Abstract
In the last few months, there have been numerous reports describing a variety of cutaneous signs associated with COVID‐19. Clinicians from Italy were the first to describe the cutaneous manifestations of COVID‐19, which were later observed in other parts of the globe. In some cases, cutaneous signs were the only manifestation of COVID‐19 rather than the typical syndrome of fever and upper respiratory tract symptoms. However, there is considerable heterogeneity amongst the cutaneous signs described so far, which has been published extensively. Our aim is to summarise the latest studies that have reported the early and late cutaneous signs of COVID‐19 and compare them to the most common established viral exanthems.
Keywords: Coronavirus 19, COVID‐19, exanthem, skin
Introduction
Viruses can cause distinctive exanthems which help the clinician hypothesise a diagnosis even before the results of diagnostic investigations become available. Contrary to the initial belief, severe acute respiratory syndrome caused by a new coronavirus (SARS‐CoV2; also known as COVID‐19) doesn’t have a specific exanthem but can present with various cutaneous manifestations which are important to recognise. Erythema infectiosum, varicella, infectious mononucleosis and measles are some examples of specific viral exanthems which are well established and share some similarities with the cutaneous signs of COVID‐19. Our aims are twofold, firstly to describe the various cutaneous manifestations of COVID‐19 that have been observed so far based on their morphology and time of onset, and secondly, to compare their similarities and differences with other established viral exanthems.
Methods
A comprehensive literature review was conducted via PubMed for the search terms ‘COVID‐19 and skin’; ‘COVID‐19 and dermatology’; ‘coronavirus and skin’ and ‘coronavirus and dermatology’. Additional studies were sourced through a Google search and reference lists of a few recent review articles. A total of 576 articles were carefully screened, and 55 articles were further evaluated for cutaneous signs of COVID‐19. Only patients with a confirmed diagnosis of COVID‐19 using polymerase chain reaction diagnostic assay of nasopharyngeal (NP) swab samples and/or antibody testing were included in the study. Eight studies were further excluded as they used a clinical diagnosis of COVID‐19 without confirmatory laboratory investigations. Information on confirmed cases of COVID‐19 was extracted from the study if it reported both suspected and confirmed cases. Our search included articles in different languages, which had translations available. The exanthems were divided into broad clinical categories of (1) generalised maculopapular or morbilliform eruption (2) varicella‐like or vesicular lesions (3) vascular ischaemic lesions or chilblains (4) acute urticaria and (5) others. We only included established viral exanthems known to be associated with respiratory symptoms in prominent dermatology and virology textbooks for comparison with COVID‐19. 1 , 2 , 3
Results
Literature review identified 406 reported cases of COVID‐19 with cutaneous signs meeting the inclusion criteria (Table 1). The most common type of manifestations (Table 2) are (1) a generalised maculopapular or morbilliform presentation (39.7%), (2) vascular lesions manifesting as acral ischaemic lesions or chilblains (20.2%) (3) varicella‐like lesions (16.5%) and (4) an acute urticarial reaction (16.0%). The acral lesions affected the toes more commonly than fingers and the vesicular and maculopapular lesions tend to be widespread and usually seen on the trunk, face and neck. There is significant heterogeneity in the timing of onset of the exanthems and the respiratory symptoms. Some reports have suggested that the cutaneous manifestation was the only symptom of COVID‐19 in some patients (1.7%). 4 , 5 A histopathological diagnosis was included in 11 (23%) studies.
Table 1.
