Abstract
Emerging evidence has underscored the potential usefulness of red blood cell distribution width (RDW) measurement in predicting the mortality and disease severity of COVID‐19. This study aimed to assess the association of the plasma RDW levels with adverse prognosis in COVID‐19 patients. A comprehensive literature search from inception to September 2020 was performed to harvest original studies reporting RDW on admission and clinical outcomes among patients hospitalized with COVID‐19. RDW levels were compared between cases (patients who died or developed more severe symptoms) and controls (patients who survived or developed less severe symptoms). A total of 14,866 subjects from 10 studies were included in the meta‐analysis. Higher levels of RDW were associated with adverse outcomes in COVID‐19 patients (mean differences = 0.72; 95% CI = 0.47–0.97; I 2 = 89.51%). Deceased patients had higher levels of RDW compared to patients who survived (mean differences = 0.93; 95% CI = 0.63–1.23; I 2 = 85.58%). Severely ill COVID‐19 patients showed higher levels of RDW, as opposed to patients classified to have milder symptoms (mean differences = 0.61; 95% CI = 0.28–0.94; I 2 = 82.18%). Elevated RDW levels were associated with adverse outcomes in COVID‐19 patients. This finding warrants further research on whether RDW could be utilized as a simple and reliable biomarker for predicting COVID‐19 severity and whether RDW is mechanistically linked with COVID‐19 pathophysiology.
Keywords: biomarkers, COVID‐19, erythrocyte indices, red blood cell distribution width, severe acute respiratory syndrome coronavirus 2
Abbreviations
- CAD
coronary artery disease
- CBC
complete blood count
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- ED
emergency department
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RBC
red blood cell
- RDW
red blood cell distribution width
- ROC
receiver operating characteristic
- SD
standard deviation
1. INTRODUCTION
Red blood cells (RBC) serves as the vehicle for delivering oxygen to peripheral tissues in the human body. Unequal size of RBCs in the circulation, termed anisocytosis, is observed in several conditions, such as nutritional deficiencies, anemias, sickle cell anemia, hemolytic anemia, myelodysplastic syndrome, and other hematological disorders. 1 , 2 As such, characteristics of human RBCs are utilized in the differential diagnosis of various clinical settings. 3 The coefficient of variation of RBC distribution width (RDW), calculated from dividing the standard deviation (SD) of corpuscular volume by the mean corpuscular volume, is a commonly used measure to quantify the variation of individual RBC volumes as it circulates during the approximate lifespan of 115 days. 4 An increase in RDW can be attributed to several factors. First, increased RDW may reflect an imbalance between hematopoiesis and RBC survival. 5 Specifically, delayed clearance of senescent RBCs from the circulation leading to RBC underproduction, resulting in an increase in the plasma levels of RDW. 5 , 6 Second, elevated RDW may suggest an underlying inflammation through multiple mechanisms. For instance, proinflammatory cytokines, such as interferon γ and tumor necrosis factor α, may affect iron metabolism and the capacity of RBC production by the bone marrow, which leads to anemia and increased RDW. 7 Alternatively, RDW may increase due to shortened RBC lifespan and premature release of RBCs from the bone marrow in the presence of increased oxidative stress associated with inflammation. 8 Third, RDW could also increase in other physiologic events, such as aging, pregnancy, or following erythropoietin stimulation and exercise. 1
In practice, elevated RDW levels are utilized as a diagnostic tool for differentiating an early stage of nutritional deficiency or megaloblastic anemias from thalassemia. The potential value of RDW as a rapid and easy prognostic tool among high‐risk patients, if effective, will vastly benefit timely intervention because RDW levels are measured as part of routine measures of complete blood count (CBC) by automated instruments in hematology laboratories. In the pre‐coronavirus disease 2019 (COVID‐19) era, a large‐scale prospective cohort study indicated that RDW was a predictor for all‐cause mortality independent of the presence of inflammation. 9 Nevertheless, inflammation may, at least in part, interact with the association of RDW with mortality. In the context of the COVID‐19 pandemic, emerging evidence supports the usefulness of biomarkers (e.g., C‐reactive protein, troponin, D‐dimer) in predicting mortality, disease severity, or thrombotic complications among patients hospitalized for COVID‐19. 10 , 11 However, the association of RDW with adverse prognosis in COVID‐19 has not been well‐established.
