Abstract
Objective
Recent cohort studies have identified obesity as a risk factor for poor outcomes in coronavirus disease 2019 (COVID‐19). To further explore the relationship between obesity and critical illness in COVID‐19, the association of BMI with baseline demographic and intensive care unit (ICU) parameters, laboratory values, and outcomes in a critically ill patient cohort was examined.
Methods
In this retrospective study, the first 277 consecutive patients admitted to Massachusetts General Hospital ICUs with laboratory‐confirmed COVID‐19 were examined. BMI class, initial ICU laboratory values, physiologic characteristics including gas exchange and ventilatory mechanics, and ICU interventions as clinically available were measured. Mortality, length of ICU admission, and duration of mechanical ventilation were also measured.
Results
There was no difference found in respiratory system compliance or oxygenation between patients with and without obesity. Patients without obesity had higher initial ferritin and D‐dimer levels than patients with obesity. Standard acute respiratory distress syndrome management, including prone ventilation, was equally distributed between BMI groups. There was no difference found in outcomes between BMI groups, including 30‐ and 60‐day mortality and duration of mechanical ventilation.
Conclusions
In this cohort of critically ill patients with COVID‐19, obesity was not associated with meaningful differences in respiratory physiology, inflammatory profile, or clinical outcomes.
Study Importance.
What is already known?
-
►
Obesity has been associated with altered pulmonary mechanics and a heightened inflammatory milieu, yet patients with obesity have similar or improved outcomes in critical illness compared with patients without obesity.
-
►
The Centers for Disease Control and Prevention has identified obesity as a risk factor for the development of critical illness in COVID-19.
What does this study add?
-
►
Respiratory physiology, including respiratory system compliance and oxygenation, was similar between patients with and without obesity.
-
►
Patients with obesity did not have elevated inflammatory markers compared with their counterparts without obesity, and they tolerated standard acute respiratory distress syndrome therapies.
-
►
In this critically ill cohort, there were no differences in 30- or 60-day mortality, duration of mechanical ventilation and ICU admission, or need for tracheostomy between patients with or without obesity.
How might these results change the direction of research or the focus of clinical practice?
-
►
This study supports the continued application of standard ICU therapies including consideration of prone ventilation in acute respiratory distress syndrome patients with and without obesity. More research is needed in larger cohorts to determine whether outcomes are different for patients with obesity once they are critically ill.
Introduction
In the United States, from 1999 to 2018, the prevalence of obesity (conventionally defined as a BMI of 30 kg/m2 or greater) rose from 30.5% to 42.4%, and the prevalence of class 3 obesity (BMI 40 kg/m2 or greater) rose from 4.7% to 9.2% (1). Obesity is associated with an increased incidence of chronic medical conditions including heart disease, stroke, and type 2 diabetes (1), but the relationship between obesity and critical illness remains complex (2). Although obesity is often considered a hyperinflammatory state, investigators have observed improved outcomes among critically ill patients with obesity when compared with patients without obesity, an observation termed the “obesity paradox” (3). The effect of body mass on clinical features and outcomes in coronavirus disease 2019 (COVID‐19) also remains unclear. The Centers for Disease Control and Prevention has listed class 3 obesity (BMI > 40) as a risk factor for severe illness from COVID‐19 (4) based on observational studies. Early reports demonstrated that obesity is associated with higher rates of intensive care unit (ICU) admission and need for mechanical ventilation (5, 6, 7), as well as overall increased mortality, in COVID‐19 (5, 7, 8). Although obesity may be a risk factor for developing COVID‐19 critical illness, once in the ICU, the effects of obesity on pulmonary pathophysiology and clinical outcomes remain unknown.
In this single‐center study, we examined the characteristics of patients with and without obesity admitted to the ICU with COVID‐19 critical illness. We compared baseline characteristics, ICU interventions, respiratory parameters including gas exchange and mechanics, laboratory markers of inflammation, and clinical outcomes between BMI groups.
Methods
Participants
In this retrospective, observational cohort study, we examined the first 311 consecutive adult patients admitted to an ICU at Massachusetts General Hospital in Boston, Massachusetts from March 14, 2020, to May 3, 2020. The Massachusetts General Brigham Institutional Review Board provided ethical approval for this study. We included patients aged 18 years or older with laboratory‐confirmed severe acute respiratory syndrome coronavirus 2 infection within the 14 days prior to ICU admission. We excluded patients who were transitioned to comfort‐focused care shortly after hospital admission (n = 11). Patients transferred from an external hospital ICU (n = 23) were also excluded, leaving a cohort of 277 patients. We divided patients into four groups according to BMI class (patients without obesity, BMI ≤ 29.9 kg/m2; patients with class 1 obesity, 30 to 34.9 kg/m2; class 2 obesity, 35 to 39.9 kg/m2; class 3 obesity ≥ 40 kg/m2).
