Abstract
The purpose of this systematic review and meta‐analysis was to examine clinical outcomes associated with convalescent plasma therapy in COVID‐19 patients. We performed a literature search on PubMed, medRxiv, Web of Science, and Scopus to identify studies published up to December 10th, 2020 that examined the efficacy of convalescent plasma treatment for COVID‐19. The primary endpoints were mortality, clinical improvement, and hospital length of stay. We screened 859 studies that met the search criteria, performed full‐text reviews of 56 articles, and identified 15 articles that fulfilled inclusion criteria for meta‐analysis. The odds of mortality were significantly lower in the convalescent plasma group compared to the control group (OR = 0.59 [95% CI = 0.44; 0.78], P < .001), although results from two key randomized controlled trials did not support the mortality benefit. The odds of clinical improvement were significantly higher in the convalescent plasma group compared to the control group (OR = 2.02 [95% CI = 1.54; 2.65], P < .001). There was no difference in hospital length of stay between the convalescent plasma group and the control group (MD = −0.49 days [95% CI = −3.11; 2.12], P = .713). In all, these data indicate that a mortality benefit with convalescent plasma is unclear, although there remain benefits with convalescent plasma therapy for COVID‐19.
Keywords: convalescent plasma, coronavirus, COVID‐19, meta‐analysis
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has affected over 79 million individuals worldwide, with 1.7 million deaths attributed to the disease as of December 27, 2020. 1 Convalescent plasma (CP) treatment has been used for previous viral outbreaks (eg, Spanish influenza, SARS) 2 , 3 and may represent an effective treatment strategy for COVID‐19. Initial studies have noted beneficial effects of CP to treat COVID‐19, especially CP containing high titers of neutralizing antibodies (NAbs) administered early in the disease course. 4 , 5 However, existing data come from small observational, non‐randomized studies, which are underpowered and suffer from study bias at various levels (eg, patient selection, lack of appropriate controls, additional therapies). 6 Therefore, it is essential to compile and analyze the collective data obtained from smaller studies in order to better characterize the treatment outcomes associated with CP therapy. Here, we performed a systematic review and meta‐analysis to determine the efficacy of CP for the treatment of COVID‐19.
2. METHODS
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines were adhered to for this review article. We conducted a literature search on PubMed, medRxiv, Web of Science, and Scopus through December 10, 2020 using the following keywords: “COVID‐19 OR SARS‐CoV‐2 OR novel coronavirus” and “convalescent plasma”. Inclusion criteria for article selection consisted of the following: (a) study contains convalescent plasma and standard therapy arms, and (b) study provides clinical outcomes data resulting from convalescent plasma therapy. We excluded reviews, case reports, guidelines, nonhuman studies, commentaries, opinions, editorials, news, and studies that did not report outcomes data. We also excluded studies that consisted of groups (CP, control) that exhibited substantial differences in disease severity at baseline. For example, we would not include studies that compared outcomes in critically ill patients that received CP with minor‐moderately ill patients that received standard treatments at baseline as differences in outcomes would not necessarily be a product of the treatment.
2.1. Risk of bias and quality assessment
The risk of bias and levels of evidence of each study was scored using the Scottish Intercollegiate Guidelines Network (SIGN) checklists for controlled clinical trials and cohort studies. 7 As such, individual items on checklists were categorized as follows:
“Well addressed” or “Yes”
“Adequately addressed”
“Poorly addressed” or “No”
“Not reported”
“Not applicable (N/A)”
The risk of bias among individual studies was coded as follows:
High quality (++)
Adequate quality (+)
Low quality (−)
Unacceptable (0)
Individual levels of evidence among studies were graded as follows:
High‐quality controlled trials (1++)
Acceptable‐quality controlled trials (1+)
Low‐quality controlled trials (1−)
High‐quality prospective cohort studies (2++)
Acceptable‐quality cohort studies (2+)
Low‐quality cohort studies (2−)
Within their separate checklists, non‐randomized controlled trials were rated no higher than 1+ and retrospective cohort studies were rated no higher than 2+.