Summary of the reported cases of the cutaneous manifestations of COVID‐19
| Type of study | Region | Author | COVID‐19 positive patients | Morphology | Location | Age of the patient | Timing of onset in relation to respiratory symptoms | Histological diagnosis |
|---|---|---|---|---|---|---|---|---|
| CS | Italy | Recalcati et al. 6 | 18 | Erythematous lesions (14), widespread urticaria (3), varicella‐like vesicles (1) | Trunk | NR | At the onset of symptoms (8), after hospitalisation (10) | NR |
| CS | Canada | Sachdeva et al. 22 | 3 | Maculopapular lesions resembling Grover disease (1), morbilliform lesions (1), papulovesicular eruption (1) | Trunk (1), trunk and hips (1), trunk and legs (1) | 71, 77, 72 | More than 10 days after symptoms (1), 5 days after symptoms(1), 4 days after symptoms (1) | NR |
| CS | Italy | Marazano et al. 23 | 22 | Varicella‐like papules | Trunk and limbs, no facial or mucosal involvement | 60 (median age) | Median latency period of 3 days after the onset of symptoms | Y |
| CR | Belgium | Kolivaras et al. 24 | 1 | Violaceous, infiltrated plaques on an erythematous background | Dorsal aspect of toes and lateral sides of the feet | 23 | 3 days after onset of symptoms | Y |
| CR | USA | Najarian et al. 25 | 1 | Morbilliform | Legs, thighs, forearms, arms, shoulders, back, chest, abdomen | 58 | 1 day after symptoms | NR |
| CR | Iran | Kamali Aghdam et al. 26 | 1 | Cutaneous mottling | NR | 15 days | 2 days after symptoms | NR |
| CR | France | Henry et al. 27 | 1 | Urticaria | Hands, face and feet | 27 | 2 days before onset of symptoms | NR |
| CS | China | Zhang et al. 28 | 2 | Urticaria | NR | 57 (median age) | NR | NR |
| CR | Spain | Estebanez et al. 29 | 1 | Confluent erythematous‐yellowish papules | Heel | 28 | 14 days after diagnosis | NR |
| CR | France | Mahe et al. 30 | 1 | Erythematous lesions | Antecubital fossa, then to the trunk and axillary folds | 64 | 4 days after symptoms | NR |
| CR | USA | Hunt 31 | 1 | Morbilliform | Trunk and extremities with sparing of the face | 20 | 6 days after symptoms | NR |
| CR | Thailand | Joob et al. 32 | 1 | Erythema with petechiae | NR | NR | NR | NR |
| CS | China | Zhang et al. 21 | 7 | Acro‐ischaemia including finger/toe cyanosis, skin bulla and dry gangrene | Extremities | 59 (median age) | Median latency period of 19 days after onset of symptoms | NR |
| CS | USA | Manalo et al. 33 | 2 |
Transient non‐ pruritic blanching unilateral livedoid patch resembling livedo reticularis (1) Unilateral asymptomatic eruption resembling livedo reticularis (1) |
Lower limbs | 67, 47 | 7 days after symptoms (1), 10 days after diagnosis (1) | NR |
| CS | France | Bouaziz et al. 34 | 14 | Maculopapular eruption (4), chicken pox‐like vesicles (2), urticaria (1), vascular lesions including cherry angiomas (6), livedo (1) | Generalised | NR | Few days after onset of symptoms, except cherry angiomas which occurred 21 days later | NR |
| CS | Belgium | Damme et al. 35 | 2 | Acute urticaria | Generalised | 71, 39 | A day before onset of symptoms (1), concomitantly with symptoms (1) | NR |
| CR | Iran | Ehsani et al. 8 | 1 | Pityriasis rosea | Trunk | 27 | 3 days after onset of symptoms | NR |
| CS | Spain | Fernandez et al. 36 | 24 | Small papules, vesicles and pustules | Disseminated vesicular lesions (18) localised vesicular eruption (6) | 40 (median age) | Median latency of 14 days after symptoms | Y |
| CR | Indonesian | Gunuwan 37 | 1 | Pruritic urticaria | face | 51 | 5 days after symptoms | NR |
| CS | Spain | Miriam Morey‐Olive ´Mar ´ıa et al. 38 | 2 |
Maculopapular lesions (1) Acute urticaria (1) |
Trunk and neck, spreading to palms and hands (1), started on the face then spread to extremities, sparing palms and soles (1) | 6 years and 2 months | 16 days after symptoms (1), at the onset of symptoms (1) | NR |
| CR | Spain | Moreno et al. 39 | 1 | Morbilliform | Generalised spread including folds and scalp, respecting the palmo‐plantar region and mucosa | 32 | 6 days after symptoms | NR |
| CR | Spain | Quintana‐castanedo et al. 5 | 1 | Acute urticaria | Thighs, arms and forearms, sparing palms and soles | 61 | Cutaneous manifestation was the only symptom | NR |
| CS | Spain | Suarez‐valle et al. 40 | 3 | Acro‐ischaemic lesions | Toes only (2) toes and soles (1) | NR | 17, 24, 28 days after symptoms | Y |
| CS | France | Adele de Masson et al. 41 | 7 | Acral ischaemic lesions | Toes | 27 (median age) | NR | Y |
