The coronavirus disease 2019 (COVID‐19) pandemic continues to spread. As I write this editorial in a quarantine hotel in Beijing on 26 October 2020, more than 38 million people worldwide have been infected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus that causes COVID‐19, and more than 1 million have died from COVID‐19, according to the WHO COVID‐19 dashboard. Before vaccines and antiviral drugs are made available, non‐pharmaceutical interventions such as physical (social) distancing and quarantine have become our only defense against this new coronavirus. Implementing an appropriate level of intervention has been difficult, partly due to our poor understanding of the transmission routes of the virus.
1. VENTILATION AND INFECTION
SARS‐CoV‐2 is not the only virus about which there is significant uncertainty regarding transmission routes. Confusion and doubt have existed for a long time over the role of inhalation in the transmission of other respiratory infections. For example, one review concluded that influenza viruses can be transmitted by inhalation, 1 whereas another concluded the opposite. 2 Significantly, both of these studies examined the same well‐known Alaska plane outbreak, 3 in which 72% of the 54 passengers were infected with influenza and the estimated maximum ventilation rate was 0.4 L/s per passenger. 4 These opposing conclusions illustrate what I would refer to as the aerosol inconsistency phenomenon.
In an earlier editorial that described basic transmission routes for respiratory infection, 5 (aerosol) inhalation transmission was defined as the infection of a susceptible individual via the inhalation of virus‐laden respiratory droplets, that is, aerosols suspended in the air. Aerosol inhalation can occur at short range (when in close contact with an infected person) or at long range (when across a room from an infected individual). In the literature, short‐range inhalation is also referred to as the short‐range airborne route, while long‐range inhalation is known as the airborne route.
In earlier outbreak studies, such as those of the Alaska plane outbreak, 3 data on close contact at time of infection were mostly absent, and thus any conclusions drawn on the role of other routes, such as long‐range inhalation, may not be reliable. Actual ventilation rates of infection venues are also generally not available. However, accurate data on occupancy, human behavior and building ventilation rate at the time of infection are pre‐requisites for determining possible exposure. Riley et al. 6 was probably both the first and the last study of a disease outbreak (measles) in which ventilation rates were measured before this COVID‐19 pandemic, although the ventilation rate is a crucial parameter in the well‐known Wells‐Riley equation. 6 The current pandemic is occurring in the age of artificial intelligence and big data, and in some countries, health authorities have conducted intensive contact‐tracing using trajectory sensors, surveillance videos and facial recognition. In our own study of the outbreak of COVID‐19 in a Guangzhou restaurant, we had access to three surveillance videos of the restaurant at the time of infection (not yet published). These videos clearly revealed that several infected individuals had no close contact with the index patient, and thus, close‐contact transmission could be ruled out.
By using these unprecedented data in combination with knowledge of the physical mechanisms of transmission, I believe that there is a good chance that the long‐standing confusion over aerosol transmission can be resolved. I have been involved in investigations of two COVID‐19 outbreaks (one in a restaurant in Guangzhou and two in buses in Hunan) in which it was found probable that long‐range inhalation transmission had occurred, due to insufficient ventilation. In both investigations, environmental studies of the transmission routes, including ventilation rate measurement by using a tracer gas decay method, were performed in the original infection venues and the spread of exhaled droplets was measured or predicted. A ventilation rate of less than 3 L/s per person, which is lower than the ventilation requirement for acceptable indoor air quality of 5.1 L/s per person in restaurant or dining rooms advocated by professional societies such as American Society of Heating, Refrigerating and Air‐Conditioning Engineers (ASHRAE), was found to lead to infection by long‐range inhalation (unpublished data). Many studies have shown that close‐range transmission of SARS‐CoV‐2 seems to be most common, which probably explains why social distancing has been effective (Jarvis et al., 2020, Dehning et al., 2020). 7 , 8 The mass wearing of face masks has also been shown to be effective (MacIntyre et al., 2020), 9 but it is known that the effectiveness is due to both (incomplete) filtration and jet blockage. That is, mask‐wearing is known to block the expiration jet, thus preventing or minimizing short‐range transmission. There is some evidence that masks are effective at stopping transmission from an infected mask‐wearer. Nevertheless, most SARS‐CoV‐2 infections occur indoors, and in the occasional reports of long‐range transmission, super‐spreading events were found to be involved, such as the aforementioned restaurant and bus outbreaks.