Registry data
We collected demographics, past medical history, presenting symptoms, laboratory values, and clinical variables from the electronic medical record. Detailed ICU parameters were collected for the first 6 days of ICU admission. Initial laboratory values were collected within the first 72 hours of ICU admission, based on the first available value. Fluid balance assessment through central venous pressure (CVP) and total body fluid balance were collected as clinically available. ICU characteristics were collected for patients managed with mechanical ventilation (n = 249; 89.9%), including ventilatory support settings, gas exchange, and respiratory mechanics. A ratio of arterial oxygen partial pressure (PaO2) to fraction of inspired oxygen (FiO2) was calculated for endotracheally intubated patients, as a measure of hypoxemia (9). Data on targeted maneuvers for treatment of acute respiratory distress syndrome (ARDS) (prone ventilation, paralysis, inhaled nitric oxide, veno‐venous extracorporeal membrane oxygenation [ECMO]) as well as incidence of renal replacement therapy and shock (defined as any vasopressor requirement) were also collected on patients managed with mechanical ventilation. Compliance of the respiratory system was calculated based on an inspiratory breath hold maneuver using the following formula: compliance = change in lung volume(VT)/(plateau pressure − positive end‐expiratory pressure [PEEP]). Clinical management occurred at the discretion of the treating physician. Hospital treatment guidelines recommended ventilation with tidal volumes less than 6 mL/kg predicted body weight, early consideration of prone ventilation for PaO2:FiO2 < 200, and conservative fluid management. As has previously been described (10, 11), a seven‐category ordinal scale was recorded at 30 days from ICU admission: dead, hospitalized on invasive mechanical ventilation or ECMO, hospitalized on noninvasive ventilation or high‐flow nasal cannula, hospitalized on supplemental oxygen, hospitalized not on supplemental oxygen, not hospitalized with limitation in activity, or not hospitalized without limitation in activity. Other outcomes recorded at 30 and 60 days included extubation status, need for reintubation, tracheostomy placement, number of days requiring ventilatory support, number of ICU days, and mortality. Reintubation for mechanical circuit dysfunction (e.g., kinked endotracheal tube, balloon rupture) were excluded. ICU admission days were recorded during the initial stay of any given admission.
Statistical analysis
Continuous variables, reported as means with standard deviation or as medians with interquartile range (IQR) for nonnormal distributions, were compared between groups with t tests or nonparametric tests, as appropriate. Categorical variables, reported as counts and percentages, were compared between groups with χ2 tests or Fisher exact tests, as appropriate. Survival rates between BMI groups were compared using Kaplan‐Meier estimates and the log‐rank test for equality of survival curves. A two‐sided P < 0.05 was considered statistically significant. All analyses were performed using Python 3.0 (Python Software Foundation, www.python.org) and GraphPad Prism version 9.0 (GraphPad Software, San Diego, California).
Results
Patients
We studied 277 patients admitted to the ICU with COVID‐19 (Table 1). There were 139 patients without obesity (BMI ≤ 29.9), 77 patients with class 1 obesity (BMI 30‐34.9), 32 patients with class 2 obesity (BMI 35‐39.9), and 29 patients with class 3 obesity (BMI ≥ 40). Median overall age was 60 years; patients without obesity were older than patients with obesity (median age 66 [IQR 54‐76] vs. 56 [IQR 46‐66], P < 0.05). There was a high prevalence of diabetes and hypertension in both groups. There was a higher prevalence of preexisting heart failure in patients without obesity compared with patients with obesity (13% vs. 9%, P < 0.05). The groups did not otherwise vary significantly with regard to sex, ethnicity, race, or smoking history.
TABLE 1.
Patient demographics and baseline comorbidities by BMI class
| Characteristic | Overall (N = 277) | Without obesity, BMI ≤ 29.9 (n = 139) | Class 1, BMI 30‐34.9 (n = 77) | Class 2, BMI 35‐39.9 (n = 32) | Class 3, BMI ≥ 40 (n = 29) | All with obesity, BMI ≥ 30 (n = 138) | P (“Without obesity” to “All with obesity”) |
|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 60 (49‐72) | 66 (54‐76) | 58 (48‐68) | 53 (41‐60) | 58 (45‐68) | 56 (46‐66) | <0.05 |
| Male | 175 (63) | 93 (67) | 48 (62) | 20 (63) | 14 (48) | 0.12 | |
| Ethnicity | |||||||
| Hispanic or Latino | 111 (40) | 48 (35) | 42 (55) | 10 (31) | 11 (38) | 63 (46) | |
| Non‐Hispanic or Latino | 123 (44) | 69(50) | 29 (38) | 12 (38) | 13 (45) | 54 (39) | 0.012 |
| Unknown | 43 (16) | 22 (16) | 6 (8) | 10 (31) | 5 (17) | 21 (15) | |
| Race | |||||||
| Black or African American | 32 (12) | 22 (16) | 4 (5) | 2 (6) | 4 (14) | 10 (7) | |
| American Indian or Alaska Native | 1 (0.4) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (<1) | |
| Asian | 10 (4) | 4 (3) | 4 (5) | 2 (6) | 0 (0) | 6 (4) | 0.19 |
| Native Hawaiian or other Pacific Islander | 2 (0.7) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 1 (<1) | |
| White or Caucasian | 93 (34) | 51 (37) | 23 (30) | 11 (34) | 8 (28) | 42 (30) | |
| Other | 139 (50) | 61 (44) | 45 (58) | 16 (50) | 17 (59) | 78 (57) | |
| Comorbidities | |||||||
| CAD | 27 (10) | 13 (9) | 11 (14) | 2 (6) | 1 (3) | 14 (10) | 0.37 |
| HF | 30 (11) | 18 (13) | 3 (4) | 2 (6) | 7 (24) | 12 (9) | <0.05 |
| HTN | 135 (49) | 69 (50) | 42 (55) | 17 (53) | 14 (48) | 73 (53) | 0.67 |
| DM | 111 (40) | 53 (38) | 32 (42) | 13 (41) | 13 (45) | 58 (42) | 0.59 |
| CKD | 40 (14) | 24 (17) | 9 (12) | 2 (6) | 5 (17) | 16 (12) | 0.37 |
| Pulmonary disease | 53 (19) | 21 (15) | 18 (23) | 4 (13) | 10 (34) | 32 (23) | 0.05 |
| Immunocompromise | 19 (7) | 12 (9) | 5 (6) | 0 (0) | 2 (7) | 7 (5) | 0.41 |
| Malignancy | 33 (12) | 17 (12) | 9 (12) | 2 (6) | 5 (17) | 16 (12) | 0.62 |
| Ever smoker (former and current) | 111 (40) | 64 (46) | 23 (30) | 11 (34) | 13 (45) | 47 (34) | 0.05 |
Data given as n (%) unless otherwise specified. CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; IQR, interquartile range.