| CR | France | Ahouach et al. 42 | 1 | Diffuse fixed erythematous blanching maculopapular lesions | Limbs and trunk, with burning sensation over the palms | 57 | At the onset of symptoms | NR |
| CR | Kuwait | Alramthan et al. 4 | 2 | Red‐purple papules (1); diffused erythema in the subungual area of the right thumb in the 2nd patient | On the dorsal aspect of fingers bilaterally | 27, 35 | Asymptomatic patients with skin lesions as the chief complaint | NR |
| CR | France | Amatore et al. 13 | 1 | Erythematous and oedematous non‐pruritic annular fixed plaques | Upper limbs, chest, neck, abdomen and palms, sparing the face and mucous membranes | 39 | AT the onset of disease | NR |
| CS | Spain | Andina et al. 10 | 1 | Chilblains | Toes | 12 (median age for the series) | Mean of 16 days after initial symptoms | Y |
| CS | Mexico | Cepeda‐Valdes et al. 43 | 2 | Urticaria | Shoulders, elbows, knees and buttocks | 20, 50 | After respiratory symptoms | NR |
| CS | Spain | Fernandez et al. 44 | 2 | Acral lesions | Distal aspect of toes and fingers | NR | Median latency of 9.2 days for the series | NR |
| CR | Italy | Genovese et al. 45 | 1 | Erythematous papules and few vesicles | Trunk | 8 | 6 days after onset of symptoms | NR |
| CS | USA | Kalner et al. 46 | 2 | Dusky red, non‐pruritic, non‐blanching periorbital dyschromia | Periorbital region | 43, 50 | 2 days prior to the onset of symptoms | NR |
| CR | Italy | Locatelli et al. 11 | 1 | Erythemato‐oedematous, partially eroded macules and plaques | Dorsal aspect of the hand | 16 | 3 days after dysgeusia and mild diarrhoea | Y |
| CR | Turkey | Naziroğlu et al. 47 | 1 | Urticaria | Generalised | 53 | Cutaneous manifestation was the only symptom | NR |
| CS | USA | Rivera‐Oyola et al. 48 | 2 | Erythematous macules coalescing into papules (1) large, disseminated, urticarial plaques (1) | Back, bilateral flanks, groyne, and proximal lower extremities (1), trunk, abdomen, head, and upper and lower extremities (1) | 60 , 60 | 3 days after symptoms (1), 9 days after symptoms (1) | Y |
| CS | Spain | Landa et al. 49 | 2 | Acral vascular lesions | Toes | 91, 24 | Asymptomatic (1), after symptoms (1) | NR |
| CR | Spain | Mayor‐Ibarguren et al. 50 | 1 | Acute leukocytoclastic vasculitis | Lower legs, feet and toes | 84 | 4 weeks after symptoms | Y |
| CR | Italy | Rossi et al. 51 | 1 | Generalised maculopapular lesions | Trunk, limbs, legs, face | 34 | Fever and cutaneous lesions only | NR |
| CS | Spain | Galvan et al. 14 | 234 | Pesudo‐chilblain (29), vesicular (17), urticarial (49), maculopapular (122), livedo/necrosis (17) | Trunk and limbs | Pseudo‐chilblain (median age: 32), vesicular (median age: 45), urticarial median age: 49), maculopapular (median age: 55), livedo/necrosis (median age: 63) | Pseudo‐chilblain (occurred later in the disease), vesicular (occurred during the course of the disease), urticarial and maculopapular lesions (happened at the same time), livedo/necrosis (late sign) | NR |
| CS | 8 countries (USA, UK, Canada, France, Italy, Mexico, The Netherlands and Iran) | Freeman et al. 52 | 23 | Pernio‐like lesions | Foot (20), hand (7) | NR | Before symptoms (4), after symptoms (11), at the onset of symptoms (3), no other symptoms (5) | NR |
| CR | Russia | Olisova et al. 53 | 1 | Erythematous lesions and purpura | Upper eyelid, eyebrow and temple region | 12 | 3 days after symptoms | NR |
| CR | Portugal | Calvao et al. 54 | 1 | Petechial lesions that evolved into haemorrhagic bullae and necrotic plaques | Hands and feet | 81 | After respiratory symptoms | Yes |
| CR | Spain | Bosche‐amate et al. 55 | 1 | Reticular purpura | Lower legs | 79 | 7 days after symptoms | Yes |
| CR | UK | Klimach et al. 56 | 1 | Multiple erythematous, tender papules, macular lesions with associated scattered petechiae | Feet and legs | 13 | 1 days after symptoms | NR |
| CR | Belgium | Verheyden et al. 57 | 1 | Symmetric livedo reticularis | Trunk and thighs | 57 | At onset of symptoms | NR |
| CR | France | Giudice et al. 58 | 1 | Acute necrosis | Bilateral leg and foot | 83 | After respiratory symptoms | NR |
| CS | Turkey | Dertlioğlu et al. 59 | 5 | Erythematous lesions | Trunk (4), feet (1) | 32, 42, 29, a teenager, 10‐month old | After respiratory symptoms (3), cutaneous lesions as the only complaint (2) | NR |
CS: case series; CR: case report; NR: not reported.
Table 2.