2.2. Statistical analysis
All data were entered into a Microsoft Excel sheet and imported to RStudio (Version 1.3.959, RStudio, PBC) running R‐4.0.2 for analysis using the “metafor” package. 8 The primary outcomes of interest were the odds of mortality, clinical improvement, and hospital length of stay. In certain studies, insufficient information was presented to extract direct measures of variance for continuous parameters. We contacted the study authors when data were missing. If data remained unavailable, the studies were either omitted, or statistical methods were used to derive estimated measures of variance. When the assumption of approximately normally distributed data was justified, means and variances were estimated using methods described by Luo et al 9 and Wan et al, 10 respectively. If summary measures from individual studies were primarily reported as quantiles (median, IQR, etc.), aggregated results were computed using the weighted median of medians method proposed by McGrath et al. 11
The magnitude of between‐study heterogeneity unrelated to sampling error was evaluated by Higgin's I 2 statistics. 12 For dichotomous data, effect sizes were computed as log‐transformed odds ratios (ORs) using the inverse of the variance method (fixed‐effects models) or using the inverse of the variance with a restricted effects maximum likelihood (REML) estimator for estimation of the between‐study variance component in random‐effects models. For continuous data, effect sizes were computed as pooled mean differences (MDs) using the Hedge's g method 13 or using Hedge's g with a REML estimator for estimation of the between‐study variance component in random‐effects models. To aid in interpretation, log‐transformed effect sizes were converted to a probability scale. Fixed‐effects models were chosen in order to make conditional inferences from aggregated results from the k studies included in the analysis; random‐effects models were chosen in order to make unconditional inferences about predicted outcomes in a hypothetical population of studies from which the k studies included in the analysis are assumed to have come from. As such, separate fixed‐effects models were used for aggregating rates of different background characteristics, comorbidities, and treatment interventions and random‐effects models were used for aggregating predicted values for patient outcomes. A separate model was fit for each summary measure. 95% confidence intervals were calculated using exact binomial intervals (dichotomous data) or normal approximation (continuous data) for fixed‐effects models or using the Q‐profile method for random‐effects models. 14 95% prediction intervals (PIs) were also calculated for each random‐effects outcome measure. 14 In brief, a 95% PI estimates where the true effects are to be expected for 95% of similar (exchangeable) studies that might be conducted in the future. 15 As recommended in the Cochrane Handbook for Systematic Reviews of Interventions, funnel plots were used to visually depict small study bias when at least 10 studies were included for outcome comparisons. Asymmetry in funnel plots will be assessed using the Harbord test. 16
3. RESULTS
A total of 859 articles were discovered that met the search criteria, out of which 56 articles were selected for full‐text review. After the full‐text screening, 15 studies 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 with a total study population of 4898 patients met our criteria for inclusion in the quantitative synthesis (Figure 1). There were 5 randomized controlled trials, 18 , 20 , 24 , 25 , 26 1 non‐randomized controlled trial, 17 2 prospective cohort studies, 19 , 27 2 ambidirectional cohort studies, 22 , 23 and 5 retrospective cohort studies. 21 , 28 , 29 , 30 , 31 Among this study population, 2247 patients (45.9%) were treated with CP plus standard of care, and 2651 (54.1%) were treated with standard of care alone (control). Standard of care could consist of antivirals, corticosteroids, antibiotics, and immunomodulators.
FIGURE 1.
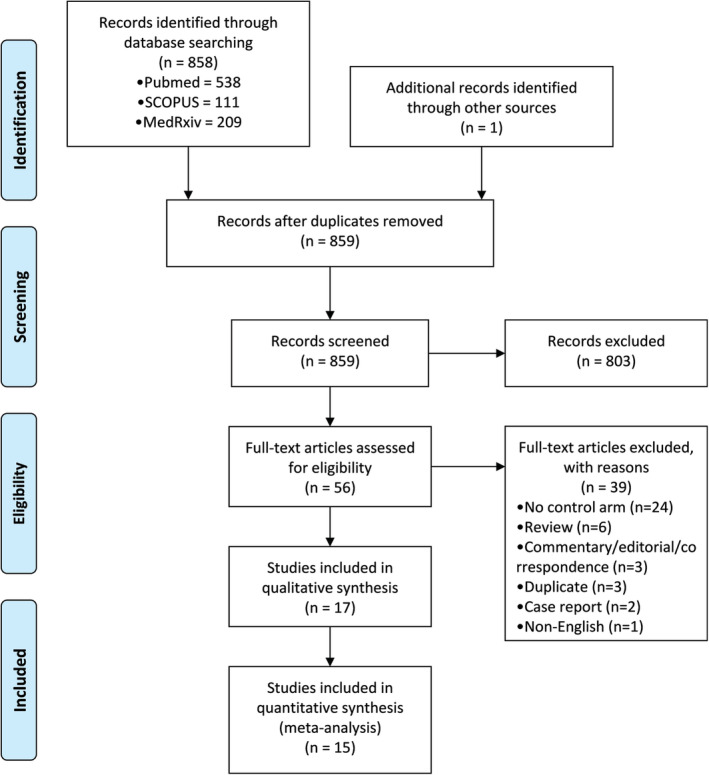
PRISMA diagram of search records and included studies
3.1. Background characteristics and comorbidities
The mean age of the CP group was 57.1 years (95% CI: 56.3; 58.0), while the mean age of the control group was 56.6 years (95% CI: 55.9; 57.3). There was no significant difference in age distributions between the CP and control groups (MD = −0.18 years [95% CI = −1.30; 0.93], P = .747; Figure S1). There was no significant difference in prevalence of male patients between the CP and control groups (OR = 0.97 [95% CI = 0.85; 1.10], P = .633). Within the CP group, the median time from symptom onset to receive CP treatment was 13.25 days (95% CI = 8.06; 18.45; Figure S2). There was no difference in utilization of immunoglobulins (OR = 1.30 [95% CI = 0.56; 3.03], P = .539), steroids (OR = 1.04 [95% CI = 0.87; 1.23], P = .849), or antivirals (OR = 0.83 [95% CI = 0.58; 1.17], P = .278) between the CP and control groups (Table 1). However, the CP group had significantly higher baseline supplemental oxygen use compared to the control group (OR = 1.73 [95% CI = 1.24; 2.42], P = .001). There was no difference in prevalence of diabetes (OR = 1.04 [95% CI: 0.92; 1.18], P = ..538), hypertension (OR = 0.95 [95% CI = 0.84; 1.07], P = .422), chronic obstructive pulmonary disease (OR = 1.02 [95% CI = 0.76; 1.37], P = .901), chronic kidney disease (OR = 0.81 [95% CI = 0.64; 1.02], P = .072), cardiovascular disease (OR = 0.90 [95% CI = 0.75; 1.06], P = .200), or cerebrovascular disease (OR = 1.30 [95% CI = 0.63; 2.68], P = .477) between CP and control groups. Comparisons of all dichotomous background characteristics and comorbidities between the CP and control groups are shown in Table 1
TABLE 1.