ICU admission characteristics
The modified Sequential Organ Failure Assessment score on ICU admission was not different between patients without obesity versus with obesity (median 6 [IQR 4‐8] vs. median 6 [IQR 4‐8], P = 0.34) (Supporting Information Table S1). On the initial day of ICU admission, patients without obesity versus patients with obesity had no difference in PaO2:FiO2 ratio (median 208.0 [IQR 135.0‐266.0] vs. 183.3 [136.6‐243.8], P = 0.16), driving pressure (median 10.5 cmH2O [IQR 9.0‐12.0] vs. 11.0 cmH2O [9.0‐13.8], P = 0.07), or static compliance of the respiratory system (36.0 cmH2O [IQR 29.0‐44.0] vs. 33.0 cmH2O [17.0‐41.0]; P = 0.06) (Figure 1, Supporting Information Table S2). Plateau pressures were lower in patients without obesity compared with patients with obesity (20.0 cmH2O [IQR 18.0‐22.0] vs. 22.5 cmH2O [IQR 20.3‐25.0], P < 0.05) on the day of ICU admission. Initial applied PEEP was also lower in patients without obesity compared with patients with obesity (10.0 cmH2O [IQR 8.0‐12.0] vs. 10.0 cmH2O [IQR 10.0‐12.0], P < 0.05). Although the median PEEP was 10.0 cmH2O in both groups, the rank‐sum was lower in patients without obesity, and this difference was confirmed using the Chernoff‐Savage statistic (12). Initial CVP measurements were lower in patients without obesity compared with patients with obesity (median 7.0 cmH2O [IQR 4.0‐10.0] vs. 9 cmH2O [IQR 7.0‐12.0]; P = 0.04) (Supporting Information Table S3). The ICU Day 1 total body balance was not different between patients without and with obesity (median 0.42 L [IQR −0.18 to 1.31] vs. 0.28 L [IQR −0.26 to 0.91], P = 0.08) (Supporting Information Table S2). COVID ordinal scale calculated on day 6 of ICU admission, the last day of detailed physiologic recording for ICU patients, was not different between patients without and with obesity (median 5 [IQR 1‐6] vs. 5 [IQR 2‐6], P = 0.62) (Supporting Information Table S4).
Figure 1.
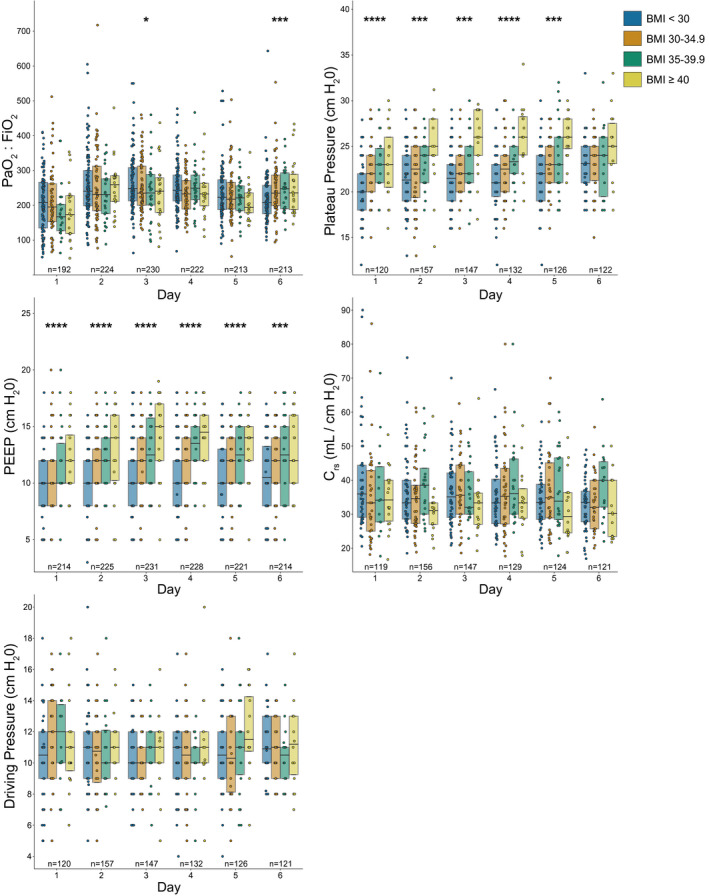
Respiratory parameters for the first 6 days of ICU admission by BMI class. Circles indicate individual patient values. N indicates the number of patients with an observation on each ICU day. Box plots show the 25th, 50th (median), and 75th percentiles. The Mann‐Whitney U test was used to compare groups without obesity (BMI < 30) and groups with obesity (BMI ≥ 30); *P < 0.05, ***P < 0.005, ****P < 0.0005. Crs, compliance of the respiratory system; FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen; PEEP, positive end‐expiratory pressure.