Proportion of analysed case reports and case series of various cutaneous manifestations observed in COVID‐19 positive patients
| Type of exanthem associated with COVID‐19 | Cases (n = 406) | Percentage (%) |
|---|---|---|
| Acral ischaemic lesions or chilblains | 84 | 20.2 |
| Varicella‐like or vesicular lesions | 67 | 16.5 |
| Generalised maculopapular or morbilliform | 161 | 39.7 |
| Urticaria | 65 | 16.0 |
| Livedo reticularis | 21 | 5.20 |
| Others | ||
| Pityriasis rosea | 1 | 0.20 |
| Petechial eruption | 1 | 0.20 |
| Confluent erythematous‐yellowish papules | 1 | 0.20 |
| Cutaneous mottling | 1 | 0.50 |
| Periorbital dyschromia | 2 | 0.50 |
| Leukocytoclastic vasculitis | 1 | 0.20 |
| Reticular purpura | 1 | 0.20 |
Discussion
COVID‐19 can present as a syndrome of dry cough, fever, rhinorrhoea, anosmia and fatigue with radiological evidence of bilateral pneumonia seen on chest x‐ray and CT chest. 6 Recalcati and colleagues were the first to describe the cutaneous manifestations of COVID‐19 infection observed in Italy in 20% of their cohort. 6 Subsequently, new reports have come from many countries confirming the widespread cutaneous signs related to the virus which has been observed sporadically in COVID‐19 patients. A recent review by Tang et al. analysed 16 studies with 88 confirmed COVID‐19 related cutaneous manifestations and concluded that they can be categorised as erythematous, urticarial, and vesicular (chicken pox‐like or varicelliform) which most commonly affected the trunk. 7 Some individual reports of a petechial eruption, livedo reticularis, pityriasis rosea and reactivation of herpes simplex virus‐1 have also been reported. 8 , 9 There has also been reports of outbreaks of peculiar perniosis‐like acral lesions (chilblains) that have occurred in Spain and Italy amidst the pandemic believed to be a late manifestation of the COVID‐19 infection; however, its relevance is questionable as discussed later in the article. 10 , 11 COVID‐19 associated Kawasaki syndrome or paediatric multisystem inflammatory syndrome temporarily associated with COVID‐19 (PIMS‐TS), also known as multisystem inflammatory syndrome in children (MIS‐C) has emerged in Europe and America, with very few cases observed in Asia, especially Japan where the usual incidence is 20 times higher than the Western world. 12 One report recorded significant differences in the COVID‐19 triggered Kawasaki disease to the traditional entity, in that COVID‐19 was associated with Kawasaki in older children (mean age: 7.5 years) and caused haemodynamic instability in 20% of the affected children as compared to the usual 7%. 12
According to the analysis done by Tang et al., the latency period between the prodromal clinical symptoms such as cough and fever and cutaneous presentation was −2 to 21 days, with some reports suggesting that the cutaneous manifestation was the only symptom of COVID‐19 in otherwise asymptomatic patients. 7 , 13 The pathogenesis of the skin signs of COVID‐19 remains poorly understood and warrants further investigation via large scale prospective studies analysing the serological profile of the antibody response to the infection supported by histopathological diagnosis through biopsies. A study reporting the clinical patterns and sequalae of COVID‐19 skin lesions suggested that chilblains affected younger patients, lasted longer and presented later in the disease and were associated with less severe disease. 14 Similar observations have been reported by Andina et al. and Recalcalti et al. 10 , 15 In comparison, urticarial and maculopapular lesions occurred earlier in the disease and were associated with more severe COVID‐19 disease. 14 Necrotic lesions mainly affected older patients who had severe COVID‐19 disease, which is also evident from the data summarised in Table 1. 14