Comparison of background characteristics and comorbidities between convalescent plasma and control
| Study characteristics | Convalescent plasma | Control | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | No. Studies | Events (n/N) | Prop (95% CI) | Events (n/N) | Prop (95% CI) | OR (95% CI) | P‐value | I 2 (95% CI) | P‐value (Q) |
| Male Sex | 13 | 1484/2196 | 0.647 (0.562; 0.723) | 1760/2615 | 0.679 (0.591; 0.755) | 0.97 (0.85; 1.10) | .633 | 0.0% (0.0; 58.9%) | .392 |
| IVIG Use | 3 | 19/287 | 0.241 (0.150; 0.364) | 25/293 | 0.298 (0.183; 0.445) | 1.30 (0.56; 3.03) | .539 | 0.0% (0.0; 79.0%) | .609 |
| Steroid Use | 10 | 806/1164 | 0.659 (0.629; 0.688) | 1027/1632 | 0.609 (0.582; 0.635) | 1.04 (0.87; 1.23) | .849 | 59.0 (14.4; 80.4%) | .012 |
| Oxygen Use | 10 | 750/929 | 0.891 (0.861; 0.914) | 1048/1271 | 0.825 (0.795; 0.851) | 1.73 (1.24; 2.42) | .001 | 80.8% (55.1; 91.8%) | <.001 |
| Antiviral Use | 10 | 1376/1682 | 0.675 (0.618; 0.728) | 1510/1761 | 0.691 (0.645; 0.733) | 0.83 (0.58; 1.17) | .278 | 0.0% (0.0; 16.6%) | .911 |
| Diabetes | 13 | 752/2207 | 0.349 (0.329; 0.370) | 904/2633 | 0.348 (0.330; 0.367) | 1.04 (0.92; 1.18) | .538 | 0.0% (0.0; 41.1%) | .716 |
| HTN | 12 | 1028/2168 | 0.480 (0.459; 0.501) | 1203/2477 | 0.489 (0.469; 0.509) | 0.95 (0.84; 1.07) | .422 | 0.0% (0.0; 57.3%) | .465 |
| COPD | 7 | 93/990 | 0.107 (0.088; 0.129) | 136/1344 | 0.120 (0.102; 0.140) | 1.02 (0.76; 1.37) | .901 | 17.1% (0.0; 61.0%) | .300 |
| CKD | 9 | 130/1897 | 0.076 (0.064; 0.089) | 202/2142 | 0.101 (0.088; 0.115) | 0.81 (0.64; 1.02) | .072 | 14.9% (0.0; 57.0%) | .309 |
| CVD | 9 | 349/1878 | 0.223 (0.203; 0.245) | 438/2130 | 0.238 (0.219; 0.259) | 0.90 (0.76; 1.06) | .200 | 0.0% (0.0; 18.3%) | .903 |
| Cerebrovascular disease | 3 | 17/351 | 0.058 (0.003; 0.544) | 20/467 | 0.029 (0.001; 0.499) | 1.30 (0.63; 2.68) | .477 | 0.0% (0.0; 87.9%) | .425 |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HTN, hypertension; OR, odds ratio.
3.2. Mortality
All studies included in the quantitative meta‐analysis had sufficient data to evaluate the relative odds of mortality between the CP and control groups. The overall mortality rate for the CP group was 0.127 (95% CI = 0.088; 0.181), while the overall mortality rate for the control group was 0.194 (95% CI = 0.139; 0.263). The odds of mortality were significantly lower in the CP group compared to the control group (OR = 0.59 [95% CI = 0.44; 0.78], p = <.001; Figure 2A). The estimated between‐study heterogeneity ranged from low to high (I 2 = 45.5% [95% CI = 0.3%; 70.2%], P = .028). Harbord's test revealed significant small study bias with respect to overall mortality rate (estimate = −1.285, SE = 0.483, intercept = −0.076, P = .020; Figure S3).
FIGURE 2.
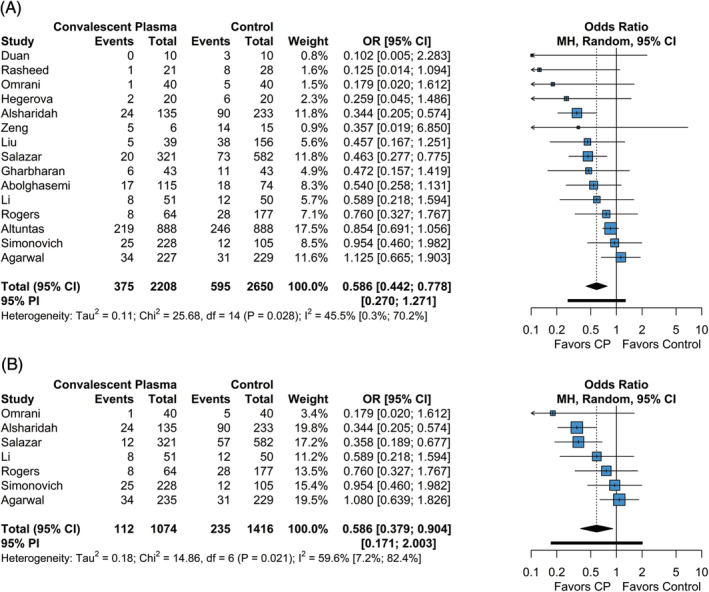
Forest plot of subgroup comparisons of mortality. A, Overall mortality across all follow‐up times. B, Mortality at 28 to 30 day follow‐up. Pooled results were computed using restricted effects maximum likelihood with 95% confidence intervals computed using the Q‐profile method. A 95% prediction interval was also computed (black bar)
To investigate the impact of follow‐up time on mortality, we performed a subgroup analysis on studies reporting mortality at standardized follow‐up times at either 28 or 30 days. Seven studies included in the meta‐analysis reported mortality at either 28‐ or 30‐day follow‐up. The mortality rate at 28‐ or 30‐day follow‐up for the CP group was 0.107 (95% CI = 0.068; 0.165), while the overall mortality rate for the control group was 0.167 (95% CI = 0.109; 0.247). The odds of mortality were significantly lower in the CP group compared to the control group (OR = 0.59 [95% CI = 0.38; 0.90], P = .016; Figure 2B). The estimated between‐study heterogeneity ranged from low to high (I 2 = 59.6% [95% CI = 7.2%; 82.4%], P = .021). Overall, results from pooled mortality assessment at standardized follow‐up times did not reduce between‐study heterogeneity and results were not significantly different than those observed across all timepoints.