The current meta‐analysis aimed to review and synthesize the current evidence on the association of RDW levels with COVID‐19 mortality and severity. We hypothesized that among patients with laboratory‐confirmed COVID‐19 infection, those who died or were severely ill would have higher levels of RDW compared to those who survived or were mildly ill.
2. METHODS
2.1. Search strategies and selection criteria
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, and the protocol was registered in PROSPERO (registration number: CRD42020211560). 12 A systematic literature search was performed in PubMed, supplemented by a hand search of references from relevant publications, to collect eligible studies from inception to September 2020. The search for identifying qualifying studies was initiated by constructing sets of relevant keywords (i.e., RDW and COVID‐19) and their synonyms. These search terms were expanded and organized in thematic building blocks, as provided in Table S1. To be included in the meta‐analysis, published studies needed to be (1) conducted in human subjects, (2) original research articles (including letters and abstracts), (3) reported RDW levels in COVID‐19 patients, where there were two or more groups of patients with mortality status (i.e., deceased or survived) or severity (e.g., mild, moderate, or severe cases of COVID‐19), and (4) published in English. Nonoriginal publications (e.g., narrative review, systematic review, meta‐analysis, editorial) and studies that did not report RDW or adverse outcomes in COVID‐19 patients were excluded. Reference lists of relevant studies and review articles were screened for potentially eligible studies. Additional searches were performed in medRxiv to identify preprints (i.e., preliminary reports of work that have not been certified by peer review) that were qualified for the analysis. 13 Duplicated publications were removed after confirming identical publication information.
2.2. Data extraction
Data extracted from each study included study design, study population, setting, patient characteristics (age, sex, body mass index, coronary artery disease [CAD], hypotension, smoking status, type 2 diabetes mellitus, cancer, chronic kidney disease), length of hospital stay, methods for RDW measurement, RDW levels (mean and SD and/or median and 25th and 75th percentiles), follow‐up visits and adverse outcomes (i.e., disease severity or mortality status). Two investigators (Jane J. Lee and Gerald Chi) independently performed the database search and completed article screening and study selection based on a prespecified standardized approach. A third investigator (Adeel Jameel) adjudicated disagreement in extracted articles. A PRISMA flow diagram depicting the process of literature search and screening is provided in Figure 1.
Figure 1.
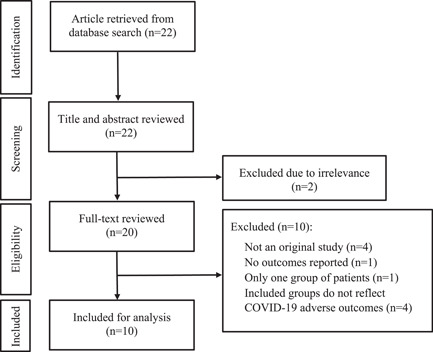
Flowchart presenting the literature search process according to the PRISMA Statement
2.3. Quality assessment
Two independent investigators (Sahar M. Montazerin and Jolanta Marszalek) assessed the quality of the included studies in accordance with the Newcastle–Ottawa Scale. Disagreement in the quality assessment was resolved by discussion and consensus. The quality assessment criteria and scores are provided in Tables S2 and S3.
2.4. Study endpoints and RDW measurements
The study endpoint was adverse clinical outcomes, defined as the composite of mortality or severe COVID‐19. If a study classified patients into three groups (i.e., mild, moderate, and severe) based on the clinical severity of COVID‐19, the group with the most severe symptoms was compared with the group with the mildest symptoms. The coefficient of variation of RDW, expressed as percentages were assessed upon hospital admission as part of the standard complete blood test from each study for assessment of association with adverse clinical outcomes.