Given the variety of cutaneous presentations and their timing with respect to stage of disease, it is likely that there are distinct underlying mechanisms potentially including direct endothelial infection, coagulopathy with microthrombosis and immune complex deposition. 16 SARS‐CoV‐2 virus shows endothelial tropism due to the cellular distribution of the angiotensin converting enzyme‐2 receptor. Direct infection and endothelial activation are likely to explain some of the severe manifestations of COVID‐19 including coagulopathy. 16 Furthermore, the deposition of immune complexes on vessels has been implicated in COVID‐19 vasculitis with some reports describing leukocytoclastic vasculitis on histopathology. 17
The cutaneous side effects of medications used to treat COVID‐19 such as hydroxychloroquine need to be reported as they can be similar to the cutaneous manifestations of COVID‐19. 18 Moreover, the pandemic has resulted in cutaneous signs for up to 97% of the frontline healthcare workers due to the strict personal protective equipment requirements, with the most common eruptions being desquamation, erythema and maceration over the nasal bridge, cheek and face from wearing the N95 facial masks. 19 , 20
Comparison of COVID‐19 with other viral exanthems
The maculopapular (Fig. 1) and morbilliform exanthem is quite commonly observed with other viral infections associated with respiratory symptoms such as infectious mononucleosis, measles, rubella, human immunodeficiency virus (HIV) and roseola, which can also present with a similar prodrome of fever, nasal congestion, cough followed by the skin signs. 1 , 2 , 3 Measles, pityriasis rosea, erythema multiforme and Kawasaki disease are some examples of non‐specific viral exanthems that are similar to the reported cutaneous signs of COVID‐19. 8 , 12 The vesicular eruption observed in COVID‐19 patients is similar to varicella, hand foot and mouth (HFM) and acute generalised exanthematous pustulosis (AGEP). 1 , 2 Chilblains, also known as pernio, are quite unusual in other viral upper respiratory tract infections and the mechanism by which SARS‐CoV‐2 leads to this manifestation is still being investigated. Chilblains can be primary (idiopathic or cold related) or secondary (connective tissue disorders, haematological malignancies, cryopathies, blood hyper viscosities and genetic conditions). 1 , 2 A study of children from Spain, reported mild symptomatic chilblains as a late manifestation of COVID‐19 based on a single case which was positive for COVID‐19 via nasopharyngeal swabs. 10 We have not included the patients with chilblains who had negative swab results and thus the prevalence of chilblains may be underrepresented in the summary we have provided. However, given that the PCR result was negative for SARS‐CoV‐2 in most patients with chilblains, many clinicians question the reliability of this clinical sign in the diagnosis of COVID‐19. Other vascular manifestations of COVID‐19 such as acro‐ischaemic lesion have been reported by Yang et al. with a median latency period of 19 days. 21 Table 3 summarises the key similarities and differences between the different viral exanthems (Fig. 2, Fig. 3). 1 , 2 , 3
Figure 1.
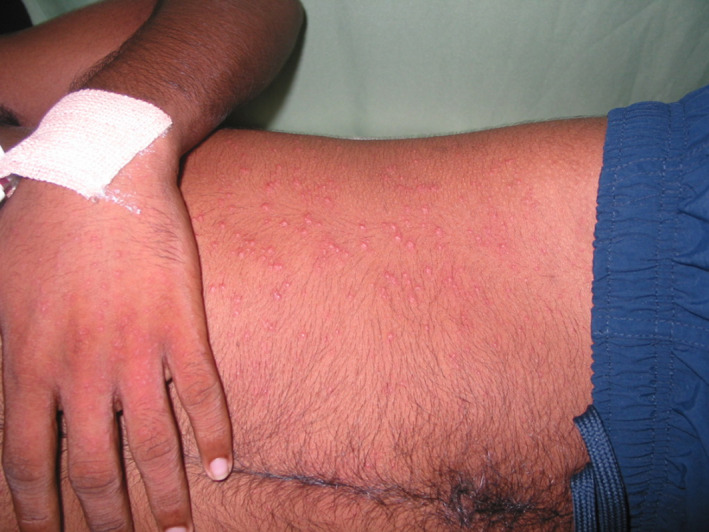
An erythematous maculopapular viral exanthem.
Table 3.
| Viral URTIs with exanthems | Cutaneous exanthem | Timing of the cutaneous manifestations |
|---|---|---|
| Measles (morbillivirus), Fig. 2 | Erythematous macules and papules that spread in a cephalocaudal direction | 2–4 days after prodrome |
| Rubella (rubella virus) | Rose‐pink macules with cephalocaudal spread | 1–5 days after prodrome |