Laboratory studies
Using available serum laboratory values during the first 72 hours of ICU admission (Table 2), ferritin values were higher in patients without obesity compared with patients with obesity (1,012 ug/L [IQR 568‐1,803] vs. 788 ug/L [IQR 405‐1,390]; P ≤ 0.05). D‐dimer was higher in patients without obesity compared with patients with obesity (1,329 ng/mL [IQR 858‐2,182] vs. 1,205 ng/mL [IQR 802‐1,920]; P = 0.05). Other inflammatory biomarkers including lactate dehydrogenase, erythrocyte sedimentation rate, C‐reactive protein, and interleukin‐6 (IL‐6) were not different between groups. Serum bicarbonate, procalcitonin, white blood cell count, and natriuretic peptide test were also not different between groups. We also examined clinically available lab values on day 6 with no significant differences (Supporting Information Table S5).
TABLE 2.
Laboratory values obtained within 72 hours of ICU admission by BMI class median (IQR)
| Lab value | Overall (N = 277) | Without obesity, BMI ≤ 29.9 (n = 139) | Class I, BMI 30‐34.9 (n = 77) | Class 2, BMI 35‐39.9 (n = 32) | Class 3, BMI ≥ 40 (n = 29) | All with obesity, BMI ≥ 30 (n = 138) | P (“Without obesity” to “All with obesity”) |
|---|---|---|---|---|---|---|---|
| LDH (U/L) | 428 (337‐544) | 431 (333‐525) | 443 (358‐581) | 396 (337‐501) | 428 (321‐563) | 427 (339‐561) | 0.24 |
| Ferritin (μg/L) | 898 (483‐1495) | 1,012 (568‐1,803) | 941 (490‐1,526) | 784 (453‐1,390) | 582 (348‐794) | 788 (405‐1,390) | <0.05 |
| ESR (mm/h) | 50 (35‐71) | 50 (33‐71) | 49 (35‐68) | 54 (32‐92) | 53 (46‐74) | 50 (35‐70) | 0.37 |
| CRP (mg/L) | 148 (102‐230) | 144 (79‐225) | 154 (102‐246) | 150 (143‐229) | 147 (116‐189) | 150 (117‐230) | 0.08 |
| IL‐6 a (pg/mL) | 69 (35‐149) | 69 (33‐129) | 69 (40‐149) | 71 (38‐214) | 70 (32‐155) | 70 (37‐166) | 0.25 |
| HCO3 (mmol/L) | 23 (20‐25) | 23 (20‐26) | 23 (20‐25) | 24 (21‐26) | 25 (22‐27) | 23 (21‐25) | 0.21 |
| D‐dimer a (ng/mL) | 1,253 (828‐2,048) | 1,329 (858‐2,182) | 1,155 (773‐1,963) | 1,261 (871‐1,652) | 1,201 (834‐2,135) | 1,205 (802‐1,920) | 0.05 |
| Procalcitonin (ng/mL) | 0.32 (0.17‐0.72) | 0.33 (0.18‐0.94) | 0.28 (0.19‐0.63) | 0.40 (0.16‐0.60) | 0.22 (0.11‐0.52) | 0.31 (0.15‐0.60) | 0.17 |
| WBC (K/uL) | 7.51 (5.73‐9.92) | 7.45 (5.54‐10.00) | 7.42 (5.88‐9.49) | 8.65 (6.27‐10.89) | 8.01 (5.92‐9.27) | 7.63 (6.03‐9.81) | 0.33 |
| NT‐proBNP (pg/mL) | 320 (89‐1,404) | 496 (98‐2,421) | 219 (105‐602) | 115 (72‐385) | 286 (72‐1,016) | 219 (86‐585) | 0.04 |
Some data reported as inequalities; these were replaced with the given absolute values (i.e., >10,000 was rewritten as 10,000).
CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; IL‐6, interleukin‐6; IQR, interquartile range; LDH, lactate dehydrogenase; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; WBC, white blood cell count.