3.3. Clinical improvement
Of the studies included in the quantitative meta‐analysis, seven studies had sufficient data to evaluate the relative odds of clinical improvement between the CP and control groups. Criteria for clinical improvement varied across studies (Table S1). The overall rate of clinical improvement for the CP group was 0.694 (95% CI = 0.508; 0.833), while the overall rate of clinical improvement for the control group was 0.492 (95% CI = 0.276; 0.711). The odds of clinical improvement were significantly higher in the CP group compared to the control group (OR = 2.02 [95% CI = 1.54; 2.65], P < .001; Figure 3). Between‐study heterogeneity with regards to clinical improvement ranged from low to moderate (I 2 = 21.6% [95% CI = 0.0%; 64.8%], P = .265). Due to limited data, small‐study bias assessments were not performed; as such, the pooled effect size for clinical improvement was assumed to be affected by small study bias to some degree.
FIGURE 3.
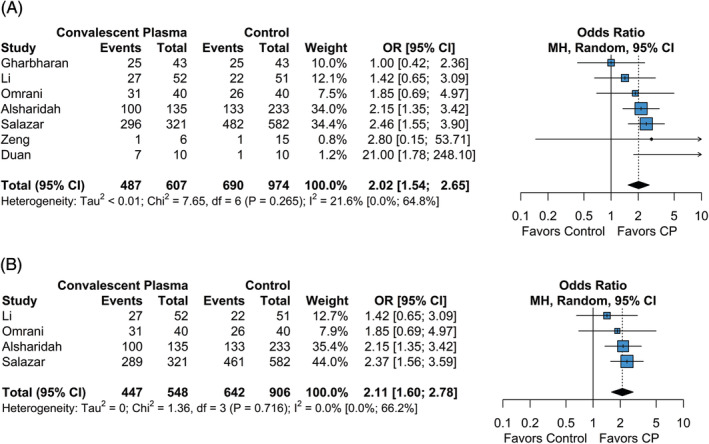
Forest plot of subgroup comparisons of clinical improvement. A, Overall clinical improvement across all follow‐up times. B, Clinical improvement at 28 to 30 day follow‐up. Pooled results were computed using restricted effects maximum likelihood with 95% confidence intervals computed using the Q‐profile method. A 95% prediction interval was also computed (black bar)
To investigate the impact of follow‐up time on clinical improvement, we performed a subgroup analysis on studies reporting clinical improvement at standardized follow‐up times at either 28 or 30 days. Four studies included in the meta‐analysis reported clinical improvement at either 28‐ or 30‐day follow‐up. The overall rate of clinical improvement at 28‐ or 30‐day follow‐up for the CP group was 0.760 (95% CI = 0.570; 0.883), while the overall rate of clinical improvement for the control group was 0.626 (95% CI = 0.459; 0.767). The odds of clinical improvement were significantly lower in the CP group compared to the control group (OR = 2.11 [95% CI = 1.60; 2.77], P < .001; Figure 3B). The estimated between‐study heterogeneity ranged from low to moderate (I 2 = 0.0% [95% CI = 0.0%; 66.2%], P = .716). Overall, results from pooled clinical improvement assessment at standardized follow‐up times did not reduce between‐study heterogeneity and results were not significantly different than those observed across all timepoints.
3.4. Hospital length of stay
Of the studies included in the quantitative meta‐analysis, six studies had sufficient data to compare hospital length of stay between the CP and control groups. The pooled mean hospital length of stay for the CP group was 9.6 days (95% CI = 6.6; 14.0), while the pooled hospital length of stay for the control group was 10.4 days (95% CI = 8.03; 13.4). The difference in hospital length of stay between the CP and control group was not significant (MD = −0.49 days [95% CI = −3.11; 2.12], P = .713; Figure 4). The estimated between‐study heterogeneity was high (I 2 = 91.1% [95% CI = 83.3%; 95.2%], P = .761). Due to limited data, small‐study bias assessments were not performed; as such, the pooled effect size for hospital length of stay was assumed to be affected by small study bias to some degree.
FIGURE 4.
