Abstract
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has spread rapidly to 185 regions and countries around the world with more than 2.8 million confirmed infections and 203,044 deaths. Respiratory diseases caused by SARS‐CoV‐2 are serious threats to human health.
Objectives
To develop a rapid detection kit for new coronavirus antibodies and use it to study the dynamic changes in antibodies in clinically confirmed SARS‐CoV‐2‐infected patients.
Methods
The SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method) was developed. Serum SARS‐CoV‐2 IgM and IgG antibodies were tested in SARS‐CoV‐2‐ and non‐SARS‐CoV‐2‐infected persons, respectively.
Results and conclusion
The sensitivities of the SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method) were 50%, 70%, 92.5% and 97.5% after 1–3 days, 4–6 days, 7–9 days and >9 days of admission, respectively, and the specificities of the IgM, IgG and IgM + IgG antibodies were all 100%. Using the SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method), the positive rates of SARS‐CoV‐2 IgM and IgG antibodies increased from 50% to 92.5% after 1–3 days, 4–6 days and 7–9 days of admission, which showed an increasing trend. The titers of the SARS‐CoV‐2 IgM and IgG antibodies in the positive specimens increased with the length of admission.
Keywords: antibody, colloidal gold immunochromatography, COVID‐19, infection, SARS‐CoV‐2
1. INTRODUCTION
SARS‐CoV‐2 causes neo‐coronary pneumonia. It belongs to the genus Coronavirus β, and it is the seventh coronavirus known to infect humans. 1 Its genome is a linear single‐stranded positive‐stranded RNA that is approximately 80% homologous to the SARS‐CoV gene. 2 SARS‐CoV‐2 infections cause respiratory tract inflammation, immune system disorders and severe pneumonia. They can be fatal in severe cases, and the risk of hospital infection increases during treatment, posing a serious threat to health and lives. 3 The incubation period of the disease is generally 3–7 days; the shortest incubation period is 1 day and the longest incubation period is 30 days. The infection is contagious during the incubation period. The disease is mainly transmitted from person to person through droplets and contact, and there may be a risk of aerosol transmission in closed unventilated places. 4 , 5 The basis for a previous diagnosis has been a positive nucleic acid test result or confirmation that the virus gene sequence is highly homologous with SARS‐CoV‐2. However, several factors have led to increased false‐negative results of nucleic acid testing, 6 which has had a huge impact on the diagnosis of SARS‐CoV‐2 infection and epidemic prevention and control. To provide enough evidence for diagnosis from a single pathology perspective, the National Health Commission’s latest announcement on 4 March 2020, entitled “New Coronavirus Infected Pneumonia Diagnosis and Treatment Guideline (Trial Version 7)”, added serological tests to the original pathology setup for confirming diagnosed cases, which include suspected cases plus “new coronavirus‐specific IgM antibody and IgG antibody positive”, “new coronavirus‐specific IgG antibody changed from negative to positive” or “antibody levels confirmed to be 4 times higher during the recovery period than the acute phase”. At the same time, the exclusion criteria for suspected cases were as follows: two consecutive tests of the novel coronavirus nucleic acid test are negative (sampling time interval of at least 24 hours), and the new coronavirus‐specific antibodies IgM and IgG are still negative 7 days after the onset of illness.
Specific proteins of the new coronavirus, such as S protein or N protein, can stimulate the immune system of an infected person and initiate an immune response, producing virus‐specific IgM and IgG antibodies. The detection of virus‐specific IgM and IgG antibodies in the serum of suspected patients, using reagents produced by recombinant S protein or N protein antigens, can compensate for the lack of pathogenic detection during diagnosis and the exclusion of suspected cases of new coronary pneumonia and complement pathogenic detection.
2. MATERIALS AND METHODS
2.1. Choice of antigen
The SARS‐CoV‐2 N antigen was obtained from two expression systems of the PET28 vector + BL21 (DE3) strain and the pCMVp‐NEO‐BAN vector + HEK293 cell line. These results were verified using the serum of SARS‐CoV‐2‐ and non‐SARS‐CoV‐2‐infected persons. The SARS‐CoV‐2 N antigen obtained from the expression system of the pCMVp‐NEO‐BAN vector + HEK293 cell line showed a better IgM antibody and IgG antibody performance than those expressed by the PET28 vector + BL21 (DE3) strain during testing of the serum.