ICU interventions
There was no difference in ICU interventions provided during the first 6 days of ICU admission (Table 3) between patients with and without obesity. These included prone ventilation (49% vs. 60%; P = 0.11), paralysis (23% vs. 29%; P = 0.39), inhaled nitric oxide (16% vs. 23%; P = 0.23), renal replacement therapy (7% vs. 12%; P = 0.18), or use of vasopressors (98% vs. 95%; P = 0.28). No patients without obesity were treated with ECMO, whereas 3% of patients with obesity received this therapy (P = 0.12).
TABLE 3.
ICU interventions for the first 6 days of ICU admission by BMI class, by number (%)
| Overall (N = 249) | Without obesity, BMI ≤ 29.9 (n = 120) | Class 1, BMI 30‐34.9 (n = 72) | Class 2, BMI 35‐39.9 (n = 29) | Class 3, BMI ≥ 40 (n = 28) | All with obesity BMI ≥ 30 (n = 129) | P (“Without obesity” to “All with obesity”) | |
|---|---|---|---|---|---|---|---|
| Prone position | 136 (55) | 59 (49) | 41 (57) | 21 (72) | 15 (56) | 77 (60) | 0.11 |
| Paralysis | 65 (26) | 28 (23) | 21 (28) | 9 (31) | 7 (26) | 37 (29) | 0.39 |
| iNO | 48 (19) | 19 (16) | 17 (24) | 9 (31) | 3 (11) | 29 (23) | 0.23 |
| RRT | 24 (10) | 8 (7) | 10 (14) | 4 (14) | 2 (7) | 16 (12) | 0.18 |
| Vasopressors a | 240 (97) | 118 (98) | 68 (94) | 27 (93) | 27 (100) | 122 (95) | 0.28 |
| ECMO | 4 (2) | 0 (0) | 3 (4) | 0 (0) | 1 (4) | 4 (3) | 0.12 |
Requirement for vasoactive pressor infusions to maintain mean arterial pressure > 65 mmHg.
ECMO, extracorporeal membrane oxygenation; iNO, inhaled nitric oxide; RRT, renal replacement therapy.
Outcomes
There was no difference in measured clinical outcomes between patients without and with obesity (Figure 2). There was no difference at 60 days between patients without and with obesity in number of days in the ICU (median 17 days [IQR 7‐23] vs. 17.5 [11‐25.75], P = 0.78), number of days on mechanical ventilation (median 17 days [IQR 9‐24] vs. 17 [13‐26.25], P = 0.70), need for reintubation (13% vs. 10%, P = 0.78), or tracheostomy placement (28% vs. 29%, P = 0.84). Survival among patients without and with obesity was similar at 30 days (72% vs. 80%, P = 0.17) and 60 days (71% vs. 78%, P = 0.23). In an analysis limited to ICU survivors, the number of days in the ICU and number of days requiring mechanical ventilation were not different between groups (Supporting Information Table S6). The median hospital days to death among nonsurvivors were also not different (Supporting Information Table S7). Univariate analyses confirmed the association between age and modified Sequential Organ Failure Assessment score on ICU admission with mortality, consistent with prior studies (13, 14) (Supporting Information Table S8).
Figure 2.
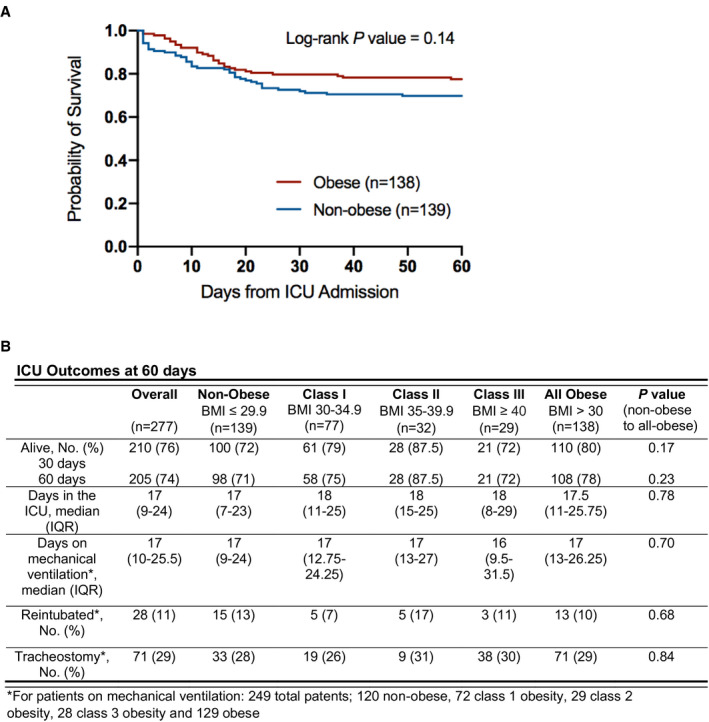
Sixty‐day Kaplan‐Meier survival estimates and ICU outcomes by BMI class. Groups without obesity (BMI < 30) and with obesity (BMI ≥ 30) are compared. There were no missing data. ICU, intensive care unit; IQR, interquartile range.
Discussion
Our observational cohort study of 277 critically ill patients with COVID‐19 found no significant differences in respiratory physiology or elevations in inflammatory markers in critically ill patients with obesity compared with patients without obesity. These findings are notable given the long history of studying the impact of obesity in critical illness and more recent data associating obesity with poor outcomes and critical illness in COVID‐19.