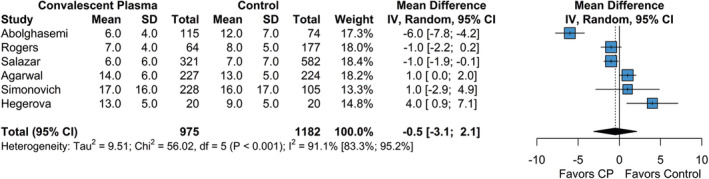
Forest plot of subgroup comparisons of hospital length of stay. Pooled results were computed using restricted effects maximum likelihood with 95% confidence intervals computed using the Q‐profile method. A 95% prediction interval was also computed (black bar)
3.5. Risk of bias and quality assessment of the included studies
Based on the SIGN method for controlled trials, 7 2 studies were rated as 1++, and 4 studies were rated as 1+. Based on the SIGN methods for cohort studies, 3 studies were rated as 2+, and 6 studies were rated as 2‐. Of note, 2 retrospective cohort studies which received “unacceptable (0)” ratings on the risk of bias assessment were excluded from the quantitative meta‐analysis. All other studies were directly applicable to the target patient population and had sufficient information for inclusion in the quantitative meta‐analysis. Risk of bias outcomes and gradings of evidence were descriptively synthesized and were not assessed for the purpose of quantitative analysis. Table S2 shows the detailed results of the risk of bias assessments as well as summaries of the conclusions from individual studies. Of the studies included in the quantitative meta‐analysis, 8 studies suggested overall benefit with CP use, 5 studies were inconclusive, and 2 studies suggested no benefit with CP use.
4. DISCUSSION
Here, we performed a systematic review and meta‐analysis of the literature to examine clinical outcomes following CP therapy for COVID‐19. Administration of CP resulted in lower odds for mortality in COVID‐19 patients, both within the global meta‐analysis and within the subgroup analysis of 28‐ or 30‐day standardized follow‐up times. However, the two largest, randomized controlled studies included in the meta‐analysis did not observe a mortality benefit with CP therapy 24 , 26 ; thus, the effects of CP on mortality are not fully understood. The odds for clinical improvement were greater in patients that received CP treatment, both within the global meta‐analysis and within the subgroup analysis of 28‐ or 30‐day standardized follow‐up times. Hospital length of stay was not significantly different between the CP and control groups. These data suggest that CP therapy may be of benefit to COVID‐19 patients; however, the disparate mortality results observed in studies with the highest levels of evidence preclude definitive conclusions regarding improved survival rates.
A principal advantage of CP therapy for COVID‐19 is that CP can be obtained from local sources (ie, recovering COVID‐19 patients) through apheresis. Moreover, the usage of CP therapy has exhibited efficacy to treat other viral illnesses, such as SARS‐CoV‐1, 3 influenza (H1N1, H5N1, H7N9), 32 , 33 , 34 , 35 Argentinian mammarenavirus/Junin virus, 36 and Middle East respiratory syndrome coronavirus (MERS‐CoV). 37 While we encountered several studies that observed lower, albeit non‐significant, odds of mortality with CP therapy, the studies 24 , 26 with the highest levels of evidence in our analysis detected similar rates of mortality between CP and control arms. Indeed, Agarwal et al had a mortality rate of 15.0% (34/227) with CP therapy vs 13.5% (31/229) in the control arm, while Simonovich et al had a mortality rate of 11.0% (25/228) with CP therapy vs 11.4% (12/105) in the control arm. Based on the complete dataset, we are uncertain if CP provides a mortality benefit for COVID‐19 patients. However, there could be inherent differences in study designs that are sufficient to confound outcome assessments, such as mortality.
The concentration of NAbs in CP likely influences the quality and/or magnitude of clinical outcome. While the U.S. Food & Drug Administration recently provided criteria for classifying CP as high or low titer using specific IgG detection kits, 38 there has not been a consensus with regards to specific cut‐offs for high and low titers (IgG, NAb) in clinical practice. Of the 15 studies included in the present analysis that assessed donor antibody titers, 4 studies assessed NAb titers, 18 , 22 , 24 , 26 4 studies assessed IgG titers, 20 , 23 , 26 , 31 and 3 studies assessed IgG indexes (Table 2). 17 , 25 , 30 In the only study that reported an increase in NAb titers following CP transfusion, Duan et al 22 noted a mortality benefit with CP therapy, albeit in small sample. Simonovich et al noted an increase in IgG titer after CP infusion; however, this study did not report a mortality benefit. This study was a randomized controlled trial consisting of 333 COVID‐19 patients. While there was a small difference in IgG titers between CP (1:400 [1:200‐1:600]) and placebo control (1:400 [1:50‐1:3200]) at day 2 (P = .044), there were no differences in IgG levels between the 2 groups at days 7 (P = .806) and 14 (P = .449). Thus, the meaningfulness of CP transfusion to mitigate disease progression in these patients was unclear as both groups exhibited a substantial rise in IgG titer over time. Agarwal et al noted that 83% of all patients possessed median NAb titers of 1:90 during enrollment, while median NAb titers of only 1:40 were present in the CP donors. 24 Moreover, there was no difference in NAb titers between groups (CP and control) following CP transfusion. These data indicate that NAb titers of both CP donors and COVID‐19 patients are important considerations when assessing the suitability of CP treatment.
TABLE 2.