2.2. Development of SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method)
The research team developed a SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method) and evaluated its sensitivity and specificity.
2.3. Test principle of SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method)
SARS‐CoV‐2 IgM/IgG was detected using the SARS‐CoV‐2 recombinant antigen and mouse anti‐human IgM/IgG antibody. SARS‐CoV‐2 IgM/IgG reacted with the colloidal gold‐bound SARS‐CoV‐2 recombinant antigen in the sample. The complex was chromatographed along a membrane, and it reached a detection line (T) with murine anti‐human IgM and IgG antibodies. When the result was positive, the colloidal gold SARS‐CoV‐2 recombinant antigen–antibody complex was bound to the IgM or IgG detection line (T), and it was purple–red. When the result was negative, the sample did not contain any SARS‐CoV‐2 recombinant antigen–antibody complex that could bind to the IgM/IgG detection line (T), and the color was not visible (shown in Figure 1).
FIGURE 1.
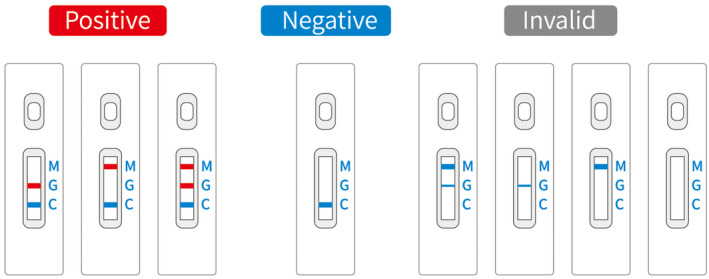
Colloidal gold immunochromatographic test results
2.4. Verification of SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method)
The sensitivity and specificity of the SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method) were determined using serum samples from 40 clinically confirmed COVID‐19 patients and 94 non‐COVID‐19 populations. Forty COVID‐19 patients were enrolled from the First Affiliated Hospital of Anhui Medical University. All the COVID‐19 patients were diagnosed based on the results from the nucleic acid reverse transcription‐polymerase chain reaction (RT‐PCR) test as well as the pathological changes observed in computed tomography (CT) images. The 94 samples with pharyngeal swab or sputum SARS‐CoV‐2 nucleic acid negative results were obtained from the First Affiliated Hospital of Anhui Medical University, including 24 samples of pregnant women (serum of pregnant women at 15–30 weeks), 25 serum samples of patients with other respiratory infections (non‐COVID‐19 patients with respiratory symptoms, such as mycoplasma pneumoniae, parainfluenza virus, adenovirus and influenza B virus), 24 serum samples of individuals with increased rheumatoid factor (more than one time above the upper limit of reference value; reference value: 0–14 IU/mL), and 21 hemolytic samples. The anti‐229E (alphacoronavirus) IgG‐positive (five samples), anti‐NL63 (alphacoronavirus) IgG‐positive (five samples), anti‐OC43 (betacoronavirus) IgG‐positive (five samples), anti‐HKU1 (betacoronavirus) IgG‐positive (five samples) and anti‐rhinovirus IgG‐positive serum samples (five samples) were detected using the SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method). All the results were negative.
2.5. Research object
Forty COVID‐19 patients, aged between 21 and 71 years, with a median age of 46 years were enrolled from the First Affiliated Hospital of Anhui Medical University. All the COVID‐19 patients were diagnosed based on the results from the nucleic acid reverse transcription‐polymerase chain reaction (RT‐PCR) test as well as the pathological changes observed in computed tomography (CT) images. The 1061 samples with pharyngeal swab or sputum SARS‐CoV‐2 nucleic acid and serum SARS‐CoV‐2 IgM and IgG antibody negative results were obtained from the First Affiliated Hospital of Anhui Medical University. They included the following: 281 patients with other respiratory infections (non‐COVID‐19 patients with respiratory symptoms), aged between 2 and 99 years, with a median of 51 years; 252 non‐respiratory patients (including 30 cases of rheumatic immune system diseases and 20 cases of severe liver disease), aged between 1 and 90 years, with a median of 50 years; 416 pregnant women (serum of pregnant women at 15–30 weeks), aged 18–34 years, with a median of 27 years; 112 cases of normal physical examination population, aged 23–72 years, with a median of 50 years.
