Abstract
The COVID‐19 pandemic has led to a reorganization of health systems to prioritize the fight against the virus. The adoption of social distancing interfered with the flow of existing policies, and may thus negatively affect the most vulnerable groups, such as the rare disease community. Aimming at characterizing the perception of the impact of COVID‐19 on the health care of the Brazilian rare disease community, an online questionnaire addressed to patients with rare diseases and their caregivers was disseminated in the Brazilian territory between June 1st to July 5th, 2020. The questions dealt with the sanitary measures adopted; access to medical services; and mental suffering during the pandemic. The survey was answered by 1,466 participants (<18 yo = 53.3%) representing 192 rare diseases. Regarding physical distancing, 1,372 (93.6%) participants did not leave their residence, or did so only when essential; 1,321 (90.1%) always wore masks when leaving home. 1,042 (71.1%) and 995 (67.9%) participants, respectively, referred medical genetics appointments and rehabilitation therapies were postponed/canceled. Telemedicine was experienced by 1,026 (70%), and 68.3% agreed this is a good strategy for health care. Patients with Inborn Errors of Metabolism (IEM, n = 624, 42.5%) appear to have more access to information and ability to overcome difficulties, and feel less threatened, lonely and depressed than the non‐IEM group (p < .05). There was an increment of the rare disease patients' vulnerability in the pandemic scenario. The cooperation of patients/caregivers along with adaptation of the health system is crucial and may be so even post‐pandemic.
Keywords: Brazil, coronavirus, COVID‐19, genetic disorders, rare diseases
1. INTRODUCTION
The COVID‐19 pandemic, in addition to transforming people's daily lives and shaking the world economy, brought new challenges for various sectors, including education and health. Concerns about the effect of the pandemic in the rare disease community have also been expressed. As an example, there is the statement “31 March, Paris—EURORDIS‐Rare Diseases Europe is alarmed by reports from member organizations and individuals that people living with a rare disease are being discriminated against in critical care guidelines” (EURORDIS, 2020a). Some papers highlighted health‐care concerns for individuals with rare or uncommon genetic diseases such as deaf children (Pattisapu et al., 2020); care of patients with Duchenne, Becker, and other muscular dystrophies (Veerapandiyan et al., 2020); Down Syndrome (Cammarata‐Scalisi, Tadich, Medina, & Callea, 2020); Epidermolysis Bullosa (Murrell et al., 2020). The French Rare Health Care for Neuromuscular Diseases Network (FILNEMUS) has established guidance in an attempt to homogenize the management of neuromuscular (NM) patients within the French territory; the main concern was with management of the interruption of physical therapy support (Solé et al., 2020). Guidelines for patients with inherited cardiomyopathies and channelopathies were detailed by Limongelli and Crotti (2020). Some publications were made available also in Brazil to educate rare disease patients and caregivers about COVID‐19 (BRASIL, 2020a; FEBRARAS & Observatório de Doenças Raras, 2020; SBGM, 2020).
The first notification of a confirmed case of COVID‐19 in Brazil was on February 26, 2020. Between March and July 2020, the conduct of the fight against COVID‐19 was intensely politicized (Campos, 2020). The Minister of Health was replaced three times. Disagreements between state governors and the federal government have become public. There was no adequate understanding about protocols or the impact of social distancing measures on schools and commercial or industrial activity. And the spread of fake news, the use of drugs without evidence for the treatment of COVID‐19 and scientific denialism contributed to the population's doubts, fears and uncertainties (Campos, 2020).
In order to provide a panorama about the actions taken to face the COVID‐19 epidemic in Brazil, we highlight that the Brazilian health system has peculiar characteristics: it is a public, regionalized and hierarchical system. This means there are significant differences in public health actions between the three levels of government in the country. Social distancing measures, interruption of services and testing of the population varied widely, depending on the region (Baqui, Bica, Marra, Ercole, & van der Schaar, 2020). It is also important to remember that the Brazilian epidemiological scenario is complex. In addition to public health management issues, the country has other important characteristics: the coexistence of communicable diseases (dengue fever, chikungunya fever, etc.) with COVID‐19, the high prevalence of chronic non‐communicable diseases in large urban centers and other demographic and environmental issues (size and diversity of the Brazilian population, the immensity of the territory and the diversity of social and historical determinants in each region) (GBD 2016 Brazil Collaborators, 2018).
Thus, it is necessary to make efforts to systematize epidemiological information about the pandemic in this diverse and complex country, which has led us to join some researchers and people with rare diseases, to understand the real effects of the epidemic. This article sought to assess the impact of the current COVID‐19 pandemic in the Brazilian community of patients with rare diseases.
2. METHODOLOGY
2.1. Editorial policies and ethical considerations
This was an observational, cross‐sectional, population‐based study with a convenience sampling strategy, approved by the Ethics Research Committee of the Hospital de Clínicas de Porto Alegre‐RS (reference number 2020‐0160). All participants granted their informed consent.
2.2. Study design and participants
An online survey addressed to patients/caregivers with rare diseases was widely disseminated through social media in Brazil between June first to July fifth, 2020. Caregivers were so considered when living in the same house as the patient.
The questionnaire was adapted from the EURORDIS English released form (Rare Barometer Program), with permission, and translated to Brazilian Portuguese. In relation to the original form, two additional questions were included: (1) the name of the rare disease of the patient, and (2) the Brazilian region where the participant lived. In the end, the Brazilian version was composed of 52 questions (three open‐ended), and consisted of five groups of questions related to the pandemic: (1) general characterization of the patient and the rare disease; (2) sanitary measures adopted and perception of the level of health threat; (3) access to services and support; (4) infection by SARS‐CoV2; (5) mental and social status. The questionnaire is available for consultation upon request.
The sample size was calculated to take in account the estimate that between 3.5% and 5.9% of the population is affected by rare diseases (Nguengang Wakap et al., 2020). Brazil has about 212 million inhabitants, which means between 7.4 and 12.5 million Brazilians may have some rare disease. We estimated a minimum of 384 individuals for confidence level 95% and a margin of error of 5%. To get a higher number of answers, national rare diseases patient and family associations were contacted and asked to help in the dissemination of the questionnaire. Physicians from all over the country, who were known to deal with rare diseases, were also contacted.