CP administration and antibody concentration
| Study | Timing | Antibody concentration | Vol | Doses | Notes | |||
|---|---|---|---|---|---|---|---|---|
| CP | Control | Donor | (ml) | |||||
| Pre | Post | |||||||
| Abolghasemi et al 17 # | ≤7 days from symptom onset | — | — | — | >1.1 | 500 | 1‐2 (24 hours apart) | — |
| Agarwal et al 24 | 8 (6–11) days from symptom onset to enrollment | — | — | — | 40 (30‐80) | 200 | 2 (24 hours apart) | Baseline NAb (50%) titer for all pts: 90 (30‐240). NAb titers similar between groups at days 0, 3, and 7. |
| Alsharidah et al 27 | CP transfusion within 24 hours of admission | — | — | — | — | 200 | 1‐2 (24 hours apart) | — |
| Altuntas et al 29 | — | — | — | — | — | ≤600 | — | — |
| Duan et al 22 | 16.5 (11.0‐19.3) days from symptom onset to CP transfusion | 320 (320‐640) | 640 (640‐640) | — | >640 | 200 | 1 | NAb (50%) |
| Gharbharan et al 18 | 10 (6‐15) days from symptom onset to time of inclusion | 320 (20‐1280) | — | 80 (20‐640) | 640 (320‐1280) | 300 | 1–2 (5 days apart) | NAb (50%) |
| Hegerova et al 19 | 2 (1‐4.3) days from hospitalization to CP transfusion | — | — | — | — | — | — | — |
| Li et al 20 * | 30 (20‐39) days from symptom onset to randomization | — | — | — | ≥640 | 4–13 mL/kg | — | — |
| Liu et al 23 * | 7 (range 0‐14) days from symptom onset to initial presentation. 4 (range 0‐7) days from admission to CP transfusion | — | — | — | ≥320 | 500 | — | — |
| Omrani et al 28 |
10 (9‐10) days from symptom onset to CP transfusion |
— | — | — | — | 400 | — | — |
| Rasheed et al 25 # | 14.8 ± 7.5 days infected to study inclusion | — | — | — | ≥1.25 | — | — | — |
| Rogers et al 30 # | 7 (5‐9) days from symptom onset to CP transfusion | — | — | — | Variable | — | 1‐2 |
50% of pts received CP with AI ≥1.4. 28% of pts received CP with AI ≥5.0. 13% of pts received CP with AI <1.4. |
| Salazar et al 31 * | — | — | — | — | ≥1350 | 300 | 1‐2 | 7% of pts received CP with IgG 150‐1350 and 2% received CP with IgG <150. |
| Simonovich et al 26 * | 8 (5‐10) days from symptom onset to enrollment | 50 (0‐800) | 400 (200‐1600) | 50 (0–1600) |
IgG:3200 (800‐3200) NAb: 300 (136‐511) |
500 (415‐600) | — | 65% of total pts had baseline IgG data. 56% of donors had NAb (80%) titers evaluated. |
| Zeng et al 21 | 21.5 (17.8‐23) days of viral shedding before treatment | — | — | — | — | 300 (200‐600) | — | — |
Note: Timing of CP administration is presented as median (IQR) or mean ± SD unless otherwise indicated. Neutralizing antibody (NAb) titers are presented unless otherwise indicated (*IgG, # IgG index). Titers (NAb, IgG; 1:#) or IgG indexes are expressed as median (IQR) or as a single value expressing the minimum concentration used for transfusion (Donor). Data from patients in the convalescent plasma cohort (CP) are provided as baseline (Pre) or after treatment (Post) levels. NAb (%): percentage of virus neutralized.
Abbreviations: AI, antibody index; Pts, patients.
NAb levels in COVID‐19 patients may be detected within 2 to 3 weeks after symptom onset. 39 , 40 By the time these antibodies develop in patients with severe disease, significant lung injury may have already occurred. Early introduction of NAbs to COVID‐19 patients could attenuate viremia and prevent immune‐mediated lung damage. Therefore, it is believed that prompt administration of CP could improve treatment efficacy through this mechanism. 41 On the other hand, late administration of CP may not be beneficial in patients that have high NAb levels and have already developed significant lung injury. In SARS‐CoV‐1, it was reported that seronegative patients experienced better clinical outcomes with CP therapy as compared to seropositive patients. 3 In the present study, the median time from symptom onset to CP therapy was approximately 13 days, which would likely be too late to be of benefit for many of these patients. Early CP therapy occurred infrequently, and the sole importance of early CP administration was unclear. Alsharidah et al 27 and Hegerova et al 19 reported fewer deaths in patients that received CP therapy within 24 and 48 hours, respectively, of hospital admission. Moreover, Hegerova et al reported all deaths in the CP group occurred in patients that received CP after 7 days from hospitalization. Rasheed et al noted that the most favorable outcomes occurred in CP patients treated no more than 3 days upon entrance to the respiratory care unit. 25 In contrast, the median time from onset of symptoms to enrollment was approximately 1 week in Agarwal et al 24 and Simonovich et al, 26 which both failed to observe clinical benefits with CP treatment. Perhaps the most thorough examination of therapeutic timing of CP treatment was performed by Salazar et al. 31 In this propensity‐score matched study that consisted of 935 patients, high IgG titer (anti‐RBD, ≥1:1350) CP infusion within 44 hours from hospital admission resulted in the greatest mortality benefit. Importantly, the mortality benefit was not observed in patients that were intubated at day 0 or in patients who were transfused >72 hours after hospital admission, even when transfused with high IgG titer CP. Taken together, these data indicate that both high titer CP and early administration are important considerations for effective CP therapy. Additional studies that ideally utilize standardized, short‐term approaches are required to identify the potential benefits of early CP treatment.