2. INHALATION ROUTE CONTINUUM
A key question is whether long‐range aerosol transmission co‐exists with close‐range transmission. In a recent mechanistic study, the short‐range inhalation route was shown to dominate respiratory‐infection exposure during close contact, and spray transmission 5 (traditionally referred to as large droplet transmission) was only significant at very close contact (<0.5 m) (Chen et al., 2020). 10 This result suggests that short‐range inhalation through close contact may be the dominant mode of transmission of respiratory viruses, such as influenza and SARS‐CoV‐2, rather than spray transmission, as has traditionally been believed.
The next question is how the long‐range and short‐range inhalation routes are linked. A potential link can be easily shown by the following thought experiment, which is also supported by theoretical analysis. The concentration of exhaled droplets in an infected person's exhaled jet continually decreases with distance from the mouth of the infected person, and is sufficiently weakened at a distance of approximately 1.5 m that it merges into the background air of the room. During this concentration decay process, the entrainment of the surrounding air provides dilution. If the surrounding air is clean, then the dilution effect becomes stronger such as in an outdoor setting. The concentration of exhaled droplet nuclei in the air in a room is determined by the source strength and ventilation rate. When the ventilation rate in a room is sufficiently low, the average nuclei concentration throughout the room is as high as that within 1.5 m of an expired air jet. In this situation, the above‐mentioned dilution effect in the short‐range becomes less. Thus, long‐ and short‐range transmission through the inhalation route is a continuum. Building ventilation affects both long‐ and short‐range transmission. We infer that SARS‐CoV‐2 transmission via long‐range inhalation may become possible if the ventilation in a room is insufficient, despite the fact that transmission would not normally occur by long‐range inhalation if the room were reasonably well ventilated. For convenience, we refer this scenario as the extended short‐range inhalation route. Epidemiologists cannot distinguish between spray transmission and short‐range inhalation transmission, and the existence of the extended short‐range inhalation route provides direct evidence of the short‐range inhalation route.
The fact that transmission by long‐range inhalation has been only observed occasionally for influenza viruses and SARS‐CoV‐2 suggests that these respiratory viruses are normally not transmitted by long‐range inhalation, and that their dominant inhalation transmission route is mostly short‐range in nature. However, as mentioned, insufficient ventilation makes long‐range inhalation transmission possible. This deduction explains the co‐existence of limited long‐range inhalation transmission and dominant close‐range transmission. Mechanistic evidence shows that the role of spray transmission may be secondary, except if the virus or pathogen exists mostly in drops larger than 50 μm.
It may be useful to visualize two zones in a room: a zone within close range of the expired jet of the infected person, and a zone comprising the remainder of the room, as shown in Figure 1. Recognizing the existence of these two zones is useful for devising interventions. Wearing a mask would block the expired jet of the infected person and minimize infection risk in the close‐range zone. In theory, a face shield would also produce a similar jet‐blocking effect, but this may not be as effective as a face mask. The concentration of infectious virus aerosols rapidly decays with distance from the mouth of an infected person, as larger drops fall and settle and fine droplets rapidly evaporate. Some viruses may even be deactivated during this process. A 10‐µm droplet fully evaporates and shrink to droplet nuclei in 66 ms in a dry environment (0% relative humidity), while a 50‐µm droplet does it in 1.7 s and a 100‐µm droplet in 6.6 s. 11 Thus, an expired jet with a typical exhalation velocity of 2 m/s would travel for only a few seconds within the 1.5‐m close‐range zone, and the evaporation of droplets smaller than 50 µm would be complete within this zone. Viruses in aerosols that remain suspended within an expired jet due to its relatively high velocity will survive longer in the close‐range zone than beyond it. A 10‐µm droplet has a stopping distance of 2.3 mm and a terminal velocity of 2.96 mm/s, while for a 50‐µm droplet these values are 40 mm and 74 mm/s, respectively, and for a 100‐µm droplet they are 130 mm and 0.25 m/s, respectively. 11 This means that droplets smaller than 50 µm are mostly carried within the airflow in the expired jet, whereas larger droplets, or drops, deviate from their flow path to either settle 1–2 m away, or are deposited on the face.
FIGURE 1.