2.5. Statistical analysis
The statistical analysis for this meta‐analysis included primary and subgroup analyses. The primary analysis was performed to compare the mean levels of RDW between cases (patients with adverse outcomes, defined as those who died or developed more severe symptoms) and controls (patients without adverse outcomes, defined as those who survived or developed less severe symptoms). In the subgroup analyses, the difference in RDW levels was calculated by comparison between (1) nonsurvivors versus survivors and (2) patients with more severe symptoms versus patients with milder symptoms. In addition, a sensitivity analysis was performed to compare the effects of RDW stratified by geographic regions (China vs. non‐China [including the United States and the Netherlands]).
RDW levels were uniformly expressed in percentage for all included studies. For analysis purposes, RDW levels reported in median and 25th and 75th percentiles were converted to mean and SD. 14 For each study, the mean level of RDW was used as an effect size statistic, and the inverse variance of the mean RDW levels was used as study weight. Confidence intervals (CIs) of RDW levels were calculated by normal approximation. The summary effect size was then computed by fitting a random‐effects model using the DerSimonian and Laird method. 15 Heterogeneity across the studies was assessed using the Cochran's Q test (with the threshold of p < .10, indicating the presence of heterogeneity) and I 2 statistic (I 2 > 50%: significant heterogeneity; I 2 ≤ 50%: insignificant heterogeneity). Funnel plots were used for visual inspection of publication bias, and the Egger test was used for detecting small‐study effect for endpoints with a study number of 10 or more. 16
All the analysis was performed using the metan and metaninf packages in the STATA software of version 16.1 (Stata Corporation).
3. RESULTS
A total of 14,866 subjects from 10 studies were included in the meta‐analysis. Summary of study characteristics and patient characteristics were provided in Tables 1 and 2. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 The mean age ranged from 38 to 77 years. The proportion of males ranged from 42.4% to 69.2%. The status of CAD, hypertension, and diabetes ranged from 0% to 28%, 2.9%–68.8%, and 5.7%–70.6%, respectively. The majority of the included studies were retrospective and observational. The quality of the studies was generally high, with scores ranging from 6 to 9, as evaluated with the Newcastle‐Ottawa Scale (Tables S2 and S3).
Table 1.
Summary of study characteristics
| Author, Year | N | Country | Study design | Study Population | Setting | Length of hospital stay (days) | RDW measurement | Follow‐Up | Comparisons | When was the CBC obtained? |
|---|---|---|---|---|---|---|---|---|---|---|
| Foy et al., 2020 17 | 1641 | United States | Retrospective observational | Adults diagnosed with COVID‐19 and admitted | Ward | 16.7 (15.5) versus 11.8 (11) | Complete blood counts (including RDW) were performed on an XN‐9000 Automated Hematology System (Sysmex Corporation). | Between March 4, 2020, and April 28, 2020 | Survivors (N = 1376) versus nonsurvivors (N = 276) | At the time of admission |
