Abstract
The COVID‐19 pandemic has resulted in the cancellation of many elective surgical procedures. This has led to reports of an increase in mortality for patients with non‐Covid health conditions due to delayed definitive management. Patients with severe aortic stenosis have a high annual mortality if left untreated. These patients are at risk due to the reduced number of surgical aortic valve replacements and competition for intensive care facilities during the COVID‐19 pandemic. This case series suggests that the minimally invasive transcatheter aortic valve implantation is safe to continue during the COVID‐19 pandemic with adjustments to the patient pathway to minimize hospital stay and to reduce patient and staff exposure. This helps to reduce the delay of definitive treatment for patients with severe aortic stenosis.
Keywords: aortic repair endovascular, aortic valve disease, percutaneous repair
1. INTRODUCTION
Symptomatic severe aortic stenosis (AS) managed medically carries an annual mortality of approximately 50%. 1 TAVI has become a well‐established treatment for severe aortic stenosis in patients with a high surgical risk 2 , 3 and is increasingly considered across a spectrum of more moderate surgical risks. 4
The current pandemic of the highly infectious coronavirus COVID‐19 has posed significant challenges to health care systems internationally. COVID‐19 is associated with a high incidence of primary respiratory failure requiring invasive mechanical ventilation, putting increased pressure on intensive care units (ITU). 5 This has led to the cancellation of a huge number of elective operations. 6 In particular, most cardiac centres have had to suspend or significantly reduce their elective cardiothoracic surgery services including surgical AVR. 5 However, a significant proportion of the increased population mortality during the COVID‐19 pandemic is not directly attributable to the virus itself. Delayed presentation and deferred definitive management of non‐Covid health conditions are likely to account for much of this excess mortality. 6 , 7
Due to the dire prognosis of untreated severe stenosis, our cardiac centre made the decision to continue TAVI procedures throughout the COVID‐19 pandemic to date but with adjustments to the process aimed at rapid patient discharge, minimizing hospital stay, reducing potential staff and patient exposure and avoiding use of ITU and theater (including hybrid) facilities (Figure 1). The following changes were made:
A virtual (video conferenced) TAVI multidisciplinary team was maintained to review all cases, including surgical cases referred because of clinical deterioration
Pre‐TAVI investigations focusing on annular dimensions and orientation, femoral access and with selection of TAVI prosthesis (chosen from four designs (Edwards group, Evolut family and Boston Scientific family (Lotus Edge and Accurate Neo) according to anatomical criteria)
Patients were pre‐counseled remotely to minimize direct contact required during procedural consent
Once testing available, patients swab‐PCR tested for COVID‐19 2 weeks prior to TAVI admission and asked to isolate until their procedure
Patients admitted on the day of their procedure with minimization of pre‐procedural contact and appropriate patient and staff personal protective equipment (PPE)
All TAVI procedures conducted under local anesthetic without transoesophageal echocardiography and via a transfemoral route with percutaneous arterial closure devices (Proglide®; Abbott Vascular inc.) with in‐lab removal of sheathes. Mild sedation was only required occasionally and achieved with low dose IV opiates or low dose IV benzodiazepines. This was administered under the direction of the TAVI operator. An anaesthesiologist was not present during the procedure.
Procedures performed in cardiac catheter laboratory as hybrid theater reassigned as COVID‐19 ventilation/ICU area
Aim for immediate permanent pacing where signs of higher degree AV‐block at the end of the TAVI procedure –continuous ECG monitoring maintained on transfer to ward care
Aim for rapid mobilization, next‐day discharge and early remote follow‐up
FIGURE 1.