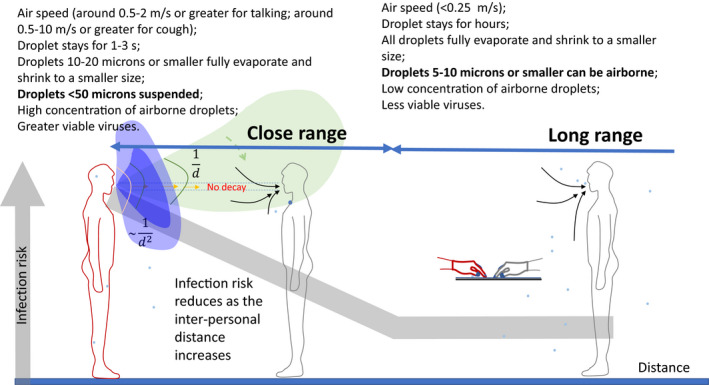
Illustration of the infection risk in the close‐range zone (in light green, representing a breathing case) and that in the remainder of a room (long‐range zone). The infection risk reduces as the inter‐personal distance increases, as shown by the thick gray line. The spread of the expired jet depends on head/body movement. When the jet spread angle is narrow, passive tracer‐gas decay follows the 1/d rule, where d is the distance; when the angle is wide, the 1/d2 rule applies
Obviously, close‐range inhalation carries a much greater risk than long‐range inhalation, and the risk of infection via inhalation is greater within 1–2 m of an infected person.
The possibility of an extended short‐range inhalation SARS‐CoV‐2 infection route has significant implications in the design of interventions for the current COVID‐19 pandemic. It reveals the importance of building ventilation, and of knowing the rate of ventilation that is required in various settings, such as offices, schools, and hotel rooms. Hence, in the absence of any solid evidence, it would be prudent to ensure a minimum indoor‐ventilation rate of, for example, 8.5 L/s per person in office spaces, as currently required by professional societies such as ASHRAE. However, it should be noted that current ventilation standards do not consider respiratory infection control. There is an urgent need to study required minimum ventilation rate for respiratory infection control, 12 which probably differs from that for acceptable indoor air quality. Care needs to be taken on optimizing air distribution, that is, how efficient the air at any point in a room is replaced by outdoor air from the ventilation system, and how efficient the exhaled droplet nuclei at any point in a room is transported and removed. Air distribution matters on both distributing the outdoor air and transporting/removing the exhaled droplet nuclei. In terms of transporting infectious droplet nuclei, a right airflow direction is needed, for example, for minimizing transport of infectious droplet nuclei to the occupied zones in a room.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ina.12806.
ACKNOWLEDGEMENT
This work is supported by a Hong Kong RGC GRF project (no. 17202719).
REFERENCES
- 1. Li Y, Leung GM, Tang JW, et al. Role of ventilation in airborne transmission of infectious agents in the built environment – a multidisciplinary systematic review. Indoor Air. 2007;17:2‐18. [DOI] [PubMed] [Google Scholar]
- 2. Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7(4):257‐265. [DOI] [PubMed] [Google Scholar]
- 3. Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP, Ritter DG. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 4. Rudnick SN, Milton DK. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13:237‐245. [DOI] [PubMed] [Google Scholar]
- 5. Li Y. Basic routes of transmission of respiratory pathogens – a new proposal for transmission categorisation based on respiratory spray, inhalation and touch. (Editorial). Indoor Air. 2021;31(1):3‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riley EC, Murphy G, Riley RL. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 1978;107(5):421‐432. [DOI] [PubMed] [Google Scholar]
- 7. Jarvis CI, Van Zandvoort K, Gimma A, et al. Quantifying the impact of physical distance measures on the transmission of COVID‐19 in the UK. BMC Med. 2020;18:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dehning J, Zierenberg J, Spitzner FP, et al. Inferring change points in the spread of COVID‐19 reveals the effectiveness of interventions. Science. 2020;369(6500):eabb9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacIntyre CR, Chughtai AA, Seale H, Dwyer DE, Quanyi W. Human coronavirus data from four clinical trials of masks and respirators. Int J Infect Dis. 2020;96:631‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen W, Zhang N, Wei J, Yen HL, Li Y. Short‐range airborne route dominates exposure of respiratory infection during close contact. Build Environ. 2020;176:106859. [Google Scholar]
- 11. Wei J, Li Y. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build Environ. 2015;93:86‐96. [Google Scholar]
- 12. Zhu S, Jenkins S, Addo K, et al. Ventilation and laboratory confirmed acute respiratory infection (ARI) rates in college residence halls in College Park, Maryland. Environ Int. 2020;137:105537. [DOI] [PMC free article] [PubMed] [Google Scholar]


