Dear Editor,
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been associated with different cutaneous manifestations, including acral lesions similar to chilblains. 1 News of the possible association between chilblains and COVID‐19 in the media and on social networks generated consternation among the general public in Spain and a considerable increase in medical consultations. A direct association between pseudo‐chilblains and COVID‐19, however, has not been unequivocally demonstrated, as microbiologically confirmed SARS‐CoV‐2 infection has been reported in very few cases. 2 , 3
We had the opportunity to study 14 patients with pseudo‐chilblain lesions during the state of emergency declared in Spain on March 14, 2020 (Fig. 1). Their mean age was 13.28 years (range, 7–20 years). The clinical and analytical data are summarized in Table 1.
Figure 1.
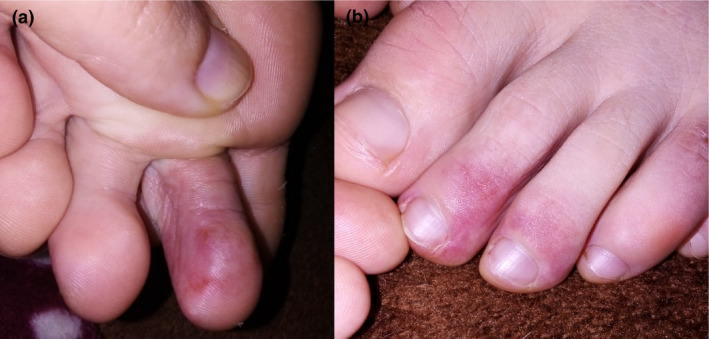
Pseudo‐chilblain lesions. Erythema and swelling on the third left toe (a); purpuric macules on the second and third right toes (b)
Table 1.
Clinical features and laboratory tests
| Case | Sex | Age (years) | Comorbidities | Family background | Site of lesions | COVID‐19 symptoms | COVID‐19 PCR | IgA + IgM SARS‐CoV‐2 | IgG SARS‐CoV‐2 | ANA | ANCA | D‐dimer | ACL | Cryoglobulins | Cryoagglutinins | IgG/M PB19 | IgG/M Enterovirus | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 12 | ADHD | No | Feet | No | Negative | Negative | Negative | Negative | Negative | High | Negative | Negative | Negative | IgG positive | Negative | Topical corticosteroids, pentoxifylline |
| 2 | F | 12 | Adenoidectomy | No | Left foot | Malaise, low‐grade fever | Negative | Negative | Negative | 1/80 speckled pattern | Negative | Normal | Negative | Positive, cryocyte <1% | Negative | IgG positive | Negative | No |
| 3 | M | 14 | No | No | Feet | Malaise, low‐grade fever | Negative | Negative | Negative | Negative | Negative | Normal | Negative | Negative | Negative | IgG/M negative | Negative | No |
| 4 | F | 7 | No | No | Feet | No | Negative | Negative | Positive | Negative | Negative | Normal | Negative | Negative | Negative | IgG positive | Negative | Topical corticosteroids |
| 5 | F | 12 | No | No | Feet | No | Negative | Negative | Negative | Negative | Negative | High | Negative | Negative | Negative | IgG positive | Negative | Topical corticosteroids and antibiotic |
| 6 | F | 13 | No | Father SARS‐CoV‐2+ | Hands | No | Negative | Negative | Negative | Negative | Negative | Normal | Negative | Negative | Negative | IgG positive | Negative | No |
| 7 | M | 17 | ADHD | No | Feet >>>hands | No | Negative | Positive | Negative | Negative | Negative | High | Negative | Positive, cryocyte <1% | Negative | IgG positive | Negative | Pentoxifylline |
| 8 | F | 20 | Raynaud's disease | No | Hands | No | Negative | Negative | Negative | 1/80 speckled pattern | Negative | Normal | Negative | Negative | Negative | IgG positive | Negative | No |
| 9 | F | 13 | No | No | Heels | No | Negative | Negative | Negative | Negative | Negative | Normal | Negative | Positive, cryocyte <1% | Negative | IgG/M negative | Negative | Topical corticosteroids and antibiotic |
| 10 | M | 15 | No | No | Hands and feet | No | Negative | Negative | Negative | 1/40 speckled pattern | Negative | Normal | Negative | Negative | Negative | IgG positive | Negative | Topical corticosteroids |
| 11 | F | 17 | No | No | Hands and feet | No | Negative | Negative | Negative | Negative | Negative | Normal | Negative | Negative | Negative | IgG positive | Negative | Topical corticosteroids |
| 12 | M | 12 | Hypothyroidism, GHD | No | Feet >>>hands | Low‐grade fever | Negative | Negative | Negative | Negative | Negative | Normal | Negative | Negative | Negative | IgG/M negative | Negative | No |
| 13 | M | 11 | Atopic dermatitis | No | Heels | No | Negative | Negative | Negative | Negative | Negative | Normal | Negative | Negative | Negative | IgG positive | Negative | No |
| 14 | M | 11 | No | Mother SARS‐CoV‐2+ | Feet | No | Negative | Negative | Negative | Negative | Negative | Normal | Negative | Negative | Negative | IgG/M negative | Negative | No |
ADHD, attention deficit hyperactivity disorder; ACL, anticardiolipin antibodies; ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies; F, female; GHD, growth hormone deficiency; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; M, male.