Obesity is a known risk factor for the development of ARDS (15, 16). The increased chest wall and abdominal weight in patients with obesity can lead to decreased lung volumes and decreased compliance of the respiratory system. Patients with obesity are more likely to have regional atelectasis, which results in lower lung volumes and lower measured respiratory system compliance, as well as hypoxemia due to ventilation‐perfusion mismatch or shunt. Patients with obesity also have an increased risk of aspiration and complicated intubations which can precipitate and worsen lung injury (2, 17). Additionally, although many targeted maneuvers for treatment of ARDS, including prone ventilation and neuromuscular blockade, are feasible in patients with obesity, patients with very high BMI have traditionally been excluded from large clinical trials in ARDS (18, 19).
Patients with obesity can also have elevated resting levels of inflammatory markers (20), which may suggest a state of chronic inflammation and oxidative stress. Adipose cells can contribute up to 30% of circulating levels of IL‐6 in the resting state (21); additionally, patients with obesity have elevated levels of tumor necrosis factor, IL‐8 (20), endothelin‐1 (22), and von Willebrand’s factor (23). Given this resting inflammatory milieu, some authors have proposed that patients with obesity are at increased risk for acute lung injury in the setting of respiratory insults (24).
Despite the heightened inflammation and potential management challenges associated with obesity, some studies in ICUs have demonstrated better outcomes for critically ill patients with obesity; this finding has been termed the “obesity paradox” (3). Early retrospective studies examining the relationship between obesity and critical illness outcomes generally found patients with obesity to have an increased risk of critical illness (25, 26, 27, 28) but similar (29) mortality compared with patients without obesity. More recent publications have had contradictory results. Meta‐analyses (30, 31, 32) and cohort studies (33) examining outcomes in ICU patients with obesity have shown either no effect on mortality or a protective effect. Conversely, a large cohort study of patients with sepsis found increased risk for development of ARDS and duration of mechanical ventilation among patients with obesity (34). These contradictory results raise questions about confounding factors and comparability of background disease states. Moreover, patients with very high BMI are often not included in ARDS trials (18, 19).
Several hypotheses have been proposed to explain the “obesity paradox.” Patients with obesity may be misclassified as having ARDS when instead they may have atelectasis from elevated pleural pressures (2) and heavy chest walls, which can be mistaken for lung infiltrates on imaging. Thus, they may be included in ARDS trials despite having a more benign cause for hypoxemia. Alternatively, patients with obesity may represent a distinct patient population because of underlying medical comorbidities (3). It has also been hypothesized that patients with obesity may receive less fluid resuscitation, leading to less lung injury (35), and that patients with obesity may have higher metabolic reserve to sustain them during critical illness (3). Another interesting hypothesis is that patients with obesity may be preconditioned to inflammation due to higher resting inflammatory state and are thus able to withstand critical illness (36). For example, patients with obesity and respiratory failure have been reported to have lower levels of inflammatory cytokines such as IL‐6 and IL‐8 during periods of respiratory failure (37) and thus may tolerate the critical illness better. Lastly, it has been posited that patients with obesity undergo fewer interventions in the ICU and are therefore protected from iatrogenesis. All of these hypotheses remain largely speculative.
It has been recently reported that obesity is a risk factor for both developing critical illness and mortality in COVID‐19 patients. Cohort studies from multiple countries have found patients with obesity (BMI > 30) are more likely to develop critical illness (5, 6, 38, 39, 40), require mechanical ventilation (5, 6, 39, 40, 41), and have overall higher mortality rates (5, 8, 41). A recent cohort study by Anderson et al. noted higher rates of intubation or death among patients with class 3 obesity, which was primarily observed in patients younger than 65 years (42). This study also did not find differences between patients with and without obesity in their measurement of inflammatory markers including C‐reactive protein, erythrocyte sedimentation rate, troponin, or D‐dimer (42).
Given these prior findings in patients with obesity and COVID‐19, it is notable that in our critically ill cohort, patients with and without obesity had similar severity of illness based on respiratory pathophysiology variables and similar outcomes, including 60‐day mortality and ICU length of stay. Our cohort is distinct from prior studies. First, much of the prior data on obesity in COVID‐19 describes the risk of patients developing severe illness. Our study instead examines outcomes among a select population that is already critically ill. Although obesity appears to be a risk factor for severe illness in COVID‐19, our data suggest that once critically ill, patients with obesity demonstrate similar respiratory physiology and inflammatory profiles as patients without obesity. Second, our patients without obesity were older than the patients with obesity; otherwise, our patients had largely similar baseline characteristics. It is not clear whether this finding represents a relatively older, unhealthy group without obesity or a relatively younger, healthy group with obesity, but this distribution may have placed patients at similar risk for poor outcomes irrespective of BMI. In the cohort study by Anderson et al., the increased mortality observed with class 3 obesity was largely in patients under 65 years old (42). Our cohort without obesity was significantly older, which may have outweighed the effects of obesity. Third, our cohort without obesity had lower CVP values and thus may have experienced fewer adverse effects of volume overload. However, it is also possible that the difference in CVP values between the groups was due primarily to different PEEP and pleural pressures, which can affect CVP.