One thousand six hundred and five (n = 1,605) replies were obtained. Out of them, 139 (8.7%) were excluded for different reasons: (1) the patient was mentioned as not having a rare disease (n = 45); (2) the same participant answering more than one form (n = 35); (3) the patient had no diagnosis (n = 15); (4) other reasons (n = 44). Hence, a total of 1,466 participants from the five different Brazilian Regions were included (North, Northeast, Center‐West, Southeast and South) (Figure 1).
FIGURE 1.
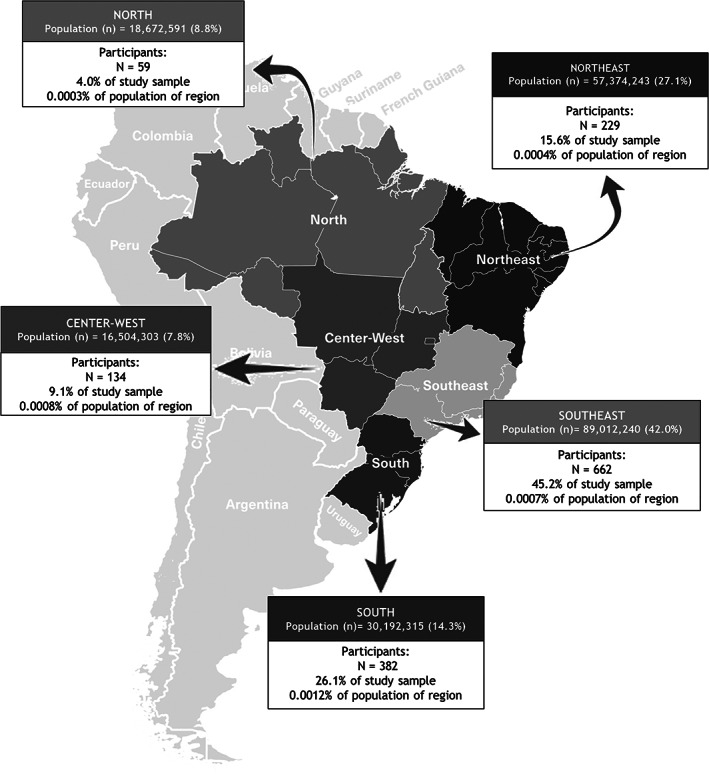
Rare diseases in Brazil: distribution of the survey participants (n = 1,466) according to their region of origin
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
2.3. Data extraction and statistical analysis
A descriptive statistical analysis was carried out and categorical variables were presented as counts and percentages. To analyze the answers, we cross‐tabulated the recorded data, grouping the answers according to etiology of the rare disease (genetic or non‐genetic), type of genetic diseases [Inborn Errors of Metabolism (IEM) plus mitochondrial disorders vs others, that is, non‐IEM], and type of treatment (pharmacological, nutritional or rehabilitation). The definition adopted for rare diseases was the one proposed by the Brazilian Policy for Rare Disorders: rare diseases include those with a prevalence of <65/100,000 individuals (BRASIL, 2014). According to this Policy, rare genetic diseases can broadly be categorized in those (1) with congenital malformations, or with late‐onset, or (2) with cognitive impairment, or (3) IEM; while the non‐genetic are due to (1) infectious, (2) inflammatory, or (3) auto‐immune causes. Pharmacological treatment refers to any strategy including the use of oral or IV drugs, such as enzyme replacement therapy, nitrogen scavengers, etc. Nutritional treatment includes dietary modifications, such as the use of a hypoproteic diet, use of a metabolic formula or vitamin supplementation; and rehabilitation or symptomatic only including physical therapy, speech therapy, psychology, psychiatry, pain management, etc.). When the disease was treated by more than one strategy, we classified them according to the highest level of intervention, for example, drugs > diet > rehabilitation. For instance, urea cycle disorders were classified as genetic diseases, IEM type, treated pharmacologically (most patients in Brazil receive oral sodium benzoate). Phenylketonuria (PKU), on the other hand, was classified as a genetic disease, IEM type, treated by nutritional strategies (since oral BH4 is still not widely available in the country). And Williams syndrome, as a genetic disease, non‐IEM type, treated by rehabilitation therapy.
When necessary, Pearson correlation coefficient and Kruskal‐Wallis test were used to evaluate nonparametric data. Statistical analyses were performed in SPSS Version 22.0 (IBM Corp., Armonk, NY, USA), with support of Excel (Office 365—Microsoft). To support the analysis of our results, we used the epidemiological data from reference research centers in Brazil as FIOCRUZ (2020) and IBGE (2020a), in addition to official data from the Brazilian Ministry of Health (MOH) (BRAZIL, 2020c) and results of the EURORDIS survey (2020b).
3. RESULTS
3.1. General characteristics of participants and rare diseases (Table 1)
Among the 1,466 questionnaires received and included in the study, 493 (33.6%) were answered by patients and 973 (66.4%), by their caregivers. Most participants came from the Southeast and South regions of Brazil (Figure 1), and most patients were <18 yo (n = 781, 53.3%). In relation to the participant's household composition, the majority was represented by a total of three or four individuals living in residence (Table 1); one or two children (63.4%); and one patient with a rare disease (92.1%).
TABLE 1.
