Scale 3 months after acute lung injury. J Crit Care. 2015;30(4):793‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361‐370. [DOI] [PubMed] [Google Scholar]
- 29. Blevins CA, Weathers FW, Davis MT, et al. The posttraumatic stress disorder checklist for DSM‐5 (PCL‐5): Development and initial psychometric evaluation. J Trauma Stress. 2015;28:489‐498. [DOI] [PubMed] [Google Scholar]
- 30. Ashbaugh AR, Houle‐Johnson S, Herbert C, et al. Psychometric validation of the English and French versions of the posttraumatic stress disorder checklist for DSM‐5 (PCL‐5). PLoS One. 2016;11(10):e0161645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosendahl J, Kisyova H, Gawlytta R, et al. Comparative validation of three screening instruments for posttraumatic stress disorder after intensive care. J Crit Care. 2019;53:149‐154. [DOI] [PubMed] [Google Scholar]
- 32. Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Metitieri T, Geroldi C, Pezzini A, et al. The Itel‐MMSE: An Italian telephone version of the mini‐mental state examination. Int J Geriatr Psychiatry. 2001;16(2):166‐167. [DOI] [PubMed] [Google Scholar]
- 34. Jaffri A, Jaffri UA. Post‐Intensive care syndrome and COVID‐19: Crisis after a crisis? Heart Lung. 2020;49(6):883‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biehl M, Sese D. Post‐intensive care syndrome and COVID‐19 – Implications post pandemic. Cleve Clin J Med. 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36. Stam HJ, Stucki G, Bickenbach J. Covid‐19 and post intensive care syndrome: A call for action. J Rehabil Med. 2020;52(4):jrm00044. [DOI] [PubMed] [Google Scholar]
- 37. Hosey MM, Needham DM. Survivorship after COVID‐19 ICU stay. Nat Rev Dis Primers. 2020;6(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valent A, Dudoignon E, Ressaire Q, et al. Three‐month quality of life in survivors of ARDS due to COVID‐19: A preliminary report from a French academic centre. Anaesth Crit Care Pain Med. 2020;39(6):740‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garrigues E, Janvier P, Kherabi Y, et al. Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. J Infect. 2020;81(6):e4‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landoni G, Moro M, Belletti A, et al. Recent exposure to smoking and COVID‐19. Crit Care Resusc. 2020;22(3):253‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herridge MS, Chu LM, Matte A, et al. The RECOVER program: Disability risk groups and 1‐year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194(7):831‐844. [DOI] [PubMed] [Google Scholar]
- 43. Schelling G, Stoll C, Haller M, et al. Health‐related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998;26(4):651‐659. [DOI] [PubMed] [Google Scholar]
- 44. Utzon‐Frank N, Breinegaard N, Bertelsen M, et al. Occurrence of delayed‐onset post‐traumatic stress disorder: A systematic review and meta‐analysis of prospective studies. Scand J Work Environ Health. 2014;40(3):215‐229. [DOI] [PubMed] [Google Scholar]
- 45. Wintermann G‐B, Rosendahl J, Weidner K, et al. Risk factors of delayed onset posttraumatic stress disorder in chronically critically Ill patients. J Nerv Ment Dis. 2017;205(10):780‐787. [DOI] [PubMed] [Google Scholar]
- 46. Apfelbacher C, Brandstetter S, Blecha S, et al. Influence of quality of intensive care on quality of life/return to work in survivors of the acute respiratory distress syndrome: prospective observational patient cohort study (DACAPO). BMC Public Health. 2020;20(1):861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically Ill patients in the seattle region — case series. N Engl J Med. 2020;382:2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grasselli G, Greco M, Zanella A, for the COVID‐19 lombardy ICU Network . Risk factors associated with mortality among patients with COVID‐19 in Intensive Care Units in Lombardy, Italy. JAMA. Intern Med. 2020;180(10):1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Consensus statement on the triage of critically ill patients . Society of Critical Care Medicine Ethics Committee.JAMA 1994;271(15):1200‐1203. [PubMed] [Google Scholar]
- 50. Sprung CL, Danis M, Iapichino G, et al. Triage of intensive care patients: identifying agreement and controversy. Intensive Care Med. 2013;39(11):1916‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lim ZJ, Subramaniam A, Reddy MP, et al. Case fatality rates for COVID‐19 patients requiring invasive mechanical ventilation: A meta‐analysis. Am J Respir Crit Care Med. 2021;203(1):54‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sprung CL, Joynt GM, Christian MD, et al. Adult ICU triage during the coronavirus disease 2019 pandemic: Who will live and who will die? recommendations to improve survival. Crit Care Med. 2020;48(8):1196‐1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rovere Querini P, De Lorenzo R, Conte C, et al. Post‐COVID‐19 follow‐up clinic: Depicting chronicity of a new disease. Acta Biomed. 2020;91(9‐S):22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