| Erythema Infectiosum (parvovirus B19 (PVB19)) | Bright red macular erythema of the cheeks (slapped cheeks), followed by lacy reticular pattern of macules and papules on the extremities | 7–10 days after prodrome |
| Roseola Infantum (human herpesvirus (HHV) 6B and HHV‐7) | Rose‐pink macules and papules on the trunk, neck and proximal extremities | 3–4 days later |
| Unilateral laterothoracic exanthem (Epstein–Barr virus, adenovirus and PVB19, HHV‐7, parainfluenza) | Morbilliform eruption which is initially unilateral, affecting mainly the axilla and lateral trunk | Few days after the prodrome |
| Varicella (varicella‐zoster virus, VZV) | Erythematous macules and papules on the scalp and face that spread to the trunk and extremities. Lesions evolve into 1–3 mm clear vesicles that evolve into pustules and crust | 12 h after the prodrome |
| Kawasaki disease | Macular and papular erythematous lesions in a morbilliform pattern | Early in the illness |
| Pityriasis Rosea (multiple causes; HHV‐6 and HHV‐7, but can also be triggered by hepatitis C, HINI influenza, HHV‐8) | Starts with a herald patch (single oval macule) followed by a generalised maculopapular eruption | Herald patch appears 1–20 days before the generalised exanthem |
| Erythema Multiforme (parapoxvirsuses, HIV, CMV, VZV, hepatitis viruses) | ‘target‐like’ lesions, which can involve mucous membranes | Abrupt onset, within 24 h |
| Human parechoviruses (HPeV −1, 2) | Maculopapular exanthem | Skin signs appear 3 days after febrile illness |
| Togaviruses (esp. Chikungunya) and bunyavirus haemorrhagic fevers (including Lassa) | Generalised maculopapular petechial exanthem. Often pruritic and may be accompanied by oral or genital aphthous ulceration | 2–3 days after onset of fever |
| Hand, foot and mouth disease (coxsackievirus 16, 4, 5, A7, A9, A10, B2, B5 and enterovirus 71), Fig. 3 |
Oral lesions begin as erythematous macules and papules on the hard palate, tongue, cheeks and gums then progress to vesicles, which may burst and may form painful ulcers surrounded by a red halo Skin lesions start as erythematous macules or papules which quickly turn into small, grey vesicles surrounded by a red halo |
Variable timing, usually early in the illness |
| Papular pruritic gloves and socks syndrome (PVB19, EBV, CMV, HHV‐6, HHV‐7, hepatitis B, rubella, measles) | Macular and papular erythema associated with oedema affecting the hands, wrists, feet and ankles. Oral inflammation with petechiae, vesicopustules and ulceration is also common. | Onset of the eruption occurs a few days before fever and malaise |
| Toxoplasma gondii, ‘others’ including syphilis, rubella, cytomegalovirus and herpes simplex types 1 and 2 (TORCH) (‘Others’ now also includes: coxsackie, enteroviruses, PVB19, VZV, HIV, hepatitis B, Zika virus) | Purpura and petechiae associated with oral vesicles and mucosal inflammation if caused due to herpes virus | Variable onset depending on the cause |
| Zika virus (flavivirus) | Morbilliform or scarlantiniform eruption | Starts on the face on the first day and then spreads to trunk and limbs |
Figure 2.
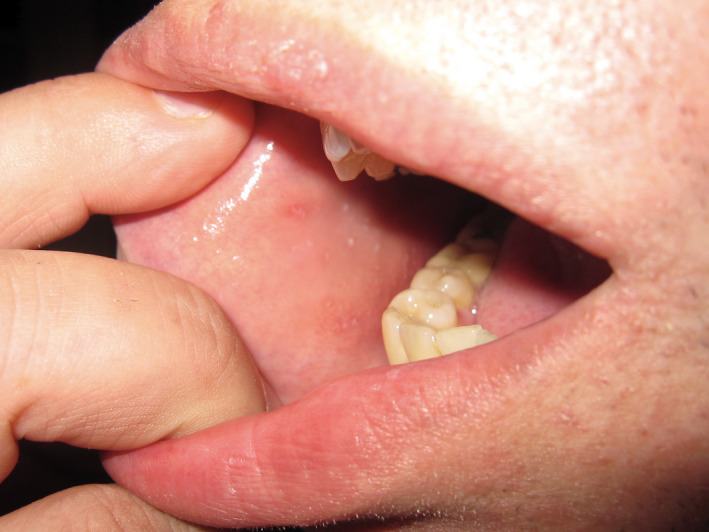
Koplik’s spots seen in measles.
Figure 3.
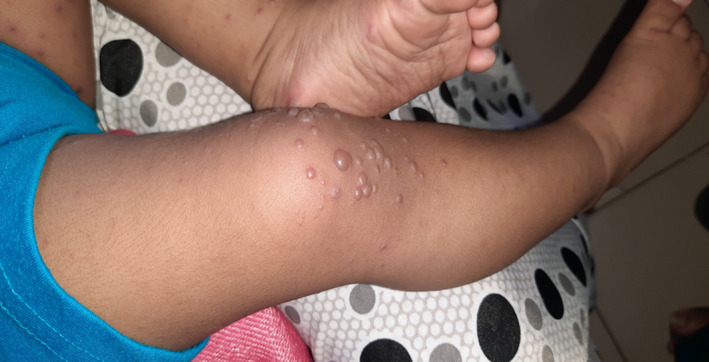
Vesicular eruption seen in hand, foot and mouth disease.
Conclusion
Viral exanthems provide early diagnostic cues for the clinician. COVID‐19 seems to have various cutaneous manifestations, none of which are specific or diagnostic for the disease. It is unclear what proportion of COVID‐19 infected patients develop cutaneous manifestations and what pathological mechanisms lead to this. Physicians and dermatologists around the world need to be vigilant about the possibility of COVID‐19 as the causative agent of a cutaneous sign in a patient with a viral prodrome, which should prompt testing for COVID‐19 where available.