PCR for SARS‐CoV‐2 was negative in 100% of patients. Serological testing (chemiluminescent immunoassay, Vircell, Spain, and enzyme‐linked immune assay, Euroimmun, Germany) revealed two positive cases: a 13‐year‐old girl with a positive result for SARS‐CoV‐2 IgG antibodies and a 17‐year‐old boy with a positive result for SARS‐CoV‐2 IgA and IgM antibodies. Additional tests were performed to rule out other possible causes of acrocyanosis (Table 1). The only significant pathological finding was cryoglobulinemia in three patients, including the 17‐year‐old boy with SARS‐CoV‐2 IgA and IgM antibodies. All the patients progressed favorably.
Chilblains represent an inflammatory skin reaction resulting from a maladaptive vascular response to very cold weather. 4 The accumulation of cases several weeks after the peak in COVID‐19 cases in Spain raised questions about a possible association with SARS‐CoV‐2. Pseudo‐chilblain lesions have been reported as a late manifestation of COVID‐19 and have been mostly observed in young patients with mild symptoms. 1 , 4 However, a causative link between pseudo‐chilblain lesions and SARS‐CoV‐2 infection has not been proven. It has been suggested that immune‐mediated inflammation or microthrombosis might have a role in their pathogenesis. 4 Another theory is that SARS‐CoV‐2 infection might induce the expression of type 1 interferon, leading to microangiopathic changes. 5 Hubiche et al 3 demonstrated a significantly higher INF‐alpha response in patients with pseudo‐chilblain lesions. They suggested that the type of immune response is a key factor explaining the different clinical manifestations of SARS‐CoV‐2 infection. 3
The patient in our series who tested positive for SARS‐CoV‐2 IgM antibodies also tested positive for cryoglobulins, which could also have a pathogenic role, as has been observed in patients with hepatitis C virus infection. 6 Three months later, serological test was repeated again in this patient showing the same result as previously with no seroconversion for SARS‐CoV‐2 IgG. It is probably that SARS‐CoV‐2 IgM and IgA antibodies detected were a false positive result possibly associated with cryoglobulins.
Nonetheless, it is striking that molecular and serological tests were positive for SARS‐CoV‐2 in just two (14.2%) of the 14 patients in our series. 1 , 2 , 4 In a review of the literature, Docampo‐Simón et al 2 found a similar rate (14.8%) for patients with chilblain‐like lesions and a positive PCR for SARS‐CoV‐2. On the other hand, the rate observed by Galván‐Casas et al 1 was considerably higher at 41%; although, in this survey participating dermatologists relocated to the care of patients diagnosed with COVID‐19. These studies have very different inclusion criteria, which could justify the discrepancy in the percentage of positives. It is important to note that very few patients with pseudo‐chilblain lesions have undergone serological testing for SARS‐CoV‐2, and, similarly to in our study, most of the results have been negative. 2 , 3 This raises several hypotheses. First, the low sensitivity of the rapid IgG/IgM tests performed at the start of the pandemic may have resulted in high false‐negative rates, and second, circulating antibody levels may drop rapidly, impeding their detection. 4 It has also been hypothesized that pseudo‐chilblain lesions may not be an exclusive marker of SARS‐CoV‐2 infection and may actually be because of other factors, such as trauma, other viral infections (mainly B19 parvovirus infection), and, in particular, a sedentary lifestyle, which would reduce acral blood flow, favoring the development of chilblain‐like lesions in certain patients. 1 Finally, Hubiche et al 3 explained that patients with pseudo‐chilblain could clear SARS‐CoV‐2 infection before humoral immunity occurs because of an exaggerated type I interferon response. This could justify the low rate of seropositivity in these patients. 3
Considering the information available to date, we believe that further research is needed to investigate the causes and pathogenic mechanisms underlying pseudo‐chilblain lesions to confirm a definitive association with SARS‐CoV‐2 infection.
Conflict of interest: None.
Funding source: None.
References
- 1. Galván Casas C, Catalá A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Docampo‐Simón A, Juan G, Poveda‐Montoyo I, et al. Are chilblain‐like acral skin lesions really indicatative of COVID‐19? A prospective study and literature review. J Eur Acad Dermatol Venereol 2020; 34: e445–e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hubiche T, Cardot‐Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon‐alpha response characteristics of patients with chilblain‐like lesions during the COVID‐19 pandemic. JAMA Dermatol 2020:e204324. 10.1001/jamadermatol.2020.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freeman EE, McMahon DE, Lipoff JB, et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J Am Acad Dermatol 2020; 83: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathological findings. JAAD Case Rep 2020; 6: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dammacco F, Lauletta G, Russi S, et al. Clinical practice: hepatitis C virus infection, cryoglobulinemia and cryoglobulinemic vasculitis. Clin Exp Med 2019; 19: 1–21. [DOI] [PubMed] [Google Scholar]