Characteristics of participants (n = 1,466): respondent, age of patient, number of persons in household, and health‐care access
| Variable | Region of Brazil | Total n = 1,466 | ||||
|---|---|---|---|---|---|---|
| North n = 59 (4.0%) | Northeast n = 229 (15.6%) | Center‐West n = 134 (9.1%) | South n = 382 (26.1%) | Southeast n = 662 (45.2%) | ||
| Participant (n) | ||||||
| Patient | 16 (27.1%) | 82 (35.8%) | 39 (29.1%) | 126 (33.0%) | 230 (34.7) | 493 (33.6%) |
| Caregiver | 43 (72.9%) | 147 (64.2%) | 95 (70.9%) | 256 (67.0%) | 432 (65.3%) | 973 (66.4%) |
| Age of patient (years) | ||||||
| <18 | 38 (64.4%) | 115 (50.2%) | 78 (58.2%) | 213 (55.7%) | 337 (50.9%) | 781 (53.3%) |
| 18–64 | 21 (35.6%) | 113 (49.4%) | 53 (39.6%) | 163 (42.7%) | 317 (47.9%) | 667 (45.5%) |
| ≥65 | 0 (0.0%) | 1 (0.4%) | 3 (2.2%) | 6 (1.6%) | 8 (1.2%) | 18 (1.2%) |
| Persons in household (n) | ||||||
| 1 | 1 (1.7%) | 5 (2.2%) | 7 (5.2%) | 18 (4.7%) | 24 (3.6%) | 55 (3.8%) |
| 2 | 5 (8.5%) | 22 (9.6%) | 10 (7.5%) | 57 (14.9%) | 78 (11.8%) | 172 (11.7%) |
| 3 | 9 (15.3%) | 78 (34.1%) | 41 (30.6%) | 124 (32.5%) | 236 (35.7%) | 488 (33.3%) |
| 4 | 30 (50.8%) | 79 (34.5%) | 53 (39.6%) | 109 (28.5%) | 203 (30.7%) | 474 (32.3%) |
| 5 | 10 (16.9%) | 28 (12.2%) | 11 (8.2%) | 55 (14.4%) | 77 (11.6%) | 181 (12.3%) |
| ≥6 | 4 (6.8%) | 17 (7.4%) | 12 (8.9%) | 19 (5.0%) | 44 (6.6%) | 96 (6.6%) |
| Restricted access to (n) | ||||||
| Testing | 33 (55.9%) | 131 (57.2%) | 73 (54.5%) | 183 (47.9%) | 484 (58.6%) | 808 (55.1%) |
| Geneticist follow‐up | 45 (76.2%) | 168 (73.3%) | 94 (70.1%) | 251 (65.7%) | 296 (73.1%) | 1,042 (71.1%) |
| Pharmacotherapy | 28 (47.4%) | 116 (50.6%) | 56 (41.8%) | 150 (39.3%) | 238 (44.7%) | 646 (44%) |
| Psychiatric follow‐up | 19 (32.2%) | 91 (39.7%) | 44 (32.8%) | 115 (30.1%) | 457 (5.9%) | 507 (34.6%) |
| Rehabilitation | 32 (54.2%) | 160 (69.9%) | 92 (68.6%) | 254 (66.5%) | 133 (69.0%) | 995 (67.9%) |
| Medicines | 8 (13.5%) | 64 (27.9%) | 26 (19.4%) | 74 (19.4%) | 133 (20.0%) | 305 (20.8%) |
| Surgery or transplant | 18 (30.5%) | 79 (34.5%) | 42 (31.3%) | 90 (23.6%) | 215 (32.5%) | 444 (30.3%) |
| Telemedicine (n) | ||||||
| Had the experience | 44 (74.6%) | 164 (71.6%) | 93 (69.4%) | 269 (70.4%) | 456 (68.9%) | 1,026 (70.0%) |
| Rated the experience as | 47 (79.7%) | 185 (80.8%) | 102 (76.1%) | 277 (72.5%) | 483 (72.9%) | 1,094 (74.6%) |
| Positive | 32 (68.1%) | 116 (62.7%) | 68 (66.7%) | 193 (69.7%) | 338 (70.0%) | 747 (68.3%) |
| Negative | 9 (19.1%) | 34 (18.4%) | 11 (10.8%) | 45 (16.2%) | 79 (16.3%) | 178 (16.3%) |
| Not sure | 6 (12.8%) | 35 (18.9%) | 23 (22.5%) | 39 (14.1%) | 66 (13.7%) | 169 (15.4%) |
Regarding employment, participants referred to be employed (43.3%); self‐employed (12.3%); housewife/househusband (18.4%); retired (9.3%); unemployed and fit to work (7.0%); unemployed but unable to work (4.2%); and other status (17.8%). Regarding the rare disease presented by the patient, 192 different diseases (or disease groups) were reported: 143 were genetic (n = 1,318, 89.9%) and 49, non‐genetic conditions (n = 148, 10.1%). Among the patients with genetic diseases, 624 (47.3%) presented IEM. The most frequent conditions, here defined as being those with at least 30 participants, are shown in Table 2.
TABLE 2.
COVID‐19 and rare diseases in Brazil: most frequently reported diseases (n = 1,466) a
| Disease | n (%) |
|---|---|
| Phenylketonuria | 95 (6.5) |
| Porphyrias | 83 (5.7) |
| Acute intermittent | 55 (66.3) |
| Cutanea tarda | 5 (6.0) |
| Erythropoietic | 4 (4.8) |
| Variegata | 4 (4.8) |
| Hereditary coproporphyria | 2 (2.4) |
| Not specified | 13 (15.7) |
| Hepatic glycogen storage disease (GSD) | 81 (5.5) |
| GSD I, subtype not specified | 47 (58.0) |
| GSD III | 5 (6.2) |
| Type not specified | 29 (35.8) |
| Spinal muscular atrophy (SMA) | 80 (5.5) |
| Mucopolysaccharidosis (MPS) | 66 (4.5) |
| VI | 15 (22.7) |
| I | 14 (21.2) |
| II | 14 (21.2) |
| IV, subtype not specified | 11 (16.7) |
| Type not specified | 12 (18.2) |
| Congenital adrenal hyperplasia | 47 (3.2) |
| Classical | 14 (29.8) |
| Non‐classical | 1 (2.1) |
| Type not specified | 32 (68.1) |
| Prader‐Willi syndrome | 47 (3.2) |
| Ataxias | 45 (3.1) |
| Spinocerebellar (SCA) | 29 (64.5) |
| SCA 1 | 1 (3.4) |
| SCA 2 | 1 (3.4) |
| SCA 3 | 24 (82.8) |
| SCA 6 | 1 (3.4) |
| Type not specified | 2 (7.0) |
| Friedreich | 6 (13.3) |
| Ataxia–telangiectasia | 1 (2.2) |
| Type not specified | 9 (20) |
| Cystic fibrosis | 40 (2.7) |
| Gaucher disease | 33 (2.2) |
| Williams syndrome | 31 (2.1) |
| Osteogenesis imperfecta | 30 (2.0) |
| Other (n = 180) | 788 (53.8) |
Disease groups corresponding to a minimum of 30 participants were cited individually.
The main strategy of treatment was pharmacological for 707 (48.2%) individuals, rehabilitation for 55 (34.4%) and nutritional for 254 (17.3%). Twelve different ICD‐Groups were represented, with distribution in 157 different ICD‐10 codes. The groups E—endocrine, nutritional and metabolic diseases (44.9%), G—diseases of the nervous system (17%), and Q—congenital malformations, deformations and chromosomal abnormalities (27.6%) were the most frequent.