The evidence from our meta‐analysis suggests that CP therapy can improve clinical status, although improvements in clinical status were examined less frequently than mortality. It is important to note that the RCTs that failed to detect a mortality benefit with CP treatment did not assess for improvements in clinical status. 24 , 26 However, Simonovich et al did not detect a difference in clinical status (6‐category ordinal scale) between CP (~8 days from symptom onset to enrollment) and placebo control patients at 30 days. 26 The two largest studies that reported improvements in clinical status with CP therapy also reported mortality benefits. 27 , 31 Both studies assessed clinical improvement in patients that received early CP administration (~1‐2 days from hospital admission). In contrast, Gharbharan et al reported identical rates of improvement in clinical status between CP and control patients (58%); however, ~10 days had elapsed from symptom onset to time of inclusion and both groups possessed substantial NAb titers at baseline. 18 Omrani et al reported a nonsignificant improvement in clinical status, 28 but this study also involved CP administration at a later time point (10 days) as compared to the two largest studies in this analysis. 27 , 31 Altuntas et al did not specifically assess for clinical improvement; however, the study did note reductions in the duration of time spent in the ICU and the rate of mechanical ventilation with CP treatment. 29 Moreover, they noted a greater rate of mechanical ventilation in CP patients that received CP therapy 20 days after COVID‐19 diagnosis or the onset of symptoms as compared to CP patients that received treatment earlier in the disease course (<15 days). In all, these data suggest that CP therapy may improve the clinical status of COVID‐19 patients, although clinical improvement may depend on CP administration early in the disease course.
We observed no differences in hospital length of stay between COVID‐19 patients that received CP therapy and those that received control therapies. Abolghasemi et al reported a lower length of stay with CP treatment, 17 while Hegerova et al reported a lower length of stay in control. 19 Administration of CP occurred ≤7 days from symptom onset in Abolghasemi et al, although the specific data on timing were not provided. Hegerova et al utilized early administration of CP relative to the time of hospitalization (~2 days); however, it is important to note that 50% of control patients received remdesivir treatment as compared to only 5% of CP patients. 19 Remdesivir is effective at reducing the amount of time needed for recovery from COVID‐19 42 ; thus, the greater use of remdesivir in control patients may have confounded length of stay results from Hegerova et al. Nevertheless, the collective data indicate that CP therapy does not reduce length of stay for hospitalized COVID‐19 patients.
There remain obstacles to the widespread use of CP therapy for the treatment of COVID‐19. While availability to apheresis has increased worldwide, effective CP therapy also depends on a sufficient number of trained professionals to perform apheresis. 43 Adequate CP storage procedures/facilities and laboratory techniques to assess IgG or NAb titers are also important considerations for effective CP treatment. Thus, the potential efficacy of CP therapy for COVID‐19 may be influenced, in part, by regional healthcare resources and infrastructure.
4.1. Limitations
Many of the studies included in the analysis were observational. Moreover, 2 of the 6 RCTs were terminated early. 18 , 20 We decided to leave early‐terminated RCTs in the final analysis due to their overall quality (eg, randomization, etc.) despite the low statistical power. While interpretation of early‐terminated, underpowered studies should be made with caution, the inclusion of these studies elevated the quality of the present meta‐analysis. The limited number of studies and the lack of patient‐level data made it difficult to explore sources of heterogeneity (eg, meta‐regression could not be performed). In addition, small study bias may have influenced the results. Standard of care varied within and across studies, which could have impacted the results. We performed subgroup analyses of mortality and clinical improvement at standardized follow‐up times to reduce bias in our primary analyses; however, reduced statistical power may impact the effect size estimates of subgroup comparisons and between‐study variability unattributable to sampling error was not reduced based on conservative estimates of I 2 around their respective confidence intervals. Lastly, the median time from symptom onset to CP therapy was approximately 13 days; thus, the window for therapeutic benefit might have been missed. Studies should utilize standardized, early CP administration to maximize treatment efficacy. To some degree, our choice of statistical model accounts for these variations in study characteristics by incorporating random effects on the study design.
5. CONCLUSIONS
We detected a mortality benefit with CP therapy; however, there was not a clear consensus in support of a mortality benefit with the randomized controlled studies included in the analysis. Therefore, we concluded that the efficacy of CP therapy to lower the odds of mortality was unclear and not fully supported by the data. We detected greater odds of clinical improvement with CP therapy, but CP therapy did not reduce hospital length of stay. There is a need for further, randomized controlled studies to better understand the efficacy of CP therapy for COVID‐19 patients, especially regarding mortality rates.
CONFLICT OF INTEREST
The authors declare no interests with the subject of this manuscript. N.M., N.R., S.K., and M.S. work for Nested Knowledge and Superior Medical Experts. J.M.P. is employed by Nested Knowledge, Superior Medical Experts, and Marblehead Medical. K.M.K. works for and holds equity in Nested Knowledge, Superior Medical Experts, and Marblehead Medical. A.R.D., K.W.E., and M.M. are employed by Superior Medical Experts.
Supporting information
Figure S1 Forest plot of subgroup comparisons of age distributions. Pooled results were computed using a fixed‐effects model. For some studies, estimation procedures described by Luo et al1 and Wan et al2 were used to derive means and variances when data were presented as quantiles.
Figure S2. Forest plot of days from symptom onset to receiving convalescent plasma. Aggregated results were computed using the weighted median of medians method proposed by McGrath et al.3
Figure S3. Funnel plot of log transformed odds of mortality. The global meta‐analysis revealed potential small study bias after using Harbord's regression test (estimate = −1.285, SE = 0.483, intercept = −0.076, P = .020).
Table S1. Clinical Improvement.
Table S2. Risk of Bias Assessments.
Vegivinti CTR, Pederson JM, Saravu K, et al. Efficacy of convalescent plasma therapy for COVID‐19: A systematic review and meta‐analysis. J Clin Apher. 2021;36:470–482. 10.1002/jca.21881
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Coronavirus disease (COVID‐19) Weekly Epidemiological Update and Weekly Operational Update; December 29, 2020; https://www.who.int/publications/m/item/weekly-epidemiological-update---29-december-2020. Accessed January 5, 2021.