2.6. Specimen collection and processing
Serum samples were used in this study. Blood samples were collected from patients with SARS‐CoV‐2 infection after 1–3 days, 4–6 days, 7–9 days and 9 days of admission. The blood samples were collected from 7 AM to 7:30 AM every day, sent for testing within 30 min and centrifuged at 3500 rpm for 5 min to obtain the sera followed by SARS‐CoV‐2 IgM and IgG antibody testing. The sera of the non‐COVID‐19 populations were obtained from the remaining samples after clinical testing. Considering the potential transmission through blood, all the blood samples were inactivated at 56°C for 30 minutes.
2.7. Antibody titer testing
The remaining serum samples of patients with non‐respiratory infections were collected, mixed and tested with the SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method) to confirm the SARS‐CoV‐2 IgM‐ and SARS‐CoV‐2 IgG‐negative cases. The COVID‐19 serum samples were diluted with the negative serum collected above and tested with the SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method) until they were found to be SARS‐CoV‐2 IgM and SARS‐CoV‐2 IgG negative. The highest dilution factor was determined (Figure 2).
FIGURE 2.
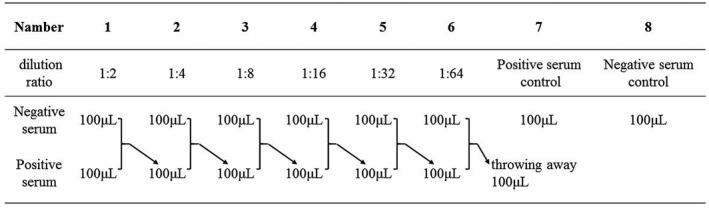
Dilution of sample
2.8. Statistical method
Chi‐squared inspection was used for comparison (chi‐square test).
3. RESULTS
3.1. Sensitivity and specificity of the SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method)
The SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method) was used to detect IgM and IgG antibodies in serum samples from 40 clinical COVID‐19 patients. The sensitivities of the IgM antibody, IgG antibody and IgM antibody + IgG antibody (IgM antibody, IgG antibody alone or both are positive) reached 97.5% after 9 days of hospitalization (the results are shown in Table 1).
TABLE 1.
Sensitivity of the SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method)
| Sensitivity | 1‐3 days (%) | 4‐6 days (%) | 7‐9 days (%) | >9 days (%) |
|---|---|---|---|---|
| IgM antibody | 50 | 70 | 92.5 | 97.5 |
| IgG antibody | 50 | 70 | 92.5 | 97.5% |
| IgM antibody + IgG antibody | 55 | 75 | 95 | 97.5 |
The SARS‐CoV‐2 IgM/IgG antibody test kit (colloidal gold method) was used to detect IgM and IgG antibodies in 94 non‐COVID‐19 serum samples. The specificities of the IgM antibody, IgG antibody and IgM antibody + IgG antibody were all 100%.
3.2. Study on kinetics of antibody from SARS‐CoV‐2‐infected population
3.2.1. Changes in IgM and IgG antibody positivity rates at different times of SARS‐CoV‐2 infection
The antibody‐positive rate increased from 50% to 92.5% after 1–3 days and 7–9 days of admission and 97.5% after 9 days of admission (the results are shown in Figure 3). However, one patient was still negative for IgM and IgG antibodies on day 22 after admission.
FIGURE 3.