Another key finding in our study was the similar inflammatory markers between the groups, with the exception of ferritin and D‐dimer, which were higher in patients without obesity. On examination of other inflammatory biomarkers in COVID‐19, some have suggested that IL‐6 (43), IL‐10 (44), and/or serum ferritin (32) were independent discriminators for severe disease. The lack of difference between patients with and without obesity in an already critically ill cohort, and the similar outcomes between the groups, again suggests that weight may not be the discriminatory factory in inflammatory status in critical illness.
There are a number of interventions that have been shown to improve outcomes in ARDS, including prone ventilation, and it is notable that there was no difference in the degree of application of these therapies between BMI classes. This finding supports the feasibility of providing the same standard of care to all patients, irrespective of BMI. The equal distribution of standard therapies for ARDS may, in part, explain the similar outcomes seen between BMI classes.
Patients with obesity did have higher PEEP application and plateau pressures during the first 6 days of ICU admission. This finding is not unexpected, given the respiratory physiology of obesity, with increased chest wall and abdominal weight leading to elevated pleural pressures. Higher PEEP is often necessary to maintain alveolar recruitment, particularly in sedated and recumbent patients. Importantly, PaO2:FiO2 ratio and driving pressure, both of which have been associated with prognosis in ARDS, were not different between the groups. These findings also suggest that the higher PEEP application in patients with obesity did not result in lung overdistention.
To our knowledge, this is the largest cohort to date of critically ill patients with obesity and COVID‐19 for which detailed, serial physiological and laboratory value measurements are available. Additionally, we provide 60‐day outcomes data, which is longer than many other studies of COVID‐19 critical illness. There are several limitations to this study. First, this is a single‐center study and ICU parameters were only measured over the first 6 days of ICU admission. Second, we could not account for outcomes after 60 days. Third, the study did not characterize the distribution of excess weight, which can alter pleural pressures and ventilatory mechanics, or to discern metabolic health outside of weight class (45). Fourth, we could only report clinically available laboratory values. Future studies should include larger numbers of patients, as well as detailed information about metabolic status and ventilator mechanics, including pleural pressures through esophageal manometry, to evaluate the effects of obesity in the COVID‐19 population.
In conclusion, in this cohort of critically ill patients with COVID‐19, obesity was not associated with meaningful differences in respiratory physiology, inflammatory profiles, or clinical outcomes.O
Funding agencies
This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541), and financial contributions from Harvard University and its affiliated academic health care centers. DZ is supported by the NIH with T32HL116275. AM is supported by the NIH with RO1 HL148436. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the NIH.
Disclosure
The authors report no conflicts of interest, including relevant financial interests, activities, relationships, or affiliations. AM reports income from Merck and LivaNova related to medical education and reports that ResMed provided a philanthropic donation to UC San Diego. ANW has received funding from the Olympus Corporation and CRICO Risk Management Foundation for research unrelated to this project.
Supporting information
Table S1‐S8
References
- 1. Centers for Disease Control and Prevention. Adult obesity facts . Updated February 11, 2021.. Accessed August 11, 2020. https://www.cdc.gov/obesity/data/adult.html
- 2. Hibbert K, Rice M, Malholtra A. Obesity and ARDS. Chest 2012;142:785‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Umbrello M, Fumagalli J, Pesenti A, Chiumello D. Pathophysiology and management of acute respiratory distress syndrome in obese patients. Semin Respir Crit Care Med 2019;40:40‐56. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. People at increased risk and other people who need to take extra precautions . Updated March 15, 2021. Accessed August 11, 2020. https://www.CDC.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html
- 5. Frank R, Mendez S, Stevensen E, Guseh J, Chung M, Silverman M. Obesity and the risk of intubation or death in patients with coronavirus disease 2019. Crit Care Med 2020;48:e1097‐e1101. doi: 10.1097/CCM.0000000000004553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rottoli M, Bernante P, Belvedere A, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID‐19 patients? Results from a single Italian centre. Eur J Endocrinol 2020;184:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta S, Hayek S, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020;180:1436‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID‐19: results from an integrated health care organization. Ann Intern Med 2020;173:773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526‐2533. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Fan G, Salam A, et al. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J Infect Dis 2019;221:1688‐1698. [DOI] [PubMed] [Google Scholar]
- 11. International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) . Accessed June 12, 2020. https://isaric.tghn.org/