- 2. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599‐609. [DOI] [PubMed] [Google Scholar]
- 3. Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (COVID‐19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211(1):80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID‐19: systematic review. J Med Virol. 2020;92:1475‐1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. SIGN 50: A guideline developer's handbook. In. 2nd ed.: Scottish Intercollegiate Guidelines Network; 2011.
- 8. Viechtbauer W. Package 'metafor'. Meta‐Analysis Package for R; 2020. https://cran.r-project.org/web/packages/metafor/metafor.pdf.
- 9. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat Methods Med Res. 2018;27(6):1785‐1805. [DOI] [PubMed] [Google Scholar]
- 10. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGrath S, Zhao X, Qin ZZ, Steele R, Benedetti A. One‐sample aggregate data meta‐analysis of medians. Stat Med. 2019;38(6):969‐984. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107‐128. [Google Scholar]
- 14. Henmi M, Copas JB. Confidence intervals for random effects meta‐analysis and robustness to publication bias. Stat Med. 2010;29(29):2969‐2983. [DOI] [PubMed] [Google Scholar]
- 15. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 16. Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443‐3457. [DOI] [PubMed] [Google Scholar]
- 17. Abolghasemi H, Eshghi P, Cheraghali AM, et al. Clinical efficacy of convalescent plasma for treatment of COVID‐19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020;59(5):102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gharbharan A, Jordans CCE, GeurtsvanKessel C, et al. Convalescent plasma for COVID‐19. A randomized clinical trial. MedRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.07.01.20139857v1. [Google Scholar]
- 19. Hegerova L, Gooley TA, Sweerus KA, et al. Use of convalescent plasma in hospitalized patients with COVID‐19: case series. Blood. 2020;136(6):759‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial. JAMA. 2020;324(5):460‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu STH, Lin HM, Baine I, et al. Convalescent plasma treatment of severe COVID‐19: a propensity score‐matched control study. Nat Med. 2020;26(11):1708‐1713. [DOI] [PubMed] [Google Scholar]
- 24. Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid‐19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial). BMJ. 2020;371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rasheed AM, Fatak DF, Hashim HA, et al. The therapeutic potential of convalescent plasma therapy on treating critically‐ill COVID‐19 patients residing in respiratory care units in hospitals in Baghdad. Iraq Infez Med. 2020;28(3):357‐366. [PubMed] [Google Scholar]
- 26. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid‐19 severe pneumonia. N Engl J Med. 2020. 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alsharidah S, Ayed M, Ameen RM, et al. COVID‐19 convalescent plasma treatment of moderate and severe cases of SARS‐CoV‐2 infection: a multicenter interventional study. Int J Infect Dis. 2020;103:439‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Omrani AS, Zaqout A, Baiou A, et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: a preliminary report. J Med Virol. 2021;93: 1678‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Altuntas F, Ata N, Yigenoglu TN, et al. Convalescent plasma therapy in patients with COVID‐19. Transfus Apher Sci. 2020;102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rogers R, Shehadeh F, Mylona EK, et al. Convalescent plasma for patients with severe COVID‐19: a matched cohort study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salazar E, Christensen PA, Graviss EA, et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID‐19) patients transfused early with convalescent plasma containing high‐titer anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike protein IgG. Am J Pathol. 2020;191(1):90‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hung IFN, KKW T, Lee CK, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double‐blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144(2):464‐473. [DOI] [PubMed] [Google Scholar]
- 33. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450‐1451. [DOI] [PubMed] [Google Scholar]
- 34. Wu XX, Gao HN, Wu HB, Peng XM, Ou HL, Li LJ. Successful treatment of avian‐origin influenza A (H7N9) infection using convalescent plasma. Int J Infect Dis. 2015;41:3‐5. [DOI] [PubMed] [Google Scholar]
- 35. Hui DS, Lee N, Chan PK, Beigel JH. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res. 2018;150:202‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Enria DA, Briggiler AM, Fernandez NJ, Levis SC, Maiztegui JI. Importance of dose of neutralising antibodies in treatment of argentine haemorrhagic fever with immune plasma. Lancet (London, England). 1984;2(8397):255‐256. [DOI] [PubMed] [Google Scholar]
- 37. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS‐CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Emergency Use Authorization (EUA) for the emergency use of COVID‐19 convalescent plasma for the treatment of hospitalized patients with Coronavirus Disease 2019 (COVID‐19). 2020. https://www.fda.gov/media/141477/download. Accessed December 22, 2020.
- 39. Okba NMA, Muller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2‐specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26(7):1478‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X, Guo X, Xin Q, et al. Neutralizing antibodies responses to SARS‐CoV‐2 in COVID‐19 inpatients and convalescent patients. Clin Infect Dis. 2020;71(10):2688‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis. 2020;20(4):398‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—final report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eichbaum Q, Smid WM, Crookes R, et al. Apheresis in developing countries around the world. J Clin Apher. 2015;30(4):238‐246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Forest plot of subgroup comparisons of age distributions. Pooled results were computed using a fixed‐effects model. For some studies, estimation procedures described by Luo et al1 and Wan et al2 were used to derive means and variances when data were presented as quantiles.
Figure S2. Forest plot of days from symptom onset to receiving convalescent plasma. Aggregated results were computed using the weighted median of medians method proposed by McGrath et al.3
Figure S3. Funnel plot of log transformed odds of mortality. The global meta‐analysis revealed potential small study bias after using Harbord's regression test (estimate = −1.285, SE = 0.483, intercept = −0.076, P = .020).
Table S1. Clinical Improvement.
Table S2. Risk of Bias Assessments.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