Conflict of interest: Clinical Professor Sujith Prasad Kumarasinghe is a current Australasian Journal of Dermatology Editorial Board member.
Surabhi Sharma, MBBS (Hons), FRACGP. Edward Raby, BMBS, FRACP, FRCPA. Sujith Prasad Kumarasinghe, MBBS, MD, FACD.
References
- 1. Griffiths C, Barker J, Bleiker T et al. Rook’s Textbook of Dermatology. West Sussex: Wiley Blackwell, 2016. [Google Scholar]
- 2. Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia: Elsevier Saunders, 2012. [Google Scholar]
- 3. Zuckerman AJ, Banatvala JE, Pattison JR et al. Principles and Practice of Clinical Virology. UK: John Wiley & Sons Ltd, 2004. [Google Scholar]
- 4. Alramthan A, Aldaraji W. Two cases of COVID‐19 presenting with a clinical picture resembling chilblain: first report from the Middle East. Clin. Exp. Dermatol. 2020; 45: 746–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quintana‐Castanedo L, Feito‐Rodríguez M, Valero‐López I et al. Urticarial exanthem as early diagnostic clue for COVID‐19 infection. JAAD Case Rep. 2020; 6: 498–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e212–e213. [DOI] [PubMed] [Google Scholar]
- 7. Tang K, Wang Y, Zhang H et al. Cutaneous manifestations of the Coronavirus Disease 2019 (COVID‐19): A brief review. Dermatol. Ther. 2020; 33: e13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e436–e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hedou M, Carsuzaa F, Chary E et al. Comment on ‘Cutaneous manifestations in COVID‐19: a first perspective’ by Recalcati S. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e299–e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andina D, Noguera‐Morel L, Bascuas‐Arribas M et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatric Dermatol. 2020; 37: 406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Locatelli AG, Robustelli Test E, Vezzoli P et al. Histologic features of long‐lasting chilblain‐like lesions in a paediatric COVID‐19 patient. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e365–e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verdoni L, Mazza A, Gervasoni A et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet 2020; 395: 1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amatore F, Macagno N, Mailhe M et al. SARS‐CoV‐2 infection presenting as a febrile rash. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e304–e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galván CC, Català A, Carretero HG et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Brit. J. Dermatol. 2020; 183: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Recalcati S, Barbagallo T, Frasin L et al. Acral cutaneous lesions in the time of COVID‐19. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e346–e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evans PC, Ed Rainger G, Mason JC et al. Endothelial dysfunction in COVID‐19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020; 116: 2177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roncati L, Ligabue G, Fabbiani L et al. Type 3 hypersensitivity in COVID‐19 vasculitis. Clinic. Immunol. 2020; 217: 108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salido M, Joven B, D'Cruz DP et al. Increased cutaneous reactions to hydroxychloroquine (Plaquenil) possibly associated with formulation change: Comment on the letter by Alarcón. Arthritis Rheumatis 2002; 46: 3392–6. [DOI] [PubMed] [Google Scholar]
- 19. Dirk EM. Occupational skin disease among health care workers during the coronavirus (COVID‐19) epidemic. J. Am. Acad. Dermatol. 2020; 82: 1085–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu K, Fan J, Li X et al. The adverse skin reactions of health care workers using personal protective equipment for COVID‐19. Medicine 2020; 99: e20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Cao W, Xiao M et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020; 41: E006. [DOI] [PubMed] [Google Scholar]
- 22. Sachdeva M, Gianotti R, Shah M et al. Cutaneous manifestations of COVID‐19: Report of three cases and a review of literature. J. Dermatol. Sci. 2020; 98: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marzano AV, Genovese G, Fabbrocini G et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: Multicentere case series of 22 patients. J. Am. Acad. Dermatol. 2020; 83: 280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kolivras A, Dehavay F, Delplace D et al. Coronavirus (COVID‐19) infection–induced chilblains: A case report with histopathologic findings. JAAD Case Reports 2020; 6: 489–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020; 6: 493–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect. Dis. (Lond). 2020; 52: 427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henry D, Ackerman M, Sancelme E et al. Urticarial eruption in COVID‐19 infection. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e244–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang JJ, Dong X, Cao YY et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 2020; 75: 1730–41. [DOI] [PubMed] [Google Scholar]