3.2. Sanitary measures adopted and individual perception of the level of health threat (Table 3)
Regarding adherence to the recommended safety measures, 93.6% of participants reported not leaving the residence or doing so only when essential, and 1,321 (90.1%) always wore masks when leaving home. In both cases, there is no significant difference between Brazilian regions.
Ninety‐two percent felt threatened or very threatened by the pandemic (Table 3). In general, caregivers from all regions reported higher levels of perceived threat. For all regions of Brazil, caregivers and patients reported having a satisfactory level of access to information about COVID‐19 and their rare disease. Indeed, 24.6% considered always having access to information about COVID‐19 and their rare disease (Table 3).
TABLE 3.
Adherence of participants to the recommended shielding measures, access to information, and level of perceived threat (n = 1,466)
| Variable | n (%) |
|---|---|
| Adherence to recommended shielding measures | |
| Does not leave home | 294 (20.1%) |
| Leaves home only when essential | 1,078 (73.5%) |
| Leaves home daily | 94 (6.4%) |
| Wears mask when outside the home | |
| Always | 1,321 (90.1%) |
| Occasionally | 78 (5.3%) |
| Never | 2 (0.1%) |
| No response | 65 (4.5%) |
| Access to information regarding COVID‐19 and rare disease | |
| Always | 361 (24.6%) |
| Often | 411 (28.0%) |
| Sometimes | 385 (26.3%) |
| Rarely | 233 (15.9%) |
| Never | 76 (5.2%) |
| Level of perceived threat | |
| To participant | |
| Very threatened | 704 (48.0%) |
| Threatened | 644 (43.9%) |
| A little threatened | 118 (8.1%) |
| To parents | |
| Very threatened | 981 (66.9%) |
| Threatened | 403 (27.5%) |
| A little threatened | 82 (5.6%) |
| To family | |
| Very threatened | 808 (55.1%) |
| Threatened | 609 (41.5%) |
| A little threatened | 49 (3.4%) |
When comparing patients with IEM and non‐IEM, there were no significant differences regarding the perception of individual threat (p = .300) or familial threat (p = .760). But for the questions that assessed the parent's threat level (if the patient feels responsible for some level of risk for his parents) and access to information, there is a significant difference between populations (p = .019 and p = .002, respectively), i.e., non‐IEM patients experience higher levels of menace to their parents and a lack of access to information (Figure 2).
FIGURE 2.
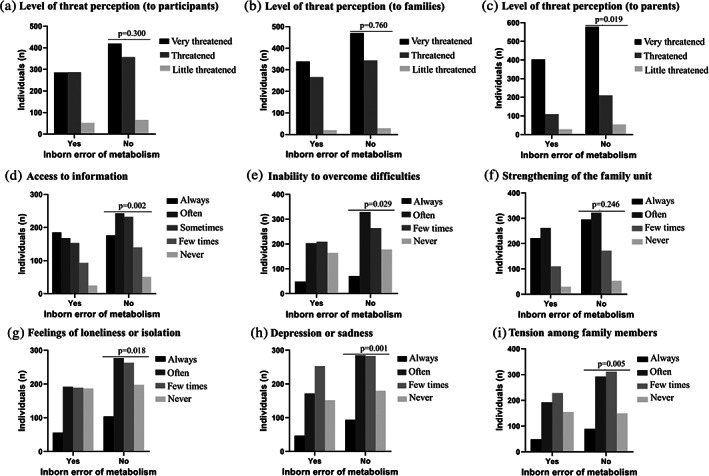
Comparison between participants from the inborn errors of metabolism group (n = 624) and other rare diseases group (n = 842) regarding their experiences during the pandemic. (a) Level of perceived threat to the participant, p = .300; (b) to the family, p = .760; and (c) to the parents, p = .019. (d) Access to information about COVID‐19 and the rare disease, p = .002. (e) Frequency of: inability to overcome difficulties, p = .029; (f) strengthening of the family unit, p = .246; (g) feelings of loneliness or isolation, p = .018; (h) depression or sadness, p = .001; and (i) tension among family members, p = .005
3.3. Access to services and support
Almost all participants (98.8%) answered there was some interruption of the public health services they needed during the pandemic. Table 1 shows the percentage of the disruption (cancellation or postponement) of medical care; rehabilitation therapies; surgery or transplant; pharmacological treatment received at hospital or at home; psychiatric follow‐up; geneticist follow‐up; and laboratory exams. For 71%, these disruptions were reported to be detrimental to their health/well‐being. Regarding the lack of medicine for the rare diseases, for 77.7% the unavailability was temporary, while 22.3% had to interrupt the treatment or seek an alternative.
One thousand and twenty‐six participants (70.0%) experienced some form of telemedicine during the pandemic (online consultations, prescription via email and online guidelines for self‐care). One thousand and ninety‐four (74.6%) participants gave their opinion on telemedicine strategies, which was positive for 747 (68.3%), as shown in Table 1.
3.4. Infection by SARS‐CoV‐2
Only 159/1,322 (12.0%) of the survey participants were tested for COVID‐19—PCR and/or antibodies testing (due to symptoms = 65, or to exposure to the virus = 47; for being in the high‐risk group = 47). Among the non‐tested (n = 1,163/1,322, 88.0%), 467 (40.1%) believe they should have been tested.
Table 4 summarizes the information about hospitalization due to COVID‐19, which reported frequency was 17 (1.2%) cases. Only one participant—presenting a Mitochondrial myopathy—reported the need for ICU admission with intubation.
TABLE 4.