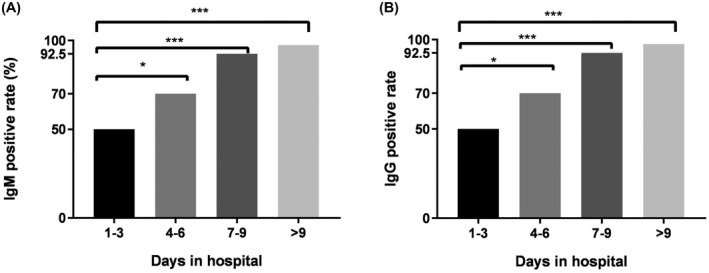
Changes in IgM and IgG antibody positive rates in patients with SARS‐CoV‐2 infection at different days of admission
3.2.2. Changes in antibody titers of SARS‐CoV‐2‐infected patients after admission
The titers of the IgM antibody‐positive patients were mainly 1–2 times after 1–3 days of admission. Two patients had IgM antibody titers of more than 4 times. The IgM antibody titers were as follows: mainly 2–8 times within 4–6 days, and two patients had titers of more than 8 times; mainly 2–16 times within 7–9 days, and four patients had titers of more than 16 times; and mainly 4–32 times after more than 9 days, and four cases reached 64 times. No patient had an IgM antibody titer exceeding 64 times (see Figure 4A). The IgG and IgM antibody titers showed similar trends (Figure 4B).
FIGURE 4.
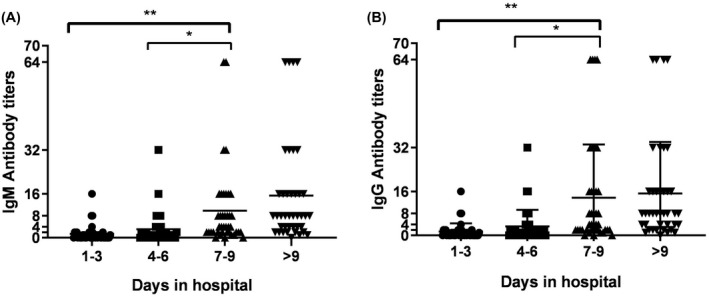
IgM antibody and IgG antibody titers in patients with SARS‐CoV‐2 infection on different days of admission
3.3. Analysis of false‐positive rates of SARS‐CoV‐2 IgM and IgG antibodies in non‐SARS‐CoV‐2‐infected people
Of the 1061 samples of non‐SARS‐CoV‐2‐infected participants, the IgM test results were positive in 6 from other respiratory infection patients and 2 from pregnant women. All 8 IgM‐positive samples were weakly positive. After 1 week, the results of the re‐sampled serum were negative (see the results in Table 2). There were no false‐positive results for IgG for the 1061 samples of the non‐SARS‐CoV‐2‐infected people. The results are shown in Table 3.
TABLE 2.
Analysis of IgM test results in 1061 non‐SARS‐CoV‐2‐infected people
| S/N | Sample type | Sample amount | IgM(+) | IgM(−) | False‐positive rate |
|---|---|---|---|---|---|
| 1 | Samples of patients with other respiratory infections | 281 | 6 | 275 | 2.1% |
| 2 | Pregnant woman sample | 416 | 2 | 414 | 0.5% |
| 3 | Non‐respiratory infection patient samples | 252 | 0 | 252 | 0 |
| 4 | Physical examination sample | 112 | 0 | 112 | 0 |
| 5 | Total | 1061 | 8 | 1053 | 0.75% |
TABLE 3.
Analysis of IgG test results in 1061 non‐SARS‐CoV‐2‐infected people
| S/N | Sample type | Sample amount | IgG(+) | IgG(−) | False‐positive rate |
|---|---|---|---|---|---|
| 1 | Samples of patients with other respiratory infections | 281 | 0 | 282 | 0 |
| 2 | Pregnant woman sample | 416 | 0 | 416 | 0 |
| 3 | Non‐respiratory infection patient samples | 252 | 0 | 252 | 0 |
| 4 | Physical examination sample | 112 | 0 | 112 | 0 |
| 5 | Total | 1061 | 0 | 1061 | 0 |
4. DISCUSSION
In this study, the SARS‐CoV‐2 N antigen obtained from the two expression systems of the PET28 vector + BL21 (DE3) strain and pCMVp‐NEO‐BAN vector + HEK293 cell line was verified. The SARS‐CoV‐2 N antigen obtained from the pCMVp‐NEO‐BAN vector + HEK293 cell line for the detection of the IgM and IgG antibodies in the serum was better than that of the PET28 carrier + BL21 (DE3) strain. It may be because the prokaryotic expression system cannot form the correct protein spatial structure. Therefore, the pCMVp‐NEO‐BAN vector + HEK293 cell line was selected to express the antigen for subsequent research. During this project, the SARS‐CoV‐2 IgM and IgG antibody kits were developed based on colloidal gold immunochromatography. After verification using the sera of the SARS‐CoV‐2‐ and non‐SARS‐CoV‐2‐infected patients, the colloidal gold immunochromatography kit had high sensitivity (see Table 1) with a specificity of 100%.