- 29. Estébanez A, Pérez‐Santiago L, Silva E et al. Cutaneous manifestations in COVID‐19: a new contribution. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e250–e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahé A, Birckel E, Krieger S et al. A distinctive skin rash associated with Coronavirus Disease 2019. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e246–e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hunt M, Koziatek C. A case of COVID‐19 pneumonia in a young male with full body rash as a presenting symptom. Clin Pract Cases. Emerg. Med. 2020; 4: 219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J. Am. Acad. Dermatol. 2020; 82: e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manalo IF, Smith MK, Cheeley J et al. A dermatologic manifestation of COVID‐19: Transient livedo reticularis. J. Am. Acad. Dermatol. 2020; 83: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bouaziz JD, Duong T, Jachiet M et al. Vascular skin symptoms in COVID‐19: a French observational study. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e451–e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Damme C, Berlingin E, Saussez S et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e300‐e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernandez‐Nieto D, Ortega‐Quijano D, Jimenez‐Cauhe J et al. Clinical and histological characterization of vesicular COVID‐19 rashes: a prospective study in a tertiary care hospital. Clin. Exp. Dermatol. 2020; 45: 872–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunawan C, Angela A, Widysanto A. Urticarial eruption in coronavirus disease 2019 infection: a case report in Tangerang, Indonesia. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e372–e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morey‐Olivé M, Espiau M, Mercadal‐Hally M et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An. Pediatr. (Engl Ed). 2020; 92: 374–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Avellana MR, Estela VLM, Avellana MV et al. Cutaneous manifestation of COVID‐19 in images: a case report. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e307–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suarez‐Valle A, Fernandez‐Nieto D, Diaz‐Guimaraens B et al. Acro‐ischemia in hospitalized COVID‐19 patients. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e455–e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masson AD, Bouaziz JD, Sulimovic L et al. Chilblains is a common cutaneous finding during the COVID‐19 pandemic: A retrospective nationwide study from France. J. Am. Acad. Dermatol. 2020; 83: 667–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahouach B, Harent S, Ullme A et al. Cutaneous lesions in a patient with COVID‐19: are they related? Brit. J. Dermatol. 2020;183: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cepeda‐Valdes R, Carrion‐Alvarez D, Trejo‐Castro A et al. Cutaneous manifestations in COVID‐19: familial cluster of urticarial rash. Clin. Exp. Dermatol. 2020; 45: 895–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A et al. Characterization of acute acral skin lesions in non‐hospitalized patients: A case series of 132 patients during the COVID‐19 outbreak. J. Am. Acad. Dermatol. 2020; 83: e61–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Genovese G, Colonna C, Marzano AV. Varicella‐like exanthem associated with COVID‐19 in an 8‐year‐old girl: A diagnostic clue? Pediatr. Dermatol. 2020; 37: 435–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of Covid‐19. JAAD Case Rep. 2020; 6: 996–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Naziroğlu T, Sözen S, Özkan P et al. A case of COVID‐19 pneumonia presenting with acute urticaria. Dermatol. Ther. 2020; 33: e13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rivera‐Oyola R, Koschitzky M, Printy R et al. Dermatologic findings in two patients with COVID‐19. JAAD Case Rep. 2020; 6: 537–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P et al. Chilblain‐like lesions on feet and hands during the COVID‐19 Pandemic. Int. J. Dermatol. 2020; 59: 739–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mayor‐Ibarguren A, Feito‐Rodriguez M, Quintana Castanedo L et al. Cutaneous small vessel vasculitis secondary to COVID‐19 infection: a case report. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e541‐e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rossi E, Lasagni C, Trakatelli M et al. Acute maculopapular eruption in Covid‐19 patient: a case report. Dermatol. Ther. 2020; 33: e13812. [DOI] [PubMed] [Google Scholar]
- 52. Freeman EE, McMahon DE, Lipoff JB et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J. Am. Acad. Dermatol. 2020; 83: 486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Olisova OY, Anpilogova EM, Shnakhova LM. Cutaneous manifestations in COVID‐19: a skin rash in a child. Dermatol Ther 2020; 33: e13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Calvão J, Relvas M, Pinho A et al. Acro‐ischemia and COVID‐19 infection: clinical and histopathological features. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e653–e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bosch‐Amate X, Giavedoni P, Podlipnik S et al. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID‐19) coagulopathy. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e548–e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Klimach A, Evans J, Stevens J et al. Rash as a presenting complaint in a child with COVID‐19. Pediatr. Dermatol. 2020; 37: 966–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Verheyden M, Grosber M, Gutermuth J et al. Relapsing symmetric livedo reticularis in a patient with COVID‐19 infection. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e684‐e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Del Giudice P, Boudoumi D, Le Guen B et al. Catastrophic acute bilateral lower limbs necrosis associated with COVID‐19 as a likely consequence of both vasculitis and coagulopathy. J. Eur. Acad. Dermatol. Venereol. 2020; 34: e679–e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dertlioğlu S. Skin manifestations in COVID‐19: A case series of 5 patients from ElazıĞ, Turkey. Dermatol. Ther. 2020; 33: e13932. [DOI] [PMC free article] [PubMed] [Google Scholar]