Summary of participants (patients with rare diseases and their family members) who were hospitalized with COVID‐19 (n = 17) a
| Participant | Age (years) | Persons in household (n) | ICD‐10 | Condition | Region of origin | Hospitalization | ||
|---|---|---|---|---|---|---|---|---|
| At dedicated COVID‐19 unit | At intensive care | Intubation | ||||||
| C1 | <18 | 4 | E70 | Phenylketonuria | Southeast | No | Yes | No |
| C2 | <18 | ≥6 | E70 | Phenylketonuria | North | Yes | No | No |
| C3 | <18 | 3 | E71.0 | Maple syrup urine disease | South | Yes | No | No |
| C4 | <18 | ≥6 | E72.4 | Ornithine transcarbamylase deficiency | Southeast | No | Yes | No |
| C5 | <18 | 4 | E75.2 | Niemann‐Pick disease type C | Northeast | Yes | No | No |
| C6 | <18 | 4 | Q78.0 | Osteogenesis imperfecta | Southeast | Yes | No | No |
| C7 | 18–64 | 3 | E27.1 | Addison disease | Southeast | No | Yes | No |
| C8 | 18–64 | 5 | E75.2 | Niemann‐Pick disease type C | Northeast | Yes | No | No |
| C9 | ≥65 | 3 | G12.2 | Amyotrophic lateral sclerosis | Southeast | No | Yes | No |
| P1 | 18–64 | 4 | D83 | Common variable immunodeficiency | Southeast | Yes | No | No |
| P2 | 18–64 | 5 | G12.2 | Amyotrophic lateral sclerosis | South | No | Yes | No |
| P3 | 18–64 | 2 | G35 | Multiple sclerosis | North | Yes | No | No |
| P4 | 18–64 | 4 | G37.3 | Acute transverse myelitis | South | Yes | No | No |
| P5 | 18–64 | 3 | G71.1 | Steinert myotonic dystrophy | Northeast | No | Yes | No |
| P6 | 18–64 | 4 | G71.3 | MELAS syndrome | Southeast | Yes | No | No |
| P7 | 18–64 | 4 | G71.3 | Mitochondrial myopathy | South | Yes | No | No |
| P8 | 18–64 | 3 | G71.3 | Mitochondrial myopathy | Southeast | No | Yes | Yes |
Abbreviations: C, caregiver; P, patient.
This table refers to the question: “Have you—or the patient you take care of—been hospitalized due to COVID‐19?”. Thus, when the questionnaire was completed by the caregiver (C1 to C9), we were unable to establish whether the patient, the caregiver, or both had COVID‐19.
When asked about their emotions during the pandemic, the majority reported frequently feeling unable to overcome the difficulties (44.4%), a strengthening between family members (73.3%), isolated or lonely (42.9%), unhappy or depressed (40.8%) and tension between family members (42.4%).
When data are aggregated by region and by disease group (IEM vs. non‐IEM), there were no significant differences between regions, but there were some differences between groups. Non‐IEM had worse results in terms of “quality of life”, reporting not being able to overcome the difficulties (p = .029), more feelings of loneliness or isolation (p = .018), unhappiness or depression (p = .001) and tension between family members (p = .005) (Figure 2).
4. DISCUSSION
This study aimed at understanding how COVID‐19 was affecting people living with rare diseases in Brazil. Our results are, in general, similar to the EURORDIS‐Rare Diseases Europe survey conducted in April 2020 (EURORDIS, 2020c): the majority of people living with a rare disease experienced interruption in care because of COVID‐19. We can highlight that it was possible to establish the most diverse cohort of patients with rare diseases in Brazil. There are no previous studies that contemplate the diversity of 192 different diseases (or disease groups) reported herein.
Brazil has a good structure of health information systems, with notification of birth defects and production of vital statistics. However, the current systems do not have sensitive, structured or automated algorithms to collect data from medical records of people with rare diseases. There are some ongoing initiatives that are being promoted by the Brazilian government that will allow a better epidemiological assessment of rare diseases (BRASIL, 2019).
Besides that, it is important to point out that the Brazilian Policy for Rare Diseases was only published in 2014 (BRASIL, 2014); before that, there was not even an agreement on the definition of rare diseases in the country, nor were the reference centers for the care of this public recognized (receiving no special funding) by the MOH. Even so, there are few reference centers for rare disorders in Brazil (about 20, which is less than one por state) and most of them are located in main cities and related to a University Hospital where access is still difficult, depending on referrals from primary care.
4.1. Brazilian regions and political situation of Brazil
Brazil has been among the three countries with the highest numbers of cases and deaths from COVID‐19 for several successive weeks (BRASIL, 2020b). The first two Brazilian patients were identified in the city of São Paulo (SP). They were two males, ages 61 and 32, that traveled from São Paulo to Italy (Lombardia region) in early February 2020. Both had returned to São Paulo. By 26th February 2020 confirmatory diagnostic through real‐time RT‐PCR had been conducted at the Instituto Adolfo Lutz (IAL), the regional reference laboratory for virus detection in SP state. In the following 2 weeks the virus was detected in all regions of the country (BRASIL, 2020c). To investigate the SARS‐CoV‐2 strains circulating in Brazil, whole genomes were collected from 10 different Brazilian states, during the first 2 months of the COVID‐19 epidemic. This Brazilian SARS‐CoV‐2 lineage was probably established during February 2020 and rapidly spread through the country, reaching different Brazilian regions by the middle of March 2020 (Resende et al., 2020).
Despite the rapid spread of the virus to all regions, the number of infected, hospitalized patients and deaths was widely heterogeneous in the country. Thus, the temporal space evolution of the epidemic between March 17 and April 24 shows a greater number of cases and deaths in the two most populous states, São Paulo and Rio de Janeiro (in the southeast), as expected. However, the large number of deaths in cities like Manaus (North of Brazil), Recife and Fortaleza (in the Northeast region) drew attention. In this period, the states of the South and the Center‐West were the least affected (Souza, Paiva, Leal, Silva, & Santos, 2020). According to the special document (BRASIL, 2020d), in epidemiological week 30 (July 19 to 25), Brazil had an incidence of 1,139 cases/100,000 inhabitants and a mortality rate of 41.1 deaths/100,000 inhabitants. The North and Northeast regions had numbers above the Brazilian average. Next was the Center‐ West region, with the Federal District presenting the highest incidence rate. Lower incidences and, in general, lower mortality, were observed in the southeastern and southern regions.
An important point in the Brazilian case is the low testing rate performed for COVID‐19. The country faced both difficulty in obtaining the necessary inputs and structural difficulty in carrying out the tests. At the end of April, Brazil performed only 0.63 tests per 1,000 inhabitants, while Italy, for example, tested 23.64 (Barrucho, 2020).
4.2. Access to services and support/infection by SARS‐CoV‐2
In this survey, 12.0% (1.2/1,000) of participants were tested for COVID‐19, that is, the testing rate in the population of people with rare diseases may be higher than the population in general. And about 1.2% (~1,200/100,000) were hospitalized, a value similar to the estimated incidence for COVID‐19 in Brazil during the survey period. Unfortunately, this study did not collect data on the number of positive tests for COVID‐19, nor on the mortality rate for COVID‐19 in the rare disease community.