| Gong et al., 2020 18 | 189 | China | Retrospective observational | Nonsevere COVID‐19 hospitalized patients | Ward | – | – | >15 days after admission | Nonsevere (N = 161) versus severe (N = 28) | On admission |
| Henry et al., 2020 19 | 49 | United States | Prospective observational | Adults (≥18 years of age) presenting to the ED with COVID‐19 confirmed by RT‐PCR | ED | – | The CBC with differential was performed using a Beckman UniCel DxH 800 Coulter Cellular Analysis System. | 30 days | Maximum severity within 30 days of presentation (Mild [N = 16] vs. Moderate [N = 17] vs. severe [N = 16]) | During the index visit to the ED |
| ED disposition severity (mild [N = 16] vs. moderate [N = 27] vs. Severe [N = 6]) | ||||||||||
| Jans et al., 2020 20 | 261 | The Netherlands | Retrospective observational | Patients who presented with PCR‐proven COVID‐19 | Ward | – | – | From March 5, 2020, until May 7, 2020 | Nonsevere (N = 162) versus severe (N = 92) | During the index visit to the ED |
| Levy et al., 2020 21 | 11,095 | United States | Retrospective and prospective cohort study | Adult patients hospitalized with a confirmed diagnosis of COVID‐19 | Ward | 5.97 (3.16–10.20) versus 7.81 (3.86–14.18) | – | 7 days | Survivors (N = 8499) versus nonsurvivors (N = 2596) | Obtained while the patient was in the ED |
| Lu, 2020 26 | 151 | China | – | Intensive care patients admitted with confirmed COVID‐19 | ICU | – | – | From January 25 to February 25, 2020 | Type D (favorable prognosis) (N = 42) versus Type C (intermediate prognosis) (N = 27) versus Type B (poor prognosis) (N = 45) versus Type A (extremely poor prognosis) (N = 37) | On the day of admission |
| Luscze et al., 2020 22 | 1022 | United States | Retrospective observational | COVID‐19 patient (PCR‐positive COVID‐19 test) admissions from 14 Midwest US hospitals | Ward/ICU | – | – | ≥14 days after admission | Clinical phenotype III (N = 173) versus phenotype II (N = 613) versus phenotype I (N = 236) | Collected within 72 h of admission |
| Nicholson et al., 2020 23 | 1042 | United States | Retrospective observational | Adult patients with confirmed COVID‐19 who were admitted for illness related to COVID‐19 | Ward | – | – | Up to July 20, 2020 | Survivors (N = 829) versus nonsurvivors (N = 211) | Performed on or within 24 h of hospital admission |
| Wang et al., 2020 24 | 45 | China | Retrospective observational | Hospitalized patients with confirmed COVID‐19 | Ward | – | Complete blood counts (BC‐6900, Mindray) were collected from the laboratory information system. | Between January 23, 2020, and February 13, 2020 | Moderate (N = 35 [131 samples]) versus severe (N = 10 [30 samples]) | Collected from the laboratory information system |
| Wu et al., 2020 25 | 71 | China | – | Hospitalized patients with confirmed COVID‐19 | Ward | – | – | – | Mild (N = 32) versus severe (N = 39) | Obtained from electronic medical record |
Abbreviations: CBC, complete blood count; ED, emergency department; RT‐PCR, reverse transcription‐polymerase chain reaction.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Summary of patient characteristics
| Author, year | Age (years) | Men (%) | BMI (kg/m2) | CAD (%) | Hypertension (%) | Smoking (%) | Diabetes (%) | Cancer (%) | CKD (%) |
|---|---|---|---|---|---|---|---|---|---|
| Foy et al., 2020 17 | 59.6 (17.6) versus 74.6 (13.4) | 53 versus 59 | 30.8 (6.8) versus 30.2 (7.2) | 8 versus 16 | 23 versus 36 | – | 17 versus 22 | – | 8 versus 21 |