A medical team from Guangzhou established a rapid IgM‐IgG antibody detection method and used the kit for clinical research. The clinical sensitivity and specificity of the test were determined using blood samples from 397 COVID‐19 patients and 128 negative patients, which were confirmed by PCR in 8 different hospitals. The detection sensitivity and specificity were 88.66% and 90.63%, respectively. This study indicates that the combined IgM‐IgG test has better practicality and sensitivity than a single IgM or IgG test. 7 Our results also show that the combined detection of IgM and IgG can increase the detection rate of infected patients (see Table 1).
Guo et al. reported that the positive rates of IgM antibodies and IgG antibodies in SARS‐CoV‐2‐infected patients increased significantly after 7–14 days of symptoms. 8 Another study reported that the IgG detection rates reached 100.0% in samples collected on day 13 or later using an immunochromatographic assay kit. 9 Considering the uncertainty of the time patients can recall the symptoms, this study used the number of days of admission as the basis for grouping. The results showed that the positive rates of IgM and IgG antibodies 1–3 days, 4–6 days, 7–9 days and more than 9 days after admission (see Figure 3) were 50%, 70%, 92.5% and 97.5%, respectively, suggesting that the antibody positivity rate increased rapidly during the early stages of infection, and antibody detection can be used as an indicator for SARS‐CoV‐2 infection. However, one infected person was negative for IgM and IgG antibodies during the third week. From the medical records, this infected person only had a history of exposure and did not show clinical symptoms. The nucleic acid test continued to be positive, suggesting that there was a delay in antibody production in the individual, which required attention. At the same time, we found an interesting phenomenon: the positive rate of IgM and IgG antibodies showed a parallel rise (see Figure 3), and further research is needed.
The serum antibody titers of COVID‐19 patients increase with the length of hospital stay, but there are individual differences. After more than 9 days of hospitalization, antibody titers of 70% of patients can rise to more than 4 times, and those of some patients can rise to 32–64 times (see Figure 4). The test results of 1061 non‐SARS‐CoV‐2‐infected people showed eight cases of weak positive SARS‐CoV‐2 IgM results: six cases of other respiratory infections and two cases of pregnant women. The results were reviewed after 1 week, and they were negative. It has been suggested that some factors interfere with the detection of SARS‐CoV‐2 IgM in other respiratory infections and pregnant women, but the result can be confirmed by a retest after 1 week. Negative health checkups and other systemic diseases, such as rheumatic immune system disease and severe liver disease, did not affect the test results.
The specificity and sensitivity of the SARS‐CoV‐2 virus serology enzyme‐linked immunosorbent assay (ELISA) kit were reported as 97.5% and 97.1%, respectively, by a previous study. 10 At present, ELISA 10 , 11 and the colloidal gold method 12 are the two main methods for detecting IgM and IgG antibodies against SARS‐CoV‐2. The advantages of the colloidal gold method are that it is rapid, simple to use, sensitive and accurate; it can be used to quickly identify infected patients with SARS‐CoV‐2 to prevent virus transmission and ensure timely treatment. Recently, chemiluminescent immunoassay (CLIA), 13 fluorescence‐linked immunosorbent assay, 14 indirect immunofluorescence assay 15 and other techniques have been developed to improve the accuracy of serological detection. Antibody tests are too insensitive to be used in the diagnosis of COVID‐19 during the first week after symptom onset; however, if antibody tests are used 9 days or more after the onset of symptoms, they may be useful for detecting previous SARS‐CoV‐2 infections. Our research on the SARS‐CoV‐2 nucleocapsid protein assay showed that the measurement of the serum SARS‐CoV‐2 N protein has a high diagnostic value for infected patients before antibodies are produced, which shortens the window for serological diagnosis. 16 Thus, antibody and antigen tests may complement other testing in individuals presenting later when RT‐PCR tests are negative or not performed.