In addition to the epidemiological variables, some of the socioeconomic aspects assessed in the research can be highlighted. Worldwide, unemployment rates increased due to the closure of several productive sectors and the necessary measures of social distancing. According to IBGE (2020b), in July 2020, the unemployment rate in Brazil was 13.8%. Only 43.3% participants of the survey made reference to being employed, 9.3% retired, and 5.3% students, which means that 42.1% of the participants have no formal income. This can be an important indicator and it can point to the need for socioeconomic support mechanisms to be directed to these families. The impoverishment of these families becomes an important factor of vulnerability in a country that presents great social inequalities. In Brazil, health care is public, universal and free, but it will be necessary to develop strategies that can mitigate the effects of the COVID‐19 pandemic, in a syndemic approach (Horton, 2020).
Our data regarding interruption of treatment in rare diseases showed a lot of similarity to the data found in the EURORDIS study. Virtually all patients reported interruption of treatment and a sense of threat to their health associated with a pandemic. On the other hand, the telemedicine strategy adopted by some regions reduced the difficulties in accessing information and health care, having been a positive aspect and was well accepted by patients and caregivers.
In the early months of the COVID‐19 epidemic, most health‐care efforts were directed at screening, diagnosis and providing supportive therapies for those infected. Primary health‐care services have been interrupted or suspended in most parts of Brazil, due to uncertainties about transmission mechanisms and the real risk to the health of patients and health teams (OPAS, 2020). There are reports that surgeries and access to elective procedures in other countries have been interrupted (Søreide et al., 2020). The results obtained in our research partially corroborate these findings. Patients with IEM undergoing enzyme replacement therapies had more access to patient support services, often paid for by the industry itself. In this case, we see a better support and attention structure for this group of patients.
4.3. Measures of social distancing, aspects of mental and social suffering during the pandemic
A strategy to deal with the pandemic, with proven effectiveness, is social distancing, quarantine and isolation. The measure whose order in Brazil was #fiqueemcasa (#stay at home) has been implemented with different results among the different states of the federation. São Paulo, is the state with the highest number of COVID‐19 cases and deaths. The Social Isolation Index (SII) in April was 58.4%. On June 14, SII in Brazil reached 48.9% and in São Paulo SII reached 50.1% (INLOCO, 2020). The data obtained here clearly demonstrate that people with rare diseases seemed to be aware of their risks (even if for some groups that risk would be low for the severe form of the disease) and strictly respected the measures of social isolation.
The measures of social isolation have revealed both individual and collective impacts. Certainly, the measure of social isolation reported by the patients had a positive impact on health and number of affected cases.
Recommendation number 31 of April 30, 2020 of the National Health Council on “people with disabilities and COVID‐19” is a very complete document in support of people with disabilities in the spheres of health, education, citizenship and the economy. In relation to health care, the MOH, State and Municipal Secretariats recommend to “regulate care provided through teleconsultations and establish a virtual network of teleorientation and telemonitoring, considering the possibility of extending the period of social isolation, in order to continue the network's policy of action for the care of people with disabilities, including rehabilitation needs” (BRASIL, 2020e).
A look at some aspects of quality of life and mental health of the participants shows that a certain degree of pessimism and mental suffering affected the patients, and this situation has been reported for other forms of chronic illness worldwide (Chudasama et al., 2020). Despite the relative access to information and care mediated by telemedicine, the interruption of services, the scenario of uncertainties about the outcome of the pandemic, and the economic impact may have aggravated the mental suffering of patients. There are several reports in the literature that an epidemic of mental illness has occurred along with the COVID‐19 pandemic (Torales, O'Higgins, Castaldelli‐Maia, & Ventriglio, 2020). We noticed differences between the groups of patients with IEM and non‐IEM, with higher percentages of mental suffering among the latter. This may be related to the fact that health care for patients with IEM takes place in a more structured way in Brazil, that is, there is a public neonatal screening policy for the most prevalent IEM and there are well‐structured referral services, with multi professional follow‐up. Interestingly, Lampe et al. (2020), studying the impact of Covid‐19 on IEM in the European Union, found that the rate of infection in this population was lower than that of general European population; they pointed out that IEM health‐care providers and organization of patients were able to work quickly and effectively together to support and protect this group of patients. We believe that this may have also happened in Brazil, explaining the differences found among the IEM and non‐IEM group. Although there are many organizations for rare disorders in the country, those ones dealing with IEM are still the majority and the better‐organized ones.
4.4. Study limitations
All the collected information consisted of self‐reported data from the survey participants and may contain potential sources of bias. The online nature of this survey, despite being the most efficient way to contact the patients, caregivers and organizations that constitute the Brazilian rare disease community, entails a series of cultural and socioeconomic restraints. Thus, the study population was narrowed to people with access to the Internet and the ability to effectively use it, which may not constitute a representative sample of the population.
In this survey we chose some population characteristics that stratify health opportunities and outcomes, to maintain the similarity with the original questionnaire from EURORDIS. Unfortunately, we recognize that there are some gaps in equity assessment questions (race/ethnicity/culture/language, occupation, gender/sex, religion, education, socioeconomic status, social capital).
We reached a sample size of 3.8× the amount of expected responses. But, if we consider the expected demographic weights (North 8.8%, Northeast 27.1%, Center‐West 7.8%, South 14.3%, Southeast 42%) to achieve an adequate distribution by different Brazilian regions, our sample has a discrete underrepresentation of populations in the North and Northeast regions and a higher representation in the South of the country.