| Gong et al., 2020 18 | 45.0 (33.0–62.0) versus 63.5 (54.5–72.0) | 44.7 versus 57.1 | 23.4 (21.4–25.7) versus 23.4 (22.3–24.4) | – | – | – | – | – | – |
| Henry et al., 2020 19 | 44 (32–50) versus 59 (39–68) versus 66 (53–71) | 68.8 versus 44.4 versus 62.5 | 28 (25–33) versus 26 (25–36) versus 29 (25–32) | 0 versus 23.5 versus 18.8 | 18.8 versus 64.7 versus 68.8 | 25.0 versus 23.5 versus 31.3 | 18.8 versus 70.6 versus 31.3 | 0 versus 5.9 versus 18.8 | 0 versus 17.6 versus 18.8 |
| Jans et al., 2020 20 | 60.3 (16.6) versus 68.2 (16.8) | 52.4 versus 63.4 | 30.2 (5.7) versus 28.4 (5.4) | 11.3 versus 16.1 | 35.7 versus 46.2 | – | 13.7 versus 23.7 | 6.0 versus 15.1 | 2.4 versus 7.5 |
| Levy et al., 2020 21 | – | 57.2 versus 63.7 | 28.30 (25.10–32.60) versus 27.40 (23.90–31.70) | 8.2 versus 15.4 | 49.6 versus 61.8 | – | 29.6 versus 35.5 | – | 6.5 versus 2.0 |
| Lu, 2020 26 | 53 (43–58) versus 65 (51–74) versus 62 (52–70) versus 77 (70–81) | – | – | – | – | – | – | – | – |
| Lusczek et al., 2020 22 | 58.6 (34.8–71.3) versus 60.9 (45.9–75.4) versus 67.2 (52.9–79.0) | 42.4 versus 46.6 versus 58.4 | 30.4 (13.4) versus 30.8 (8.2) versus 29.5 (8.9) | – | – | 10.4 versus 7.2 versus 3.8 | – | 9.2 versus 11.9 versus 12.3 | 21.4 versus 27.7 versus 39.0 |
| Nicholson et al., 2020 23 | 61 (50–71) versus 75 (66–82) | 54.1 versus 66.4 | – | 14.8 versus 28.0 | 53.6 versus 67.3 | – | 39.8 versus 52.6 | 13.6 versus 25.1 | 13.8 versus 28.0 |
| Wang et al., 2020 24 | 38 (16–62) versus 43 (28–62) | 48.6 versus 60.0 | – | – | 2.9 versus 30.0 | – | 5.7 versus 20.0 | 0 versus 10.0 | 2.9 versus 10.0 |
| Wu et al., 2020 25 | 56 (38–66) versus 62 (54–72) | 56.2 versus 69.2 | – | 9.4 versus 23.4 | 12.5 versus 30.8 | – | 18.8 versus 20.5 | 3.1 versus 12.8 | – |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.1. Association between RDW and adverse outcomes among COVID‐19 patients
Higher levels of RDW were significantly associated with severely ill COVID‐19 patients (pooled mean differences: 0.72; 95% CI, 0.47 to 0.97; Figure 2). The I 2 value of 89.51% suggested the existence of heterogeneity. No sign of publication bias was detected based on visual inspection of the funnel plot, which was symmetrically shaped (Figure 3). No small‐study effect was observed, as determined by Egger's test (Z = 1.94, p = .052). In the sensitivity analysis, elevated RDW was associated with adverse outcomes in Chinese populations (pooled mean differences: 0.55; 95% CI, 0.09–1.01) and in non‐Chinese populations (pooled mean differences: 0.83; 95% CI, 0.53–1.12). The test for subgroup difference was not significant (p = .31) (Figure S1).
Figure 2.
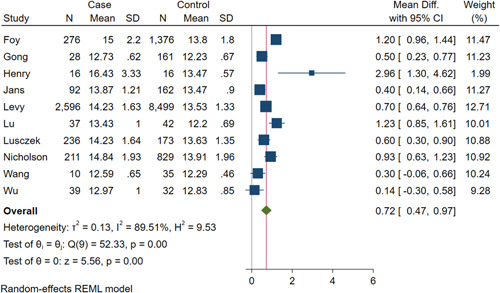
Red blood cell distribution width and adverse outcomes in COVID‐19 patients
Figure 3.
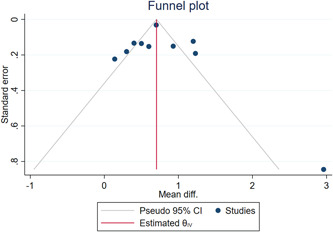
Funnel plot of red blood cell distribution width and adverse outcomes in COVID‐19 patients
3.2. Subgroup analysis
Results of the subgroup analysis on the associations with two subsets of the study participants (mortality and COVID‐19 severity) are shown in Figure 4. On the basis of three studies included in the mortality subgroup analysis, deceased patients had higher levels of RDW compared to survived COVID‐19 patients (pooled mean differences: 0.93; 95% CI, 0.63–1.23).
Figure 4.