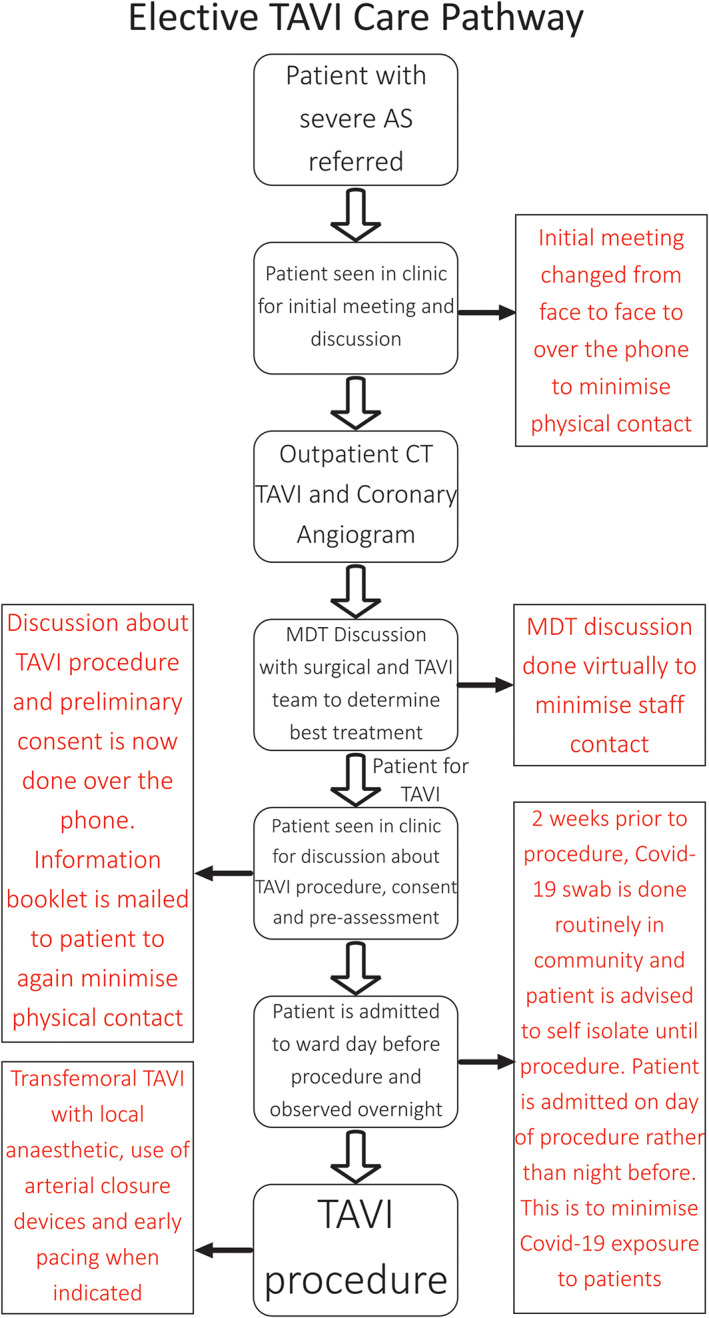
TAVI care pathway with changes made during the COVID‐19 pandemic in red
We report a series of 40 patients that underwent a TAVI procedure during a 12 week period of the COVID‐19 pandemic with the adaptions made in the standard treatment pathways for severe symptomatic aortic stenosis from a single tertiary cardiac/cardiothoracic centre. We review their procedure outcomes and complications and compare them with similar data from the 12 week period immediately before the COVID‐19 pandemic. Data collection was institutionally approved and registered (Ref No. 10589).
2. CASE SERIES
From the start of the COVID‐19 special measures, 40 TAVI procedures were performed over 12 weeks. During this period, 7 balloon aortic valvuloplasty (BAV) procedures and 15 isolated surgical AVRs were undertaken. This compares with the 12 weeks prior to lockdown when 40 TAVI procedures, 16 BAV procedures and 48 isolated AVRs were performed (Table 1).
TABLE 1.
Number of aortic valve procedures 12 weeks before COVID‐19 lockdown and during COVID‐19 lockdown
| Aortic valve procedures | Pre COVID‐19 | COVID‐19 |
|---|---|---|
| Balloon aortic valvuloplasty | 16 | 7 |
| Transcutaneous aortic valve implantation | 40 | 40 |
| Isolated surgical aortic valve repair | 48 | 15 |
Note: Indications for Isolated BAV procedures: 1. Emergency bridge to TAVI—done if there are capacity issues; 2. To assess symptomatic relief in patients with multiple comorbidities (e.g., advanced COPD or severe left ventricular systolic dysfunction; 3. For palliation.
Patient characteristics and outcomes including procedural mortality and complications are shown in Table 2. Patient baseline characteristics were similar in both groups. The median Euroscore II for the COVID‐19 group was 3.03% compared to 4.05% in the pre COVID‐19 group but was not a significant difference. Three (7.5%) patients in the COVID‐19 group required a valve‐in‐valve procedure compared to no patients in the pre COVID‐19 group. An increase in Edwards Sapien 3 device implantation was observed. Devices were selected based on patient's anatomy and access. Where valves were felt equivalent, this valve design was generally selected during the COVID‐19 pandemic due to shortened procedural times and lower anticipated requirement for pacing.
TABLE 2.