- 12. Chernoff H, Savage I. Asymptotic normality and efficiency of certain nonparametric test statistics. The Annals of Mathematical Statistics 1958;29:972‐994. [Google Scholar]
- 13. Ferreira F, Bota D, Bross A, Mélot C, Vincent J. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754‐1758. [DOI] [PubMed] [Google Scholar]
- 14. Roch A, Wiramus S, Pauly V, et al. Long‐term outcome in medical patients aged 80 or over following admission to an intensive care unit. Crit Care 2011;15:R36. doi: 10.1186/cc9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax 2010;65:44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anzueto A, Frutos‐Vivar F, Esteban A, et al. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax 2011;66:66‐73. [DOI] [PubMed] [Google Scholar]
- 17. McCallister J, Adkijs E, O’Brien J. Obesity and acute lung injury. Clin Chest Med 2010;30:495‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The National Heart, Lung, and Blood Institute PETAL Clinical Trials Network . Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med 2019;380:1997‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guérin C, Reignier J, Richard J‐C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159‐2168. [DOI] [PubMed] [Google Scholar]
- 20. Tilg H, Moschen A. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity‐associated diseases. Clin Sci 2008;114:275‐288. [DOI] [PubMed] [Google Scholar]
- 21. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115:911‐919. [DOI] [PubMed] [Google Scholar]
- 22. van Harmelen V, Eriksson A, Astrom G, et al. Vascular peptide endothelin‐1 links fat accumulation with alterations of visceral adipocyte lipolysis. Diabetes 2008;57:378‐386. [DOI] [PubMed] [Google Scholar]
- 23. Blann A, Bushell D, Davies A, Faragher E, Miller J, McCollum C. von Willebrand factor, the endothelium and obesity. Int J Obes Relat Metab Disord 1993;17:723‐725. [PubMed] [Google Scholar]
- 24. Shore S. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol 1995;108:735‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El‐Solh A, Sikka P, Bozkanat E, Jaafar W, Davies J. Morbid obesity in the medical ICU. Chest 2001;120:1989‐1998. [DOI] [PubMed] [Google Scholar]
- 26. Yaegashi M, Jean R, Zuriqat M, Noack S, Homel P. Outcome of morbid obesity in the intensive care unit. J Intensive Care Med 2005;20:147‐153. [DOI] [PubMed] [Google Scholar]
- 27. Bercault N, Boulain T, Kuteifan K, Wolf M, Runge I, Fleury J. Obesity‐related excess mortality in an adult intensive care unit: a risk‐adjusted matched cohort study. Crit Care Med 2004;32:998‐1003. [DOI] [PubMed] [Google Scholar]
- 28. Goulenok C, Monchi M, Chiche J, Mira J, Dhainaut J, Cariou A. Influence of overweight on ICU mortality: a prospective study. Chest 2004;125:1441‐1445. [DOI] [PubMed] [Google Scholar]
- 29. Frat J‐P, Gissot V, Ragot S, et al.; Association des Réanimateurs du Centre‐Ouest (ARCO) Study Group . Impact of obesity in mechanically ventilated patients: a prospective study. Intensive Care Med 2008;34:1991‐1998. [DOI] [PubMed] [Google Scholar]
- 30. Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta‐analysis. Obesity (Silver Spring) 2008;16:515‐521. [DOI] [PubMed] [Google Scholar]
- 31. Hogue CW, Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta‐analysis. Intensive Care Med 2009;35:1152‐1170. [DOI] [PubMed] [Google Scholar]
- 32.Pepper DJ, Sun J, Welsh J, Cui X, Suffredini AF, et al. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta‐analysis. Crit Care 2016;20:181. doi: 10.1186/s13054-016-1360-z [DOI] [PMC free article] [PubMed]
- 33. Garrouste‐Orgeas M, Troché G, Azoulay E, et al. Body mass index. An additional prognostic factor in ICU patients. Intensive Care Med 2004;30:438‐442. [DOI] [PubMed] [Google Scholar]
- 34. Rana A, Mansoor K, Abouzid M, et al. Influence of body mass index on the duration of ventilator use and its association with acute respiratory distress syndrome. Am J Respir Crit Care Med 2020;201:A1146. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A1146 [DOI] [Google Scholar]
- 35. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network , Wiedemann H, Wheeler A, Bernard GR, et al. Comparison of two fluid‐management strategies in acute lung injury. N Engl J Med 2006;354:2564‐2575. [DOI] [PubMed] [Google Scholar]
- 36. Fernandez‐Bustamante A, Repine J. Adipose‐lung cell crosstalk in the obesity‐ARDS paradox. J Pulm Respir Med 2013;3:2564‐2575. [Google Scholar]
- 37. Stapleton R, Dixon A, Parsons P, Ware L, Suratt B. NHLBI Acute Respiratory Distress Syndrome Network. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest 2010;138:568‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid‐19 hospital admission. Clin Infec Dis 2020;71:896‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in‐hospital outcomes, and higher in‐hospital mortality, in a cohort of patients with COVID‐19 in the Bronx, New York. Metabolism 2020;108:154262. doi:10.1016/j.metabol.2020.154262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anderson MR, Geleris J, Anderson DR, et al. Body mass index and risk for intubation or death in SARS‐CoV‐2 infection: a retrospective cohort study. Ann Intern Med 2020;173:782‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aziz M, Fatima R, Assaly R. Elevated interleukin‐6 and severe COVID‐19: a meta‐analysis. J Med Virol 2020;92:2283‐2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID‐19 patients reveals IL‐6 and IL‐10 are disease severity predictors. Emerg Microbes Infect 2020;9:1123‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stefan N, Häring H, Schulze M. Metabolically healthy obesity: the low hanging fruit in obesity treatment? Lancet Diabetes Endocrinol 2018;6:249‐258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S8