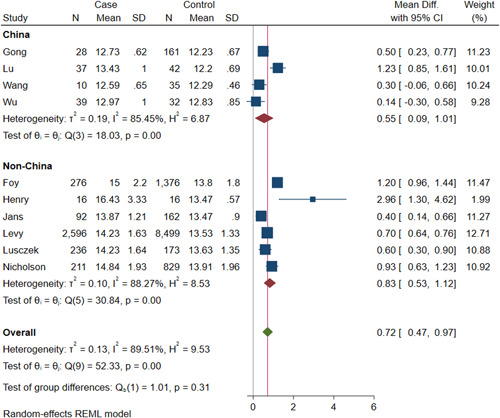
Red blood cell distribution width and subgroups of COVID‐19 patients with adverse outcomes
According to the seven studies included in the COVID‐19 severity subgroup analysis, severely ill COVID‐19 patients showed higher levels of RDW, as opposed to patients classified to have mild symptoms (pooled mean differences: 0.61; 95% CI, 0.28–0.94). There was significant heterogeneity across subgroups (I 2 for mortality subanalysis = 85.58%; I 2 for severity subanalysis = 82.18%). There was no significant difference between the subgroups (p = .17), indicating a consistent association between RDW and adverse outcomes.
4. DISCUSSION
In this meta‐analysis, higher levels of RDW were associated with unfavorable outcomes among COVID‐19 patients. Furthermore, nonsurvivors and patients with more severe symptoms had a significantly greater RDW as compared to survivors and those with less severe symptoms. Taken together, these findings suggest that RDW measurement on hospital admission provided prognostic insights among patients hospitalized for COVID‐19.
RDW has been reported as an independent marker of mortality regardless of demographic characteristics and underlying clinical conditions 1 and also in the context of COVID‐19. 17 , 20 , 21 Elevation of the RDW level has also been associated with higher mortality, such as sepsis. 27 Consistent with our findings, Henry et al. 19 demonstrated that elevated RDW at the time of emergency department visit was associated with a nine‐fold increased odds of COVID‐19 severity. This was further confirmed by another report, suggesting that RDW can be incorporated into a prediction model consisting of advanced age, lactate dehydrogenase, C‐reactive protein, blood urea nitrogen, direct bilirubin, and lower albumin (area under the receiver operating characteristic [ROC] curve, 0.912; 95% CI: 0.846–0.978], sensitivity: 85.71%, specificity: 87.58%). 18 Of clinical interest, a recent study by Wang et al. 28 reported prognostic values of hematological parameters, where an RDW cutoff value of 12.85% demonstrated a sensitivity of 73.9% and specificity of 81.9% with an area under the ROC curve of 0.870 (95% CI: 0.775–0.952) for predicting the prognosis of severe COVID‐19 patients. In light of the emergent nature of COVID‐19 infection and lack of healthcare resources, a simple and widely available tool, such as RDW, to assist with predicting disease severity and mortality among COVID‐19 patients is crucial.
The exact pathophysiology behind the association between increased RDW and adverse outcomes has yet to be elucidated. The findings may be explained by the following potential mechanisms. First, numerous reports have suggested that COVID‐19 infection was associated with an increase in the release and production of white blood cell counts and platelets from the bone marrow. The stimulation to the bone marrow may also impact the RBC kinetics, resulting in a wider range of RBC size and subsequently elevated RDW levels. 17 , 20 Another possible mechanism is the incompetent bone marrow in producing normal RBCs associated with COVID‐19 infection. Prior studies have noted the hyperinflammatory state in certain patients with COVID‐19. This overproduction of inflammatory cytokines may influence hematopoiesis by altering the release or response to erythropoietin or affecting the function and structure of RBC, thereby increasing the fragility of RBC and variability of its size. 18 In addition, systemic inflammatory conditions could have detrimental effects on iron absorption and accessibility, which are required for effective hematopoiesis. 19 Lastly, bone marrow suppression or destruction has also been attributed to immunologic dysregulation following COVID‐19 infection. In this scenario, patients typically present with anemia due to decreased production of RBC and develop a compensatory response characterized by the release of immature erythroid progenitor cells into the bloodstream that contributes to an increase in RDW levels. 24 On the contrary, RDW may serve as a nonspecific aggregate biomarker of general illness that is not mechanistically associated with the disease progression of COVID‐19.