Comparison of baseline patient characteristics, procedural characteristics and outcomes for transcutaneous aortic valve implantation between 12 week period leading up to COVID‐19 lockdown and 12 week period during COVID‐19 lockdown
| Baseline patient characteristics | Pre COVID‐19 | COVID‐19 |
|---|---|---|
| Number of patients | 40 | 40 |
| Age | 85 ± 6.75 | 80 ± 9.5 |
| Male gender | 42.5% (17) | 55% (22) |
| Chronic respiratory disease | 25% (10) | 22.5% (9) |
| Previous PCI | 15% 6 | 12.5% 5 |
| Previous CABG | 7.5% 3 | 7.5% 3 |
| eGFR <45 ml/min | 20% (8) | 12.5% 5 |
| NHYA III or IV | 85% (34) | 87.5% (35) |
| Euroscore II % | 4.05 ± 3.16 | 3.03 ± 2.87 |
| Severely impaired LVEF (≤30%) | 7.5% 3 | 7.5% 3 |
| TTE AVA (cm2) | 0.7 ± 0.23 | 0.75 ± 0.3 a |
| AV mean gradient (mmHg) | 35 ± 16.5 | 34.5 ± 20.28 |
| AV max gradient (mmHg) | 62 ± 23.25 | 62 ± 31.2 |
| Previous SAVR | 0% (0) | 7.5% 3 |
| Procedural characteristics | ||
| Elective | 95% (38) | 92.5% (37) |
| Urgent inpatient | 5% 2 | 7.5% 3 |
| Native valve | 100% (40) | 92.5% (37) |
| Valve in valve | 0% (0) | 7.5% 3 |
| Moderate or worse AR after deployment (Angio or Echo) | 7.5% 3 | 2.5% 1 |
| Edwards Sapien 3 | 32.5% (13) | 45% (18) |
| Medtronic Evolut | 35% (14) | 37.5% (15) |
| Lotus edge | 15% 6 | 12.5% 5 |
| Accurate neo | 10% 4 | 5% 2 |
| Abbott portico | 7.5% 3 | 0% (0) |
| Patient outcomes | ||
| Length of stay (days) | 2 ± 2 | 2 ± 1 |
| Inhospital mortality | 5% 2 | 0% (0) |
| Stroke | 0% (0) | 0% (0) |
| Bleeding requiring transfusion | 10% 4 | 0% (0) |
| Periprocedural myocardial infarction | 0% (0) | 2.5% 1 |
| Major vascular complications | 2.5% 1 | 2.5% 1 |
| Pacemaker implantation | 15% 6 | 15% 6 |
| 30‐day readmission | 12.5% 5 | 12.5% 5 |
| 30‐day mortality | 5% 2 | 0% (0) |
| Discharged home | 95% (38) | 100% (40) |
| COVID‐19 swab positive | N/A | 2.5% 1 b |
Five patient's AVA unable to be measured due to technical limitations.
Positive COVID‐19 swab on admission.
There were no procedural deaths in both groups of patients. Complication rates were similar in both groups, with both having 6 (15%) patients requiring pacemaker implantations following their procedure and both having 1 (2.5%) patient with a major vascular complication. 4 (10%) patients required blood transfusions in the pre COVID‐19 group compared to none in the COVID‐19 group. One patient in the COVID‐19 group suffered from a periprocedural myocardial infarction whilst none did in the pre COVID‐19 group. No patients in the COVID‐19 group required blood transfusions due to post procedural bleeding compared to 4 (10%) who did in the pre COVID‐19 group. Only 1 (2.5%) patient had moderate or worse Aortic Regurgitation (AR) after deployment of the new valve in the COVID‐19 group compared to 3 (7.5%) in the pre COVID‐19 group.
The length of stay in hospital is shown in Figure 2, more patients were discharged on day 1 or 2 in the COVID‐19 group. All patients were discharged home. There were no patients with mortality at 30‐days in the COVID‐19 group, however there were two patients with a 30‐day mortality in the pre COVID‐19 group. The first patient had an annular rupture during his TAVI procedure leading to cardiac tamponade. Although pericardiocentesis was successful, he continued to deteriorate and was started on palliative management. The second death was a patient who developed bowel ischaemia 5 days post procedure. After discharge there were 5 (12.5%) patients in both groups that required a hospital readmission after discharge. In the COVID‐19 group, 1 patient was readmitted for vascular complications, 1 for decompensated heart failure, 2 with chest infections and 1 for musculoskeletal chest pain. All repeat COVID‐19 swabs were negative. In the pre COVID‐19 group, 1 patient was readmitted for decompensated heart failure, 2 for transient limb weakness felt due to transient ischaemic attacks (TIAs), 1 for delirium due to pneumonia and 1 for shortness of breath.
FIGURE 2.