5. CONCLUSIONS
Our data is in accordance with the findings of a similar research conducted in Europe, and confirms that policy makers and authorities around the world cannot leave behind people living with rare diseases, especially during the pandemic and the post‐pandemic periods. We also believe that our data reflect the characteristics of the health system inequality in different regions of Brazil. It also surprisingly demonstrated how people and their caregivers managed to remain in social isolation and follow health recommendations as a natural mechanism of self‐protection. This certainly helped prevent more cases and higher mortality in the group. The fact that there is no precedent for a pandemic in contemporary times has left the health system more fragile and with difficulties of immediate reaction. This fact contributed to the delay in effective measures such as tele‐attendance, tele consults, home visits by the health‐care team. Such measures will remain an important tool in health care in the present and the future, especially for people affected by rare disorders.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
We acknowledge all the participants of the study, the health professionals who kindly helped us in disseminating the questionnaire, and EURORDIS for allowing translation and adaptation of the original questionnaire to Portuguese. We especially thank Dr. Taiane Vieira for the translation of the questionnaire and UG medical student Lethicia Ferraro for her collaboration in preparing and disseminating this survey. This study was supported by the Brazilian Society of Medical Genetics and Genomics and by the Rare Disease Secretariat (Brazilian Ministry of Women, Family and Human Rights). We would also like to thank the following rare disease organizations: Febrararas Federação Brasileira das Associações de Doenças Raras, Observatório de Doenças Raras (University of Brasília), Casa Hunter, Instituto Vidas Raras, Instituto Atlas BioSocial, Grupo Mães Metabólicas, Associação Brasileira de Glicogenoses—ABGLICO, Associação Brasileira da Doença de Pompe ABRAPOMPE, Associação Brasileira de Prader Willi—SPWBRasil, Associação Brasileira de Ataxias Hereditárias—ABAHE, Associação Brasileira de Amiotrofia Espinhal—ABRAME, Associação Brasileira de Porfirias Hereditária—ABRAPO, Associação Brasileira de Síndrome de Williams—SWBRASIL, Associação Nacional de Osteogenese Imperfeita—ANOI, Associação Nanismo Brasil—ANNABRA, Associação Brasileira de Fenilcetonúria—SAFE Brasil, Associação Brasileira de Assistência a Mucoviscidose—ABRAM, Associação Brasileira Síndrome Cornélia de Lange—CdLS Brasil, Associação Brasileira dos Portadores da Doença de Gaucher ABPDG, Associação Niemann Pick Batten Brasil—ANPB, Pitt‐Hopkins Brasil, Associação CNC (CFC‐Noonan‐Costello), Sociedade Brasileira de Erros Inatos e Triagem Neonatal.
Schwartz IVD, Randon DN, Monsores N, et al. SARS‐CoV‐2 pandemic in the Brazilian community of rare diseases: A patient reported survey. Am J Med Genet Part C. 2021;187C:301–311. 10.1002/ajmg.c.31883
Funding information Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brazil – (CAPES) ‐ Finance Code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brazil (CNPq).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Baqui, P. , Bica, I. , Marra, V. , Ercole, A. , & van Der Schaar, M. (2020). Ethnic and regional variations in hospital mortality from COVID‐19 in Brazil: A cross‐sectional observational study. The Lancet Global Health, 8(8), e1018–e1026. 10.1016/S2214-109X(20)30285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrucho, L. (2020). Brasil é um dos países que menos realiza testes para covid‐19, abaixo de Cuba e Chile. BBC News Brasil. Retrieved from https://www.bbc.com/portuguese/internacional52383539.
- BRASIL (2014). Ministério da Saúde. Portaria GM/MS n° 199, de 30 de janeiro de 2014. Diretrizes para Atenção Integral às Pessoas com Doenças Raras no Sistema Único de Saúde – SUS. Retrieved from https://portalarquivos2.saude.gov.br/images/pdf/2014/junho/04/DIRETRIZESDOENCAS‐RARAS.pdf.
- BRASIL (2019). Conselho Nacional de Desenvolvimento Científico e Tecnológico. Chamada CNPq/MS/SCTIE/DECIT N°25/2019 ‐ Inquérito sobre Perfil de Doenças Raras no Brasil. Retrieved from http://cnpq.br/web/guest/chamadas-publicas?p_p_id=resultadosportlet_WAR_resultadoscnpqportlet_INSTANCE_0ZaM&filtro=abertas&detalha=chamadaDivulgada&desc=chamadas&idDivulgacao=9142.
- BRASIL (2020a). Ministério da Mulher, da Família e dos Direitos Humanos. Retrieved from https://www.gov.br/mdh/pt-br/@@search?SearchableText=raras+e+covid.
- BRASIL (2020b). Ministério da saúde ‐ Painel Coronavírus. Retrieved from https://covid.saude.gov.br/ [Google Scholar]
- BRASIL (2020c). Ministério da Saúde. SVS Coronavirus Disease 2019. Retrieved from https://portalarquivos.saude.gov.br/images/pdf/2020/April/03/.
- BRASIL (2020d). Ministério da Saúde. Secretaria de Vigilância em Saúde. Boletim Epidemiológico Especial ‐ Doença pelo Coronavírus COVID‐19 ‐ Semana Epidemiológica 30 (19 a 25/07). Retrieved from http://antigo.saude.gov.br/images/pdf/2020/July/30/Boletim-epidemiologico-COVID-24.pdf.
- BRASIL (2020e). Conselho Nacional de Saúde. Recomendação n° 031, de 30 de abril de 2020. Recomenda medidas emergenciais complementares que visam a garantia dos direitos e da proteção social das pessoas com deficiência no contexto da COVID‐19. Retrieved from https://conselho.saude.gov.br/recomendacoes-cns/1146-recomendacao-n-031-de-30-de-abril-de-2020.
- Cammarata‐Scalisi, F. , Tadich, A. C. , Medina, M. , & Callea, M. (2020). Trisomy 21 and the coronavirus disease 2019 (COVID‐19). Archivos Argentinos de Pediatria, 118(4), 230–231. 10.5546/aap.2020.eng.230 [DOI] [PubMed] [Google Scholar]
- Campos, G. W. d. S. (2020). O pesadelo macabro da Covid‐19 no Brasil: entre negacionismos e desvarios. Trabalho, Educação e Saúde, 18(3), 5–9. 10.1590/1981-7746-sol00279 [DOI] [Google Scholar]
- Chudasama, Y. V. , Khunti, K. , Gillies, C. L. , Dhalwani, N. N. , Davies, M. J. , Yates, T. , & Zaccardi, F. (2020). Healthy lifestyle and life expectancy in people with multimorbidity in the UKBiobank: A longitudinal cohort study. PLoS Medicine, 17(9), e1003332. 10.1371/journal.pmed.1003332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EURORDIS (2020a). Rare disease community raises alert over discrimination in critical care guidelines during COVID‐19 pandemic [press release]. Retrieved from https://download2.eurordis.org/pressreleases/EURORDISstatement_COVID19Triage.pdf.
- EURORDIS (2020b). Rare Barometer survey on rare disease patients' experience of COVID‐19 Retrieved from https://www.eurordis.org/covid19survey.