The interaction of age on the association between RDW and mortality was investigated in two studies. In the study by Foy et al., 17 elevated RDW appeared to have a larger effect on mortality for younger patients (<70 years) than it had for older patients, suggesting a potential effect modification by age. In contrast, the study by Hornick et al. 29 showed that the relationship between RDW and mortality was consistent across the age spectrum, and there was no significant interaction between RDW and age. More research is needed to confirm the potential effect modification by age.
Of note, serial measurements of RDW could provide incremental mechanistic insights to the baseline RDW. Foy et al. 17 demonstrated that nonsurvivors had a significantly greater RDW increase during the first week of hospitalization than the survivors. Furthermore, compared to a stable RDW, an increasing RDW during hospitalization was associated with increased mortality risk among patients with a normal RDW at admission (24% vs. 6%), as well as among those with an elevated RDW at admission (40% vs. 22%). 17 Though elevated on‐admission RDW may reflect frailty or poor health at baseline, an increasing RDW during the course of infection may indicate poor hematopoietic regulation, increased RBC destruction, or increased stresses on bone marrow due to increased platelet and white cell production. Future studies should explore the prognostic value of an increasing RDW during hospitalization.
4.1. Limitations
Several limitations warrant consideration. First, only three studies were available in examining the association between RDW levels and mortality among COVID‐19 patients. Second, there were remarkable differences in patient characteristics and study settings between the included studies. Third, the definitions for disease severity of COVID‐19 vary across the studies. These variations may have contributed to the heterogeneity in the effect size. However, the consistent associations demonstrated in each study and the pooled results support the utility of RDW in predicting disease progression and mortality among COVID‐19 patients. Fourth, as patient‐level data from included studies were not available, the current meta‐analysis was performed at the study level. Although the association of RDW was robust to geographic regions, further research is warranted to explore whether the effects of RDW would remain consistent across other subgroups such as age, sex, and race. Next, differences in the characteristics and pathology of the study endpoints (i.e., mortality and severity of disease) may have under‐ or overestimated the results. To assess whether the heterogeneity of study endpoints affected the pooled results, we additionally conducted a subgroup analysis and observed consistent findings that both deceased and severely ill patients had higher levels of RDW. Further, improvement in the study endpoints and reduction of the endpoint heterogeneity could have been reached if additional studies were included by inquiring unpublished RDW values from studies of mortality and severity of COVID‐19 patients. Finally, ascertainment bias could occur when more intense surveillance or laboratory tests are arranged for critically ill patients than mildly ill patients. However, the risk of ascertainment bias may be low in the present analysis, as RDW is usually included as a part of the routine CBC test.
5. CONCLUSION
The meta‐analysis demonstrated that elevated RDW levels were associated with adverse outcomes in COVID‐19 patients. This finding warrants further research on whether RDW could be utilized as a reliable prognostic tool for predicting COVID‐19 severity. As RDW is widely available and included as a routine parameter of CBC, this simple laboratory test can be particularly useful in the context of the COVID‐19 pandemic, where identifying high‐risk patients and facilitating timely intervention with limited resources are critical. Future research should also examine whether RDW is mechanistically linked to the pathophysiology of COVID‐19.
CONFLICT OF INTERESTS
The work was not funded by industry. Dr. Chi received modest research grant support paid to the Beth Israel Deaconess Medical Center, Harvard Medical School from Bayer, Janssen Scientific Affairs, and CSL Behring. All remaining authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: Gerald Chi and Jane J. Lee. Acquisition, analysis, or interpretation of data: Gerald Chi, Jane J. Lee, Jolanta Marszalek, and Michael L. Chuang. Drafting of the manuscript: Gerald Chi, Jane J. Lee, Adeel Jamil, and Umer Jamil. Critical revision of the manuscript for important intellectual content: Sahar M. Montazerin and Michael L. Chuang. All authors have read and agreed to the published version of the manuscript.
Supporting information
Supplementary information.