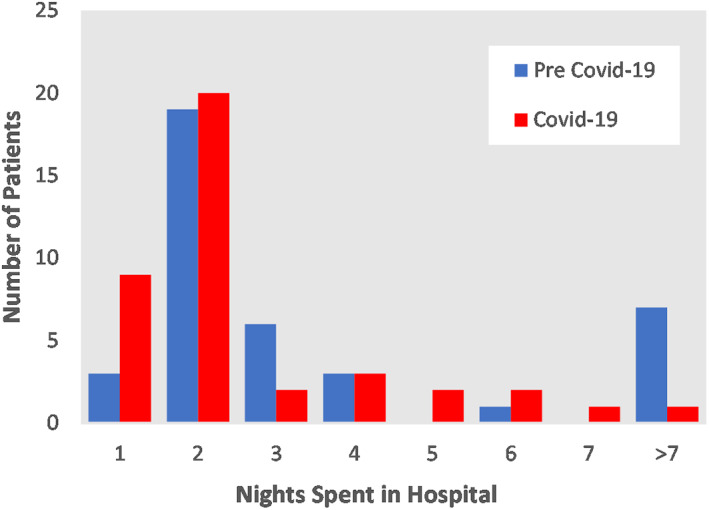
Distribution graph comparing length of stay in hospital between pre COVID‐19 group and COVID‐19 group
In the COVID‐19 group, all patients were remotely followed up with a median follow up of 43 days. No patients reported a COVID‐19 diagnosis or symptoms suggestive of COVID‐19 during follow up. One patient tested positive for COVID‐19 but was positive before his procedure, all other patients tested negative for COVID‐19.
3. DISCUSSION
During the first UK national COVID‐19 lockdown, like many tertiary centres, service pressures led to a 69% reduction in surgical AVR. However, with adjustments to minimize the risk of COVID‐19 infection for patients and staff, we have been able to maintain a similar throughput of TAVI procedures during this period, maintaining excellent results with rapid discharge. In parallel, virtual MDT monitoring of all patients awaiting surgical AVR or TAVI has continued, to facilitate transfer of more moderate risk surgical patients to TAVI later in the pandemic if this becomes necessary.
With identical vascular complication rates, pacemaker implantation rates, 30‐day readmission rates and similar 30 day mortality in the COVID‐19 cohort compared to the pre COVID‐19 cohort, these initial data suggest that the practice changes necessitated by the COVID‐19 pandemic have been adopted safely and lead to similar complication rates. This highlights the fact that modification of procedural flows, teamwork and clear guidelines can allow a continuation of a safe and effective TAVI practice despite the current pandemic. There is some potential for selection bias with lower risk patients potentially selected for procedures during the pandemic, although the patient's baseline characteristics before and after lockdown are similar. During the initial period of the lockdown, patients in the pre‐existing list were prioritized based on clinical need. A small proportion of patients chose to reject or postpone an offered date for TAVI due to concern about the in‐hospital COVID‐19 infection risk. Importantly, only one patient out of the 40 tested positive for COVID‐19 and this patient was known to be positive prior to the procedure, which was conducted after detailed MDT discussion to aid recovery.
None of the patients that underwent a TAVI procedure during COVID‐19 required ICU admission. Adoption of a fully local anesthetic, transfemoral, catheter laboratory‐based practice with mandatory arterial device closure and early pacing (where indicated) were key to avoiding an ITU requirement and ensuring rapid discharge.
4. CONCLUSION
The current COVID‐19 pandemic is a defining event that will shape the healthcare system worldwide for some time to come. With such a heavy focus on COVID‐19, it is paramount not to forget the numerous “excess deaths” not directly attributable to COVID‐19, inadvertently caused by delayed presentation and diagnosis of patients and the increased burden on healthcare systems.
This case series is suggestive that with the proper precautions, cardiac centres can continue to perform TAVI safely with good outcomes and minimal burden on stretched inpatient and ITU services.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Adlam D, Chan N, Baron J, Kovac J. Aortic stenosis in the time of COVID‐19: Development and outcomes of a rapid turnaround TAVI service. Catheter Cardiovasc Interv. 2021;98:E478–E482. 10.1002/ccd.29550
Contributor Information
David Adlam, Email: da134@le.ac.uk.
Jan Kovac, Email: jankovac2@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kapadia SR, Leon MB, Makkar RR, et al. 5‐year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485‐2491. [DOI] [PubMed] [Google Scholar]
- 2. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364(23):2187‐2198. [DOI] [PubMed] [Google Scholar]
- 3. Reardon MJ, Adams DH, Kleiman NS, et al. 2‐Year outcomes in patients undergoing surgical or self‐expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66(2):113‐121. [DOI] [PubMed] [Google Scholar]
- 4. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695‐1705. [DOI] [PubMed] [Google Scholar]
- 5. Haft JW, Atluri P, Alawadi G, et al. Adult cardiac surgery during the COVID‐19 pandemic: a tiered patient triage guidance statement. Ann Thorac Surg. 2020;110:697‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Appleby J. What is happening to non‐Covid deaths? BMJ. 2020;369:m1607. [DOI] [PubMed] [Google Scholar]
- 7. Office of National Statistics . Analysis of death registrations not involving coronavirus (COVID‐19), England and Wales: December 28, 2019 to May 1, 2020; 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/analysisofdeathregistrationsnotinvolvingcoronaviruscovid19englandandwales28december2019to1may2020/technicalannex. Accessed 13th July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