5. CONCLUSION
The positive rate and titer of the SARS‐CoV‐2 antibody showed a rapid increase with time. Patients who initially tested negative should be retested after 7 days. Patients who initially tested positive should have a titer test, which should be repeated after 7 days to determine whether it is a SARS‐CoV‐2 infection based on the titer change.
Other patients with respiratory infections had a 2.5% false‐positive rate for IgM antibodies; a 0.5% false‐positive rate for IgM antibodies was observed for pregnant women, which was identified by retesting after 1 week.
ETHICS
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethics Committee of the First Affiliated Hospital of Anhui Medical University approved this study.
CONFLICT OF INTERESTS
The antigen screening, methodological selection and reagent production of this study were completed in the research and development department of Biohit Healthcare (Hefei) Co., Ltd. Methodological evaluation and performance verification were completed in the First Affiliated Hospital of Anhui Medical University. All authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Designed the study and performed some of the statistics: T. Li and L. Wang; Contributed important reagents: L. Wang and J. Lin; Collected data and analyzed data: H. Wang, X. Li, and S. Zhang; Wrote the paper: T. Li, Y. Xu, and W. Wei.
Wang H, Li X, Li T, et al. Development of a SARS‐CoV‐2 rapid antibody detection kit and study on dynamic changes in antibodies in infected patients. Clin Respir J. 2021;15:499–505. 10.1111/crj.13331
Funding information
This work was supported by the Scientific Research Project of Anhui Province for the Prevention and Control of New Coronavirus Pneumonia (202004a07020015).
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Reference
- 1. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181‐192. 10.1038/s41579-018-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709–710. 10.1001/jama.2020.1097 [DOI] [PubMed] [Google Scholar]
- 4. Chan J‐W, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401‐402. 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin L, Guangming Y, Liangjun C, Jiajun W, Yirong L. Analysis of false‐negative results for 2019 novel coronavirus nucleic acid test and related countermeasures. Chin J Lab Med. 2020;43:E006–E006. 10.3760/cma.j.issn.1009-9158.2010.0006 [DOI] [Google Scholar]
- 7. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020;92:1518–1524. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020;71:778–785. 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chong Y, Ikematsu H, Tani N, et al. Clinical significance of SARS‐CoV‐2‐specific IgG detection with a rapid antibody kit for COVID‐19 patients. Influenza Other Respir Viruses. 2021;15:13–18. 10.1111/irv.12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao R, Li M, Song H, et al. Early detection of severe acute respiratory syndrome coronavirus 2 antibodies as a serologic marker of infection in patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:2066–2072. 10.1093/cid/ciaa523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang W, Du R‐H, Li B, et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386‐389. 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020;92(9):1518‐1524. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Infantino M, Grossi V, Lari B, et al. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti‐SARS‐CoV‐2 IgM and IgG antibodies: an Italian experience [published online ahead of print, 2020 Apr 24]. J Med Virol. 2020;92(9):1671‐1675. 10.1002/jmv.25932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo J, Wang Y, Niu S, et al. Highly sensitive fluorescence‐linked immunosorbent assay for the determination of human IgG in serum using quantum dot nanobeads and magnetic Fe3O4 nanospheres. ACS Omega. 2020;5(36):23229‐23236. 10.1021/acsomega.0c02987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edouard S, Colson P, Melenotte C, et al. Evaluating the serological status of COVID‐19 patients using an indirect immunofluorescent assay, France. Eur J Clin Microbiol Infect Dis. 2021;40:361–371. 10.1007/s10096-020-04104-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li T, Wang LI, Wang H, et al. Serum SARS‐COV‐2 nucleocapsid protein: a sensitivity and specificity early diagnostic marker for SARS‐COV‐2 infection. Front Cell Infect Microbiol. 2020;10:470. 10.3389/fcimb.2020.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