- EURORDIS (2020c). 9 in 10 people living with a rare disease experiencing interruption in care because of COVID‐19 [press release]. Retrieved from http://download2.eurordis.org/documents/pdf/PressRelease COVID19surveyresults.pdf.
- FEBRARARAS & Observatório de Doenças Raras (2020). Guidelines on the Coronavirus (Covid‐19) epidemic for people with rare diseases and their caregivers. Retrieved from http://www.sobest.org.br/arquivos/Doen__as_Raras_Covid19_v2_(3).pdf.
- FIOCRUZ . (2020). MonitoraCovid19. Retrieved from https://bigdata-covid19.icict.fiocruz.br/.
- GBD 2016 Brazil Collaborators . (2018). Burden of disease in Brazil, 1990‐2016: A systematic subnational analysis for the Global Burden of Disease Study 2016. Lancet (London, England), 392(10149), 760–775. 10.1016/S0140-6736(18)31221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, R. (2020). Offline: COVID‐19 is not a pandemic. The Lancet, 396(10255), 874. 10.1016/S0140-6736(20)32000-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBGE (2020a). O IBGE apoiando o combate à covid19. Retrieved from https://covid19.ibge.gov.br/pnad-covid/.
- IBGE (2020b). Pesquisa Nacional por Amostra de Domicílios Contínua. Retrieved from https://www.ibge.gov.br/estatisticas/sociais/trabalho/9173‐pesquisa‐nacional‐por‐amostra‐de‐domicilios‐continua‐trimestral.html?t=resultados.
- INLOCO (2020). Mapa brasileiro da Covid‐19. Retrieved from https://www.inloco.com.br/.
- Lampe, C. , Dionisi‐Vici, C. , Bellettato, C. M. , Paneghetti, L. , van Lingen, C. , Bond, S. , … Scarpa, M. (2020). The impact of COVID‐19 on rare metabolic patients and healthcare providers: Results from two MetabERN surveys. Orphanet Journal of Rare Diseases, 15(1), 341. 10.1186/s13023-020-01619-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limongelli, G. , & Crotti, L. (2020). COVID‐19 pandemia and inherited cardiomyopathies and channelopathies: A short term and long term perspective. Orphanet Journal of Rare Diseases, 15(1), 157. 10.1186/s13023-020-01444-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, D. F. , Lucky, A. W. , Salas‐Alanis, J. C. , Woodley, D. T. , Palisson, F. , Natsuga, K. , … Martinez, A. E. (2020). Multidisciplinary care of epidermolysis bullosa during the COVID‐19 pandemic—Consensus: Recommendations by an international panel of experts. Journal of the American Academy of Dermatology, 83(4), 1222–1224. 10.1016/j.jaad.2020.06.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguengang Wakap, S. , Lambert, D. M. , Olry, A. , Rodwell, C. , Gueydan, C. , Lanneau, V. , … Rath, A. (2020). Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. European Journal of Human Genetics, 28(2), 165–173. 10.1038/s41431-019-0508-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPAS (2020). Diretora da OPAS alerta para interrupções nos serviços regulares de saúde devido à COVID‐19. Retrieved from https://www.paho.org/bra/index.php?option=com_content&view=article&id=6244:diretora-da-opas-alerta-para-interrupcoes-nos-servicos-regulares-de-saude-devido-a-covid-19&Itemid=812.
- Pattisapu, P. , Evans, S. S. , Noble, A. R. , Norton, S. J. , Ou, H. C. , Sie, K. C. Y. , & Horn, D. L. (2020). Defining essential services for deaf and hard of hearing children during the COVID‐19 pandemic. Otolaryngology ‐ Head and Neck Surgery (United States), 163(1), 91–93. 10.1177/0194599820925058 [DOI] [PubMed] [Google Scholar]
- Resende, P. C. , Delatorre, E. , Gräf, T. , Mir, D. , Motta, F. C. , Appolinario, L. R. , & Siqueira, M. M. (2020). Genomic surveillance of SARS‐CoV‐2 reveals community transmission of a major lineage during the early pandemic phase in Brazil. BioRxiv, https://www.biorxiv.org/content/10.1101/2020.06.17.158006v1 [Google Scholar]
- SBGM (2020). SBGM orienta pessoas com condições genéticas raras e seus cuidadores na pandemia pelo COVID‐19. Retrieved from https://www.sbgm.org.br/notas-tecnicas-detalhe.aspx?id=3754.
- Solé, G. , Salort‐Campana, E. , Pereon, Y. , Stojkovic, T. , Wahbi, K. , Cintas, P. , … FILNEMUS COVID‐19 study group . (2020). Guidance for the care of neuromuscular patients during the COVID‐19 pandemic outbreak from the French Rare Health Care for Neuromuscular Diseases Network. Revue Neurologique (Paris), 176(6), 507–515. 10.1016/j.neurol.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søreide, K. , Hallet, J. , Matthews, J. B. , Schnitzbauer, A. A. , Line, P. D. , Lai, P. B. S. , … Lorenzon, L. (2020). Immediate and long‐term impact of the COVID‐19 pandemic on delivery of surgical services. British Journal of Surgery, 107(10), 1250–1261. 10.1002/bjs.11670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza, C. D. F. de Paiva, J. P. S. de, Leal, T. C. , Silva, L. F. da, & Santos, L. G. (2020). Spatiotemporal evolution of case fatality rates of COVID‐19 in Brazil, 2020. Jornal Brasileiro de Pneumologia: Publicacao Oficial Da Sociedade Brasileira de Pneumologia e Tisilogia, 46(4), e20200208. 10.36416/1806-3756/e20200208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torales, J. , O'Higgins, M. , Castaldelli‐Maia, J. M. , & Ventriglio, A. (2020). The outbreak of COVID‐19 coronavirus and its impact on global mental health. International Journal of Social Psychiatry, 66(4), 317–320. 10.1177/0020764020915212 [DOI] [PubMed] [Google Scholar]
- Veerapandiyan, A. , Wagner, K. R. , Apkon, S. , McDonald, C. M. , Mathews, K. D. , Parsons, J. A. , … Ciafaloni, E. (2020). The care of patients with Duchenne, Becker, and other muscular dystrophies in the COVID‐19 pandemic. Muscle and Nerve, 62(1), 41–45. 10.1002/mus.26902 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