Lee JJ, Montazerin SM, Jamil A, et al. Association between red blood cell distribution width and mortality and severity among patients with COVID‐19: A systematic review and meta‐analysis. J Med Virol. 2021;93:2513‐2522. 10.1002/jmv.26797
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Salvagno GL, Sanchis‐Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86‐105. [DOI] [PubMed] [Google Scholar]
- 2. Chi G, Ahmad A, Malik QZ, et al. Prognostic value of red cell distribution width in acute coronary syndrome. Open Access Blood Res Transfus J. 2018;1(4):555570. [Google Scholar]
- 3. Diez‐Silva M, Dao M, Han J, Lim CT, Suresh S. Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull. 2010;35(5):382‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112(10):4284‐4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgins JM, Mahadevan L. Physiological and pathological population dynamics of circulating human red blood cells. Proc Natl Acad Sci USA. 2010;107(47):20587‐20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112(10):4284‐4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133(4):628‐632. [DOI] [PubMed] [Google Scholar]
- 8. Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10(11):1923‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community‐based prospective cohort. Arch Intern Med. 2009;169(6):588‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 11. Chi G, Lee JJ, Jamil A, et al. Venous thromboembolism among hospitalized patients with COVID‐19 undergoing thromboprophylaxis: a systematic review and meta‐analysis. J Clin Med. 2020;9(8):2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. medRxiv . The preprint server for health sciences. https://www.medrxiv.org/content/about-medrxiv. Accessed January 1, 2021.
- 14. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta‐analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(pt A):139‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith GD, Egger M, Phillips AN. Meta‐analysis: Beyond the grand mean? BMJ. 1997;315(7122):1610‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foy BH, Carlson JCT, Reinertsen E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS‐CoV‐2 infection. JAMA Netw Open. 2020;3(9):e2022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gong J, Ou J, Qiu X, et al. A Tool for Early Prediction of Severe Coronavirus Disease 2019 (COVID‐19): a Multicenter Study Using the Risk Nomogram in Wuhan and Guangdong, China. Clinical Infectious Diseases. 2020;71(15):833‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henry BM, Benoit JL, Benoit S, et al. Red blood cell distribution width (RDW) predicts COVID‐19 severity: a prospective, observational study from the Cincinnati SARS‐CoV‐2 Emergency Department Cohort. Diagnostics. 2020;10(9):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jans M, Kuijper T, den Hollander J, et al. Predicting severe COVID‐19 at Presentation, Introducing the COVID Severity Score. 2020. https://ssrn.com/abstract=3627260 or 10.2139/ssrn.3627260. Accessed January 1, 2021. [DOI]
- 21. Levy TJ, Richardson S, Coppa K, et al. Estimating survival of hospitalized COVID‐19 patients from admission information. medRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.04.22.20075416v2 [Google Scholar]
- 22. Lusczek ER, Ingraham NE, Karam B, et al. Characterizing COVID‐19 clinical phenotypes and associated comorbidities and complication profiles. medRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.09.12.20193391v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholson CJ, Wooster L, Sigurslid HH, et al. Estimating risk of mechanical ventilation and mortality among adult COVID‐19 patients admitted to Mass General Brigham: the VICE and DICE Scores. medRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.09.14.20194670v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang C, Deng R, Gou L, et al. Preliminary study to identify severe from moderate cases of COVID‐19 using combined hematology parameters. Ann Transl Med. 2020;8(9):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Y, Huang X, Sun J, et al. Clinical characteristics and immune injury mechanisms in 71 patients with COVID‐19. mSphere. 2020;5(4). 10.1128/msphere.00362-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu X, Wang Y, Chen T, et al. Classification of COVID‐19 in intensive care patients. Critical Care. 2020;24(1). 10.1186/s13054-020-03127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim CH, Park JT, Kim EJ, et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit Care. 2013;17(6):R282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang C, Zhang H, Cao X, et al. Red cell distribution width (RDW): a prognostic indicator of severe COVID‐19. Ann Transl Med. 2020;8(19):1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hornick A, Tashtish N, Osnard M, et al. Anisocytosis is associated with short‐term mortality in COVID‐19 and may reflect proinflammatory signature in uninfected ambulatory adults. Pathog Immun. 2020;5(1):312‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


